Abstract
One of the most deadly neglected tropical diseases known to man is schistosomiasis. Understanding how the disease spreads and evaluating the relevant control strategies are key steps in predicting its spread. We propose a mathematical model to evaluate the potential impact of four strategies: chemotherapy, awareness programs, the mechanical removal of snails and molluscicides, and the impact of a change in temperature on different molluscicide performances based on their half-lives and the length of time they persist in contact with target species. The results show that the recruitment rate of humans and the presence of cercaria and miracidia parasites are crucial factors in disease transmission. However, schistosomiasis can be entirely eradicated by combining all of the four strategies. In the face of climate change and molluscicide degradation, the results show that increasing the temperatures and the number of days a molluscicide persists in the environment before it completely degrades decreases the chemically induced mortality rate of snails while increasing the half-life of different molluscicides increases the death rate of snails. Therefore, eradicating schistosomiasis effectively necessitates a comprehensive integration of all preventative measures. Moreover, regions with different weather patterns and seasonal climates need strategies that have been adapted in terms of the appropriate molluscicide and time intervals for reapplication and effective schistosomiasis control.
Keywords:
chemotherapy; public literacy; mechanical removal of snails; molluscicide performance; temperature; molluscicide degradation; half-life MSC:
92B05
1. Introduction
Schistosomiasis is the second-most significant neglected tropical disease (NTD), a physically debilitating and persistent disease [1,2] that leads to severe morbidity and almost 12,000 deaths globally, of which at least 90% are from sub-Saharan Africa [2]. Human schistosomiasis infection caused by Trematoda worms depends on the availability of suitable freshwater intermediate host snails (IHs) and the final human host to be transmitted [3]. The main symptoms of infection include skin rash and itching, fever, cough, muscle pain, bloody urine, and growth retardation in children [2], while severe cases can lead to damage and failure of the liver, bladder, lungs, and intestines [1,2]. Unfortunately, unlike most NTDs, including lymphatic filariasis, leprosy, and leishmaniasis, there are currently no recommendations for intensive disease management for schistosomiasis [4], and current disease control strategies rely on mass drug administration (MDA) chemotherapy, which, at times, is combined with health education [5,6]. Although there has been a success in the implementation of chemotherapy, especially in Africa [7,8], this may have limited the implementation of new methods to interrupt parasite transmission through snail control, leading to a resurgence of the disease following drug treatment and education efforts [6,9,10]. Therefore, there is a need to recast the current and future schistosomiasis control strategies to focus on integrated measures and enhanced surveillance response to both changes in infection and snail abundance.
Various methods have been employed in the control of schistosomiasis. For instance, intermediate hosts (IHs) can be controlled through biological, chemical/molluscicides, or environmental/ecological methods [11,12,13]. Biological measures include the use of predators and competitor snails [12], while chemical control measures consider the use of molluscicides to reduce IH density and abundance [14,15]. The use of molluscicides and their effectiveness has been observed to depend on their proper concentration, half-life, technique and timing of application, and length of contact time with target species, as well as the temperature of the environment where the chemical is released [16]. In addition, environmental/ecological strategies are used to lower the density and abundance of IHs and the risks of snail-to-human transmission (referred to as the mechanical control approach in this study). The approach includes excavating deep channels that act as dry buriers for snails to spread to other water outlets, increasing the flow velocity in irrigation canals, and picking IHs out of the system. Other ways include adopting appropriate cultivation methods, such as shorter fallow times, modifying irrigation techniques, and regulating flooding [11,17].
With the predicted change in global temperature due to climate change, mathematical models have become valuable tools for exploring various infection and control scenarios as they provide both theoretical and practical insights into the epidemiology of infectious diseases. In understanding the transmission and control dynamics of schistosomiasis, different models have been used to create a range of transmission and control outcomes. These models explore dynamics, including free-living parasites, miracidia, and cercariae [18], chemotherapy treatment using praziquantel drugs and the removal of snails and cercariae [19], the impact of public literacy and snail control parameters [20], as well as health education and molluscicides [21].
