Effects of NMDA Receptor Hypofunction on Inhibitory Control in a Two-Layer Neural Circuit Model
Abstract
:1. Introduction
2. Materials and Methods
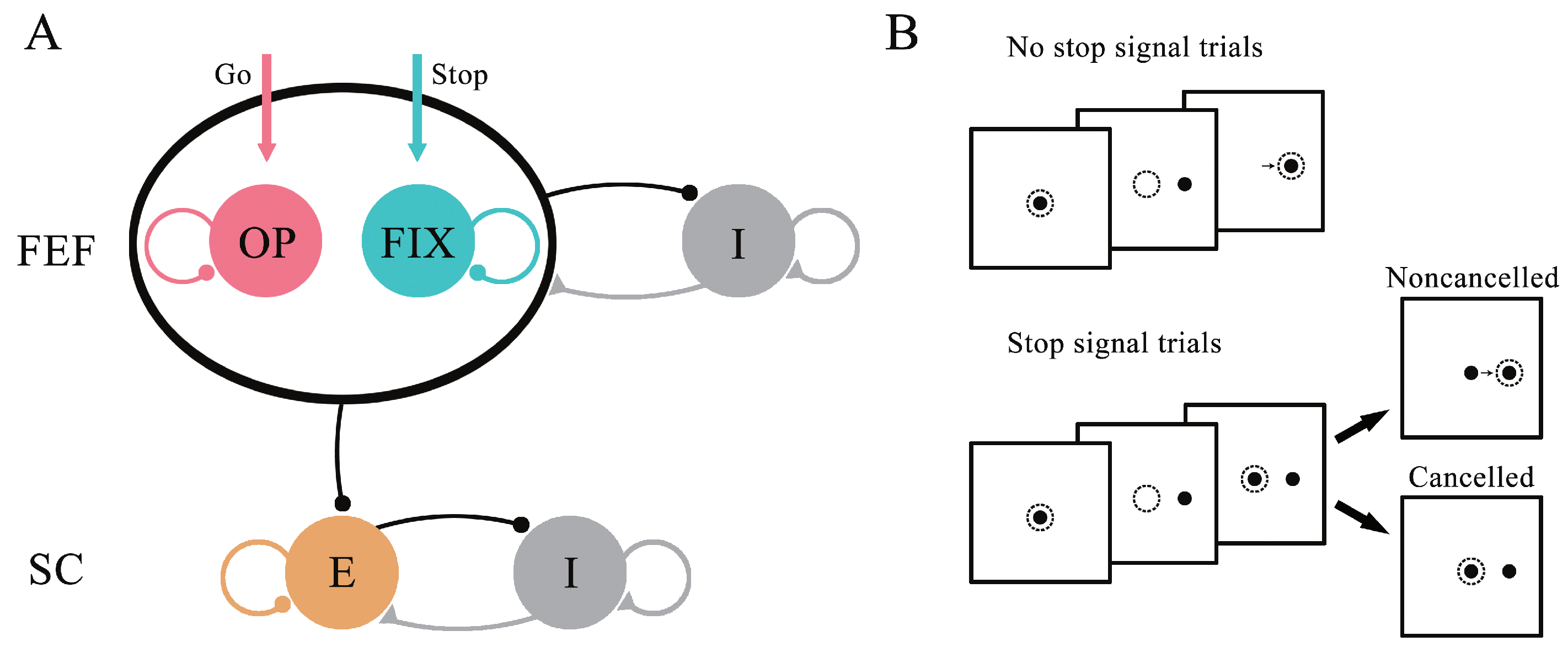
2.1. Network Model
2.2. Cognitive Tasks
3. Results
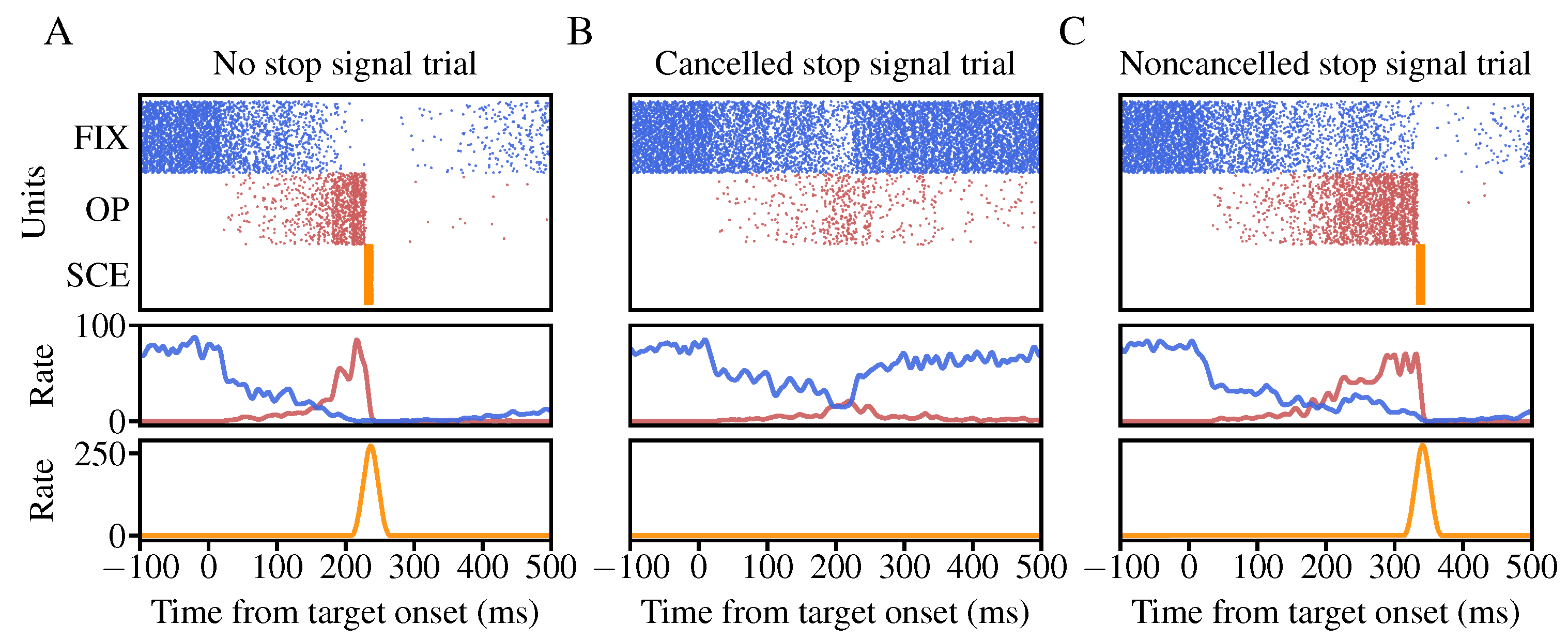
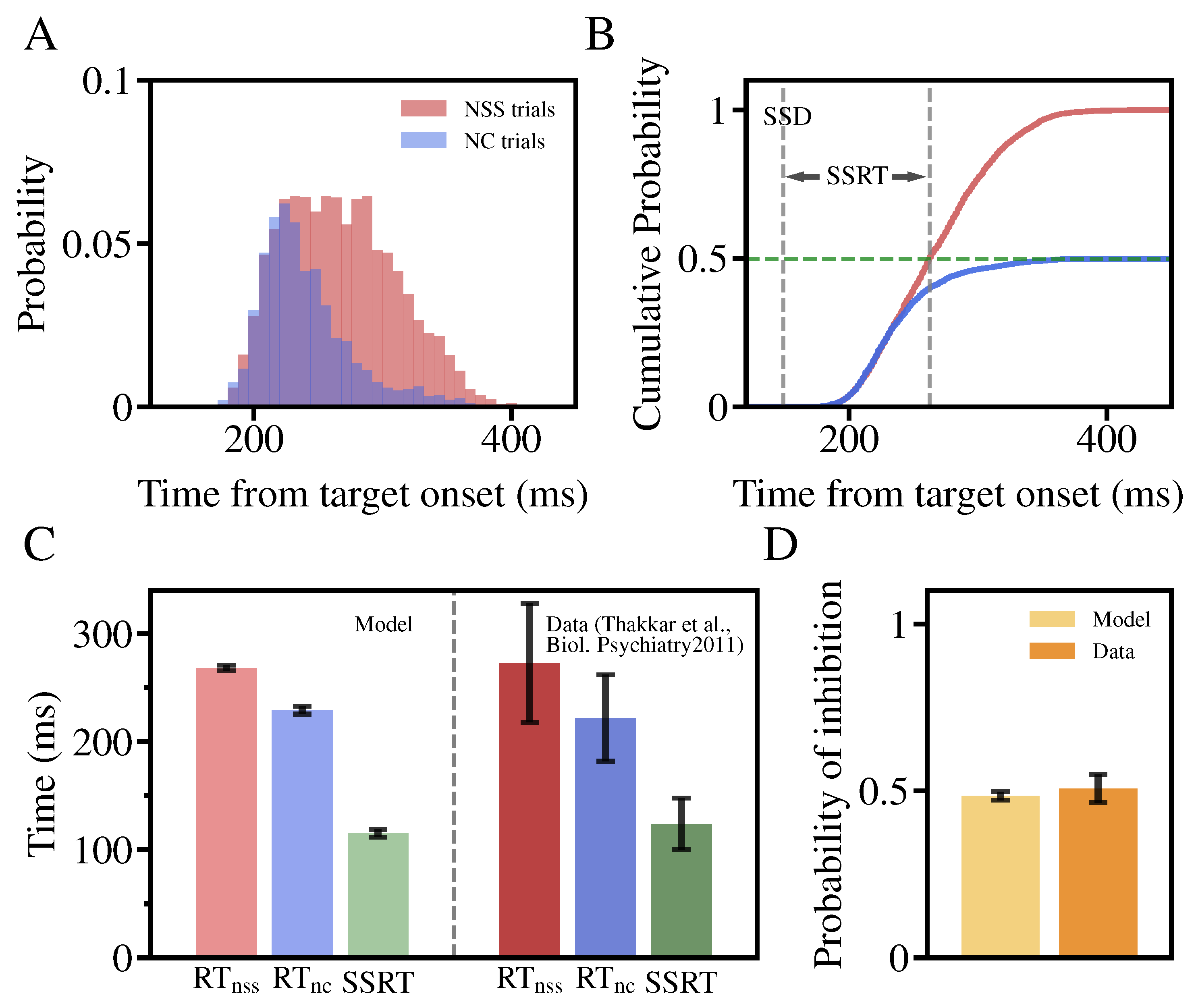
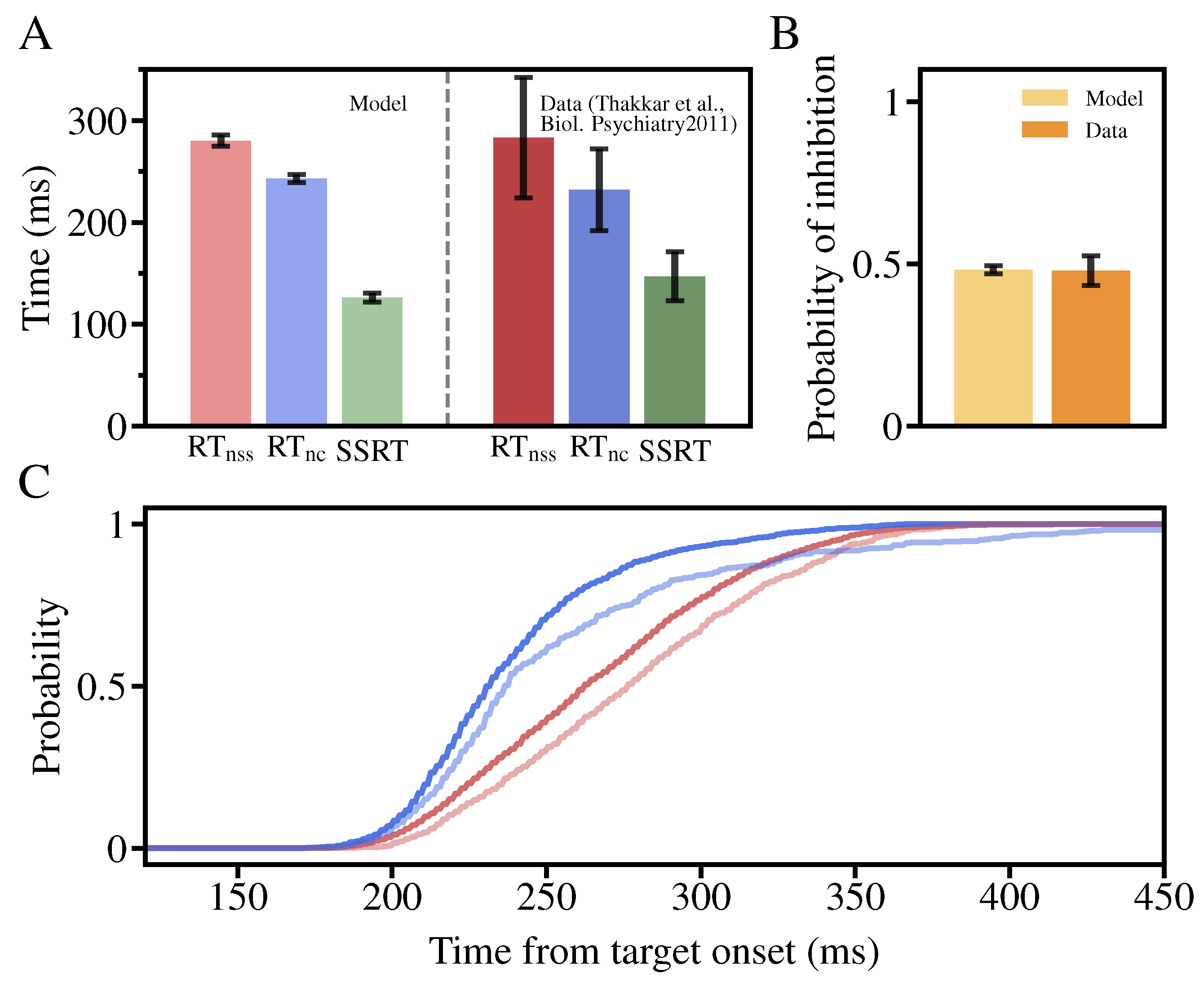
3.1. The Performance of the Neural Circuit Model in the Stop-Signal Task
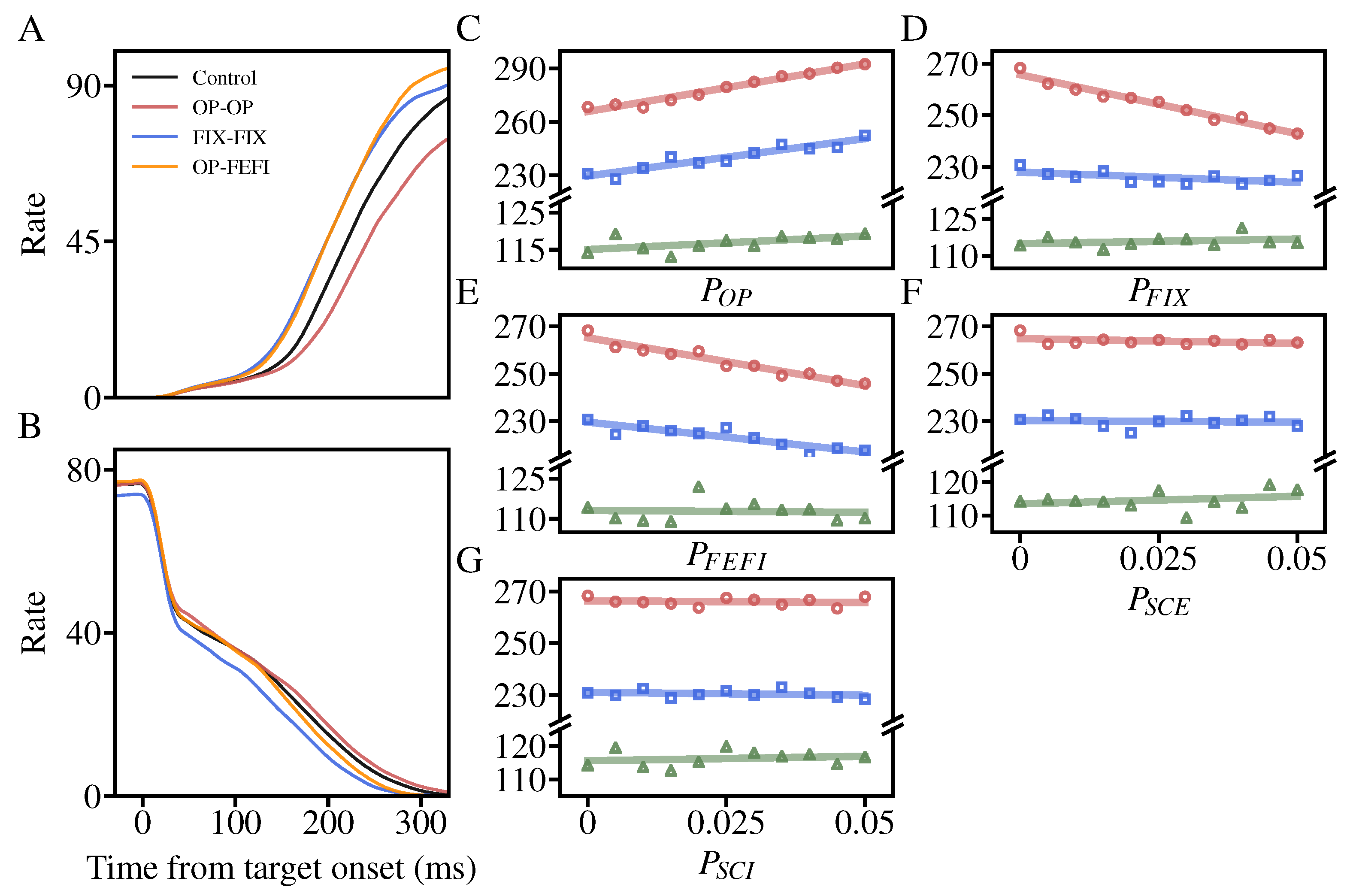
3.2. The Effects of NMDA Receptor Hypofunction on the Inhibitory Control
- (1)
- Compared to the control model, NMDA receptor hypofunction on OP-OP makes the ramping-up activity less activated and increases with a slower speed, while the corresponding activity in FIX becomes more activated because of the competition between OP and FIX. Naturally, the weakening of the excitatory input to OP results in a decrease in population activity.
- (2)
- In contrast, the NMDA receptor hypofunction on OP-FEFI and FIX-FIX mainly enhances the OP activities and reduce the FIX responses.
3.3. The Stop-Signal Task Performance in the Two-Layer Neural Circuit Model
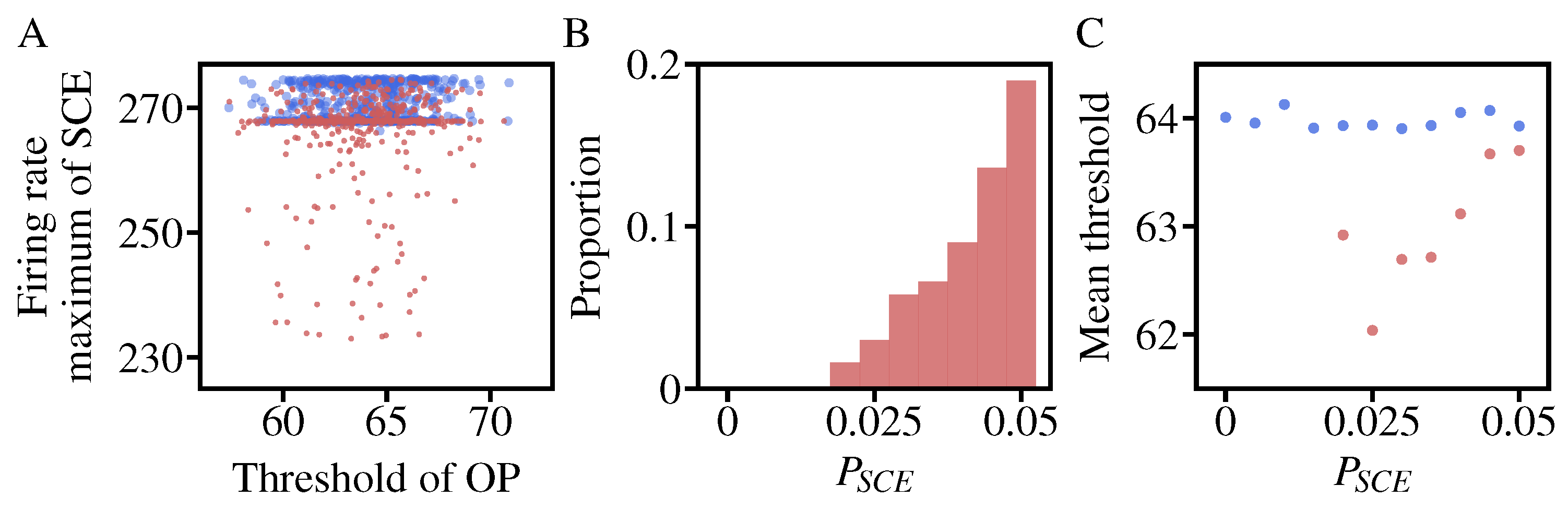
3.4. Changes of the Saccade Threshold While Varying the NMDA Hypofunction Rate
4. Conclusions and Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schall, J.; Palmeri, T.; Logan, G. Models of inhibitory control. Philos. Trans. R. Soc. Biol. Sci. 2017, 372, 20160193. [Google Scholar] [CrossRef] [PubMed]