Our study examines whether molluscicides and/or mechanical control for snail management, combined with mass drug administration and/or health education, can eliminate schistosomiasis. In addition, we postulate that the temperature rise, as predicted by climate change models [22,23,24], will strongly influence the use of chemicals/molluscicides, and their performance against the target species [25,26] and the general transmission dynamics of the disease. As such, it is important to understand how temperature affects molluscicide usage for the control of schistosomiasis. This can help in the identification and control of chemical-induced environmental hazards that could be harmful to even nontarget species and human health under various environmental conditions [27,28,29,30]. The current study will evaluate four controls: mass drug administration/chemotherapy, public literacy, mechanical measures, and chemicals/molluscicides to find the most effective way to eradicate schistosomiasis. In addition, the evaluation includes the effects of temperature rise on various molluscicide performances based on half-lives and contact durations with the targeted species.
2. Materials and Methods
2.1. Schistosomiasis Model Formulation
The dynamics of schistosomiasis [2,3] and stages of intervention strategies are the basis for model formulation (Figure 1). The deterministic model formulated and represented by ordinary differential equations (ODEs) (Equations (1)–(7)) is a modification of those presented by Abokwara and Madubueze [20] and Nur et al. [21], and it is based on the compartment diagram (Figure 1). A population is represented by state time variables that are associated with the various compartment models and change over time according to the interaction rules. : the time variable for the sizes of susceptible humans; : infected human; eggs released from infected humans; : free-living miracidia; : susceptible snail vector; : infected snail vector; : free-living cercariae (Figure 1). The parameters of the model are based on the abiotic and biotic aspects of the Schistosoma cycle and transmission, namely the contact rates between the hosts and the free-living cercaria/miracidia population in water and the effective mortality rates.
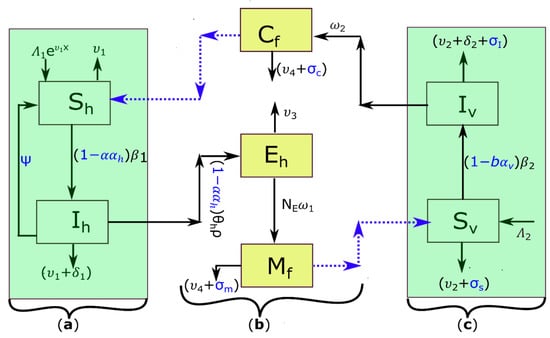
Figure 1.
Transmission dynamics of schistosomiasis in (a) human definitive hosts (susceptible human ; infected human ), (b) schistosomiasis parasite forms (cercariae ; parasite eggs ; miracidia ), and (c) intermediate host snails (susceptible snail ; and infected snail ). The blue dotted arrows represent the interaction between free-living schistosomiasis parasites and the respective hosts. All parameters are as defined in Section 2.1, and the control parameters are shown in blue.
The population of susceptible human hosts in the model system, Equation (1), increases by an exponential recruitment rate of , where is the maximum per capita birth rate/immigration rate (recruitment rate) of human individuals, is the natural mortality rate of humans, and is the initial age of infection in children. Susceptible human hosts, , are infected only by contact with cercariae in an infested freshwater environment at a rate per contact. The saturation incidence is a Holling-type II function, which relates the rate of infection transmission and the inhibitory effect, where is the saturation coefficient that represents the transmissibility of infection due to the crowding effect of the cercariae, and is the limitation of the growth velocity (density) of the cercaria in contaminated freshwater. Thus, model system, Equation (2) represents the infected humans, . Moreover, due to schistosomiasis infection, infected people die at a rate of . Thus, our model incorporates chemotherapy treatment for deworming schistosome worms in the infected human body and to stop or limit the release of parasite eggs through urine and feces. We assume that treated infected individuals recover at a rate of and return to the susceptible class because there is no lasting immunity to schistosomiasis, and reinfection is inevitable [9,31]. In addition, public literacy strategy is considered and represented by the parameters where is the proportion of people with knowledge/awareness about schistosomiasis, and is the effectiveness of acquired knowledge. This control method focuses on managing the human population by providing access to clean water and improving sanitation and hygiene (WASH) to lower the number of humans being infected [32].