- Middlebrooks, P.; Schall, J. Response inhibition during perceptual decision making in humans and macaques. Atten. Percept. Psychophys. 2014, 76, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Ethridge, L.; Soilleux, M.; Nakonezny, P.; Reilly, J.; Kristian Hill, S.; Keefe, R.; Gershon, E.; Pearlson, G.; Tamminga, C.; Keshavan, M.; et al. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr. Res. 2014, 159, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, K.; Schall, J.; Logan, G.; Park, S. Cognitive control of gaze in bipolar disorder and schizophrenia. Psychiatry Res. 2015, 225, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Schall, J.; Hanes, D.; Taylor, T. Neural control of behavior: Countermanding eye movements. Psychol. Res. 2000, 63, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Aron, A.; Band, G.; Beste, C.; Bissett, P.; Brockett, A.; Brown, J.; Chamberlain, S.; Chambers, C.; Colonius, H.; et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 2019, 8, e46323. [Google Scholar] [CrossRef]
- Thakkar, K.; Schall, J.; Boucher, L.; Logan, G.; Park, S. Response Inhibition and Response Monitoring in a Saccadic Countermanding Task in Schizophrenia. Biol. Psychiatry 2011, 69, 55–62. [Google Scholar] [CrossRef]
- Kort, N.; Ford, J.; Roach, B.; Gunduz-Bruce, H.; Krystal, J.; Jaeger, J.; Reinhart, R.; Mathalon, D. Role of N-Methyl-D-Aspartate Receptors in Action-Based Predictive Coding Deficits in Schizophrenia. Biol. Psychiatry 2017, 81, 514–524. [Google Scholar] [CrossRef]
- Lee, G.; Zhou, Y. NMDAR Hypofunction Animal Models of Schizophrenia. Front. Mol. Neurosci. 2019, 12, 1–26. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426. [Google Scholar] [CrossRef]
- Willetts, J.; Balster, R. The discriminative stimulus effects of N-methyl-d-aspartate antagonists in phencyclidine-trained rats. Neuropharmacology 1988, 27, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.; Karper, L.; Seibyl, J.; Freeman, G.; Delaney, R.; Bremner, J.; Heninger, G.; Bowers, M.B.J.; Charney, D. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Clinical Physiology and Mechanism of Dizocilpine (MK-801): Electron Transfer, Radicals, Redox Metabolites and Bioactivity. Oxid. Med. Cell. Longev. 2010, 3, 530859. [Google Scholar] [CrossRef] [PubMed]
- Coronel-Oliveros, C.; Pacheco-Calderon, R. Prenatal exposure to ketamine in rats: Implications on animal models of schizophrenia. Dev. Psychobiol. 2018, 60, 30–42. [Google Scholar] [CrossRef]
- Szlachta, M.; Pabian, P.; Kusmider, M.; Solich, J.; Kolasa, M.; Zurawek, D.; Dziedzicka-Wasylewska, M.; Faron-Górecka, A. Effect of clozapine on ketamine-induced deficits in attentional set shift task in mice. Psychopharmacology 2017, 234, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Pinto-Duarte, A.; Kappe, A.; Zembrzycki, A.; Metzler, A.; Mukamel, E.; Lucero, J.; Wang, X.; Sejnowski, T.; Markou, A.; et al. Disruption of mGluR5 in parvalbumin-positive interneurons induces core features of neurodevelopmental disorders. Mol. Psychiatry 2015, 20, 1161–1172. [Google Scholar] [CrossRef]