When infected humans indiscriminately release parasite eggs through urine/feces into the environment, their population size increases logistically, as shown in Equation (3). Where (in grams) is the portion of stool/urine per infected person due to open defecation or urination. is the number of parasite eggs per gram of stool and/or urine, and is the carrying capacity of the parasite eggs in the environment. The term represents the public literacy impact in reducing the portion of urine/feces . It describes the impact of public literacy on the density of parasite eggs directly or indirectly deposited in freshwater and lowers the likelihood of human-to-snail transmission. The indirectly deposited parasite eggs find their way into a freshwater source. Each parasite egg hatches, releasing miracidia per parasite egg at a rate under suitable conditions, or dies naturally at a rate when there is no IHs to penetrate, as represented in miracidia population dynamics in Equation (4).
In the model system, Equation (5), the snail host population is recruited at a rate and is infected due to contact with miracidia at a saturation incidence given the saturation coefficient for miracidia infectivity and the limitation of the growth velocity of the miracidia. is the rate of miracidia-to-snail transmission per contact. Both susceptible and infected IH snails die naturally at a rate , but the infected snail (in Equation (6) may also die at a rate of due to infection host. Infected snails that survive release infectious cercariae at a rate of , capable of invading and infecting humans, or dying naturally at a rate of in the absence of a human host, see Equation (7). Thus, we incorporated a snail management strategy through the mechanical measure, represented by parameter , where is the proportion of snail density removed and eliminated from the system, and is the effectiveness of such snail management practice. Thus, the impact of a mechanical measure reduces [20,21].
In addition, we assume that the application of molluscicides into the environment causes susceptible and infected IHs to die at a chemical-induced death rate of and respectively. Furthermore, molluscicides also reduce viability and cause the deaths of infective cercaria and miracidia at a rate of and , respectively.
Thus, the model equations incorporating terms for control strategies highlighted in blue are given as follows:
2.2. Temperature Control
Temperature is a crucial factor for the timing of molluscicide application [16] because molluscicide efficacy is temperature-dependent [33]. The temperature has a significant effect on how quickly the half-lives of molluscicide decrease in water [27]. This has a great influence on the chemical-induced death rates , , , and and can determine the rate of transmission of the disease. We examine and evaluate the effectiveness of temperature-dependent molluscicides on the death rates of targeted species. We derive the environmentally dependent chemical-induced deaths of species, where the effect of temperature can be reliably predicted using an Arrhenius equation [27,28,34]. The Arrhenius equation proposed and updated by the European Chemicals Agency, ECHA [29,30] to modify the half-life at any temperature of the environment is given as
where is the factory-predetermined half-life of the chemical at the experimental temperature . The preferred experimental temperature in most of the chemicals is [27,35]. Furthermore, we assume that chemically induced mortalities () decrease exponentially with time t according to the following expressions:
where , , , and are the maximum mortality rates on the first day () when the molluscicide is applied in the water. , is the exponential decay constant of the mortality rate at degradation time . The terms , , and were determined from the withdrawal terms , , and present in the dynamics of IHs and parasite populations in the absence of humans (Equations (4)–(7)). A maximum value of one (1) is assumed for each term, the same approach as in Carvalho et al. [36]. Furthermore, we link to the half-life () of the chemical released by using the following equation:
where is the typical time the chemical persists in the environment and is, therefore, in contact with the HIs, the Schistosoma parasites, and other nontarget species. The results of substituting , , and and substituting Equations (8) and (10) into Equation (9) are shown in Table 1.

Table 1.