- Boucher, L.; Palmeri, T.; Logan, G.; Schall, J. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychol. Rev. 2007, 114, 376–397. [Google Scholar] [CrossRef]
- Logan, G.; Yamaguchi, M.; Schall, J.; Palmeri, T. Inhibitory control in mind and brain 2.0: Blocked-input models of saccadic countermanding. Psychol. Rev. 2015, 122, 115–147. [Google Scholar] [CrossRef]
- Lo, C.; Boucher, L.; Pare, M.; Schall, J.; Wang, X. Proactive inhibitory control and attractor dynamics in countermanding action: A spiking neural circuit model. J. Neurosci. 2009, 29, 9059–9071. [Google Scholar] [CrossRef]
- Wang, X. Decision making in recurrent neuronal circuits. Neuron 2008, 60, 215–234. [Google Scholar] [CrossRef]
- Lundqvist, M.; Compte, A.; Lansner, A. Bistable, Irregular Firing and Population Oscillations in a Modular Attractor Memory Network. PLoS Comp. Biol. 2010, 6, e1000803. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.; Cordier, S.; Deco, G.; Mancini, S. Complexity Reduction of Rate-Equations Models for Two-Choice Decision-Making. PLoS ONE 2013, 8, e80820. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.; Sarazin, M.; Clemente, A.; Holden, S.; Paz, J.; Delord, B. Conditional bistability, a generic cellular mnemonic mechanism for robust and flexible working memory computations. J. Neurosci. 2018, 38, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Seeholzer, A.; Deger, M.; Gerstner, W. Stability of working memory in continuous attractor networks under the control of short-term plasticity. PLoS Comp. Biol. 2019, 15, e1006928. [Google Scholar] [CrossRef]
- Ye, W. Dynamics of a revised neural mass model in the stop-signal task. Chaos Solitons Fractals 2020, 139, 110004. [Google Scholar] [CrossRef]
- Li, P.; Lu, Y.; Xu, C.; Ren, J. Insight into Hopf Bifurcation and Control Methods in Fractional Order BAM Neural Networks Incorporating Symmetric Structure and Delay. Cogn. Comput. 2023, 1–43. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Li, P.; Yan, J.; Yao, L. Bifurcation Mechanism for Fractional-Order Three-Triangle Multi-delayed Neural Networks. Neural Process. Lett. 2022, 1–27. [Google Scholar] [CrossRef]
- Li, P.; Peng, X.; Xu, C.; Han, L.; Shi, S. Novel extended mixed controller design for bifurcation control of fractional-order Myc/E2F/miR-17-92 network model concerning delay. Math. Methods Appl. Sci. 2023, 1–21. [Google Scholar] [CrossRef]
- Lam, N.; Borduqui, T.; Hallak, J.; Roque, A.; Anticevic, A.; Krystal, J.; Wang, X.; Murray, J. Effects of Altered excitation–inhibition Balance on Decision Making in a Cortical Circuit Model. J. Neurosci. 2022, 42, 1035–1053. [Google Scholar] [CrossRef]
- Murray, J.; Anticevic, A.; Gancsos, M.; Ichinose, M.; Corlett, P.; Krystal, J.; Wang, X. Linking Microcircuit Dysfunction to Cognitive Impairment: Effects of Disinhibition Associated with Schizophrenia in a Cortical Working Memory Model. Cereb Cortex 2012, 24, 859–872. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.; Prigge, M.; Schneider, F.; Davidson, T.; O’Shea, D.; Sohal, V.; Goshen, I.; Finkelstein, J.; Paz, J.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Pare, M.; Hanes, D. Controlled Movement Processing: Superior Colliculus Activity Associated with Countermanded Saccades. J. Neurosci. 2003, 23, 6480–6489. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Logan, G. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen. 2008, 137, 649–672. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.; Connors, B.; Lighthall, J.; Prince, D. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 1985, 54, 782–806. [Google Scholar] [CrossRef] [PubMed]
- Brunel, N.; Wang, X. Effects of Neuromodulation in a Cortical Network Model of Object Working Memory Dominated by Recurrent Inhibition. J. Comput. Neurosci. 2001, 11, 63–85. [Google Scholar] [CrossRef]
- Wang, X. Probabilistic Decision Making by Slow Reverberation in Cortical Circuits. Neuron 2002, 36, 955–968. [Google Scholar] [CrossRef]
- Tuckwell, H.; Wan, F.; Rospars, J. A spatial stochastic neuronal model with Ornstein-Uhlenbeck input current. Biol. Cybern. 2002, 86, 137–145. [Google Scholar] [CrossRef]
- Wong, K.; Wang, X. A recurrent network mechanism of time integration in perceptual decisions. J. Neurosci. 2006, 26, 1314–1328. [Google Scholar] [CrossRef]
- Nakao, K.; Jeevakumar, V.; Jiang, S.; Fujita, Y.; Diaz, N.; Pretell Annan, C.; Eskow Jaunarajs, K.; Hashimoto, K.; Belforte, J.; Nakazawa, K. Schizophrenia-Like Dopamine Release Abnormalities in a Mouse Model of NMDA Receptor Hypofunction. Schizophr. Bull. 2018, 45, 138–147. [Google Scholar] [CrossRef]
- Van Vreeswijk, C.; Sompolinsky, H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 1996, 274, 1724–1726. [Google Scholar] [CrossRef]
- Shu, Y.; Hasenstaub, A.; McCormick, D. Turning on and off recurrent balanced cortical activity. Nature 2003, 423, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, S.; Lam, N.; Murray, J.; Hunt, L.; Kennerley, S. A circuit mechanism for decision-making biases and NMDA receptor hypofunction. eLife 2020, 9, e53664. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Wang, C.; Wang, X. Speed-accuracy tradeoff by a control signal with balanced excitation and inhibition. J. Neurophysiol. 2015, 114, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Derosiere, G.; Thura, D.; Cisek, P.; Duque, J. Trading accuracy for speed over the course of a decision. J. Neurophysiol. 2021, 126, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, X. Inhibitory Control in the Cortico-Basal Ganglia-Thalamocortical Loop: Complex Regulation and Interplay with Memory and Decision Processes. Neuron 2016, 92, 1093–1105. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Obeso, I.; Rothwell, J.; Obeso, J. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 2015, 16, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Everling, S.; Dorris, M.; Munoz, D. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J. Neurophysiol. 1998, 80, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Munoz, D.; Everling, S. Look away: The anti-saccade task and the voluntary control of eye movement. Nat. Rev. Neurosci. 2004, 5, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, A.; Ting, D.; Elangovan, K.; Gutierrez, L.; Tan, T.; Ting, D. Large language models in medicine. Nat. Med. 2023, 29, 1930–1940. [Google Scholar] [CrossRef]
- de Curto, J.; de Zarza, I.; Roig, G.; Cano, J.; Manzoni, P.; Calafate, C. LLM-Informed Multi-Armed Bandit Strategies for Non-Stationary Environments. Electronics 2023, 12, 2814. [Google Scholar] [CrossRef]
- de Zarza, I.; de Curto, J.; Roig, G.; Calafate, C. LLM Adaptive PID Control for B5G Truck Platooning Systems. Sensors 2023, 23, 5899. [Google Scholar] [CrossRef] [PubMed]
- de Zarza, I.; de Curto, J.; Roig, G.; Manzoni, P.; Calafate, C. Emergent Cooperation and Strategy Adaptation in Multi-Agent Systems: An Extended Coevolutionary Theory with LLMs. Electronics 2023, 12, 2722. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Chen, X. Effects of NMDA Receptor Hypofunction on Inhibitory Control in a Two-Layer Neural Circuit Model. Mathematics 2023, 11, 4063. https://doi.org/10.3390/math11194063
Ye W, Chen X. Effects of NMDA Receptor Hypofunction on Inhibitory Control in a Two-Layer Neural Circuit Model. Mathematics. 2023; 11(19):4063. https://doi.org/10.3390/math11194063
Chicago/Turabian StyleYe, Weijie, and Xiaoying Chen. 2023. "Effects of NMDA Receptor Hypofunction on Inhibitory Control in a Two-Layer Neural Circuit Model" Mathematics 11, no. 19: 4063. https://doi.org/10.3390/math11194063
APA StyleYe, W., & Chen, X. (2023). Effects of NMDA Receptor Hypofunction on Inhibitory Control in a Two-Layer Neural Circuit Model. Mathematics, 11(19), 4063. https://doi.org/10.3390/math11194063