The continuously exponentially decreasing mortality rates for intermediate hosts and Schistosoma parasite forms, as functions of the half-life of the chemical , the temperature of the environment in which the chemical is released, and the duration for which the targeted species are exposed to the chemicals.
Thus, chemical control guarantees the elimination of all three water-dwelling schistosomiasis agents [16]. Furthermore, based on Matthies and Beulke [27] and the European Commission [37], the chemical is nonpersistent (NP) in the environment when , persistent (P) when , and very persistent (VP) when . Based on this information, we discriminate the effects of different chemical performances based on their different half-lives , degradation , and length of chemical exposure on the death rate of the targeted species.
It is worth noting that in our model, we assume that chemotherapy treatment reduces disease morbidity among humans while molluscicide increases the mortality of IHs. Public literacy and mechanical control are management strategies for humans and snails, respectively, that directly and extrinsically prevent the likelihood of parasite-host contact for the transmission of schistosomiasis. Furthermore, there is no vertical transmission of the disease to humans nor immigration to the infected individuals. Infected snails are unable to reproduce as a side effect of infection and may die more frequently than susceptible snails.
This paper presents the model analysis first, showing that the model is epidemiologically meaningful, realistic, and of interest in a certain invariant region Ω (Appendix A). We then derive the reproduction number (Section 2.3) and show the steady states (Section 2.4).
2.3. Reproduction Number
In epidemiology, one of the most reliable indicators of infection risk is the basic reproduction number , which is the average number of new cases of infection caused by an infectious individual in a fully susceptible population [38]. We derived by rewriting the model system (Equations (1)–(7)), following Chavez et al. [39], into three components: the number of susceptible individuals (), the infected schistosomiasis agents that cannot transmit the infection (), and the infected schistosomiasis agents that transmit the disease (), as follows:
where , , and . We let represent disease-free at such that, , ), where , and . Suppose, , then A is given by
Following the next-generation matrix, an approach used by van den Driessche and Watmough [40], and the concept of reproduction numbers by Diekmann et al. [38], the matrix can be rewritten as , with (i.e., ) and , a diagonal matrix, where
The basic reproduction number is the spectral radius (dominant eigenvalue) of the matrix given by
2.4. Steady State
We show that the model system, Equations (1)–(7) have a disease-free state () and an endemic steady state , where
It always exists in provided .
is expressed in terms of and by
when = 0, we solved using Equation (6) of the model Equations (1)–(7), and this leads to the substitution of and , which yields Equation (11) below.
where
. Equation (11) has one of the solutions , which corresponds to a disease-free equilibrium. Other solutions can be determined according to the sign rule of Descartes [41], which states that depending on the change in the sign of the coefficients, , , , and , 3, 2, or 1 positive solutions exist; thus, the proof for existing conditions for for the model Equations (1)–(7) is complete.
In addition, the stability of the model system depends on the parameters used to build the model and the changes in these parameters affect , with numerous scientific and biological implications for the transmission and control of the disease. For instance, if we use as the bifurcation parameter for model Equations (1)–(7), then and
is the crucial bifurcation value for cercaria-to-human transmission. When and , for instance, only the asymptotically stable disease-free equilibrium point () can exist, and provides conditions for disease extinction. The disease-free equilibrium is unstable, whereas the endemic equilibrium ( is asymptotically stable, and both equilibrium points exist when and [40], and in this case, provides the conditions for the disease to persist. Thus, the asymptotic dynamic behavior of the infectious disease, which determines whether it will vanish or persist in the future, can be inferred from the steady states. Although we do not provide a stability analysis of the equilibrium points, we acknowledge that control strategies have a role to play in reducing and possibly eliminating schistosomiasis.
2.5. Parameter Data
For our study, we use data collected in the literature related to Bulinus and Biomphalaria (Table 2). In the absence of published data, parameter values are assumed or estimated based on expert knowledge using what is commonly known about vector and disease dynamics, as follows. The life expectancy of an adult Schistosoma worm within a human host is 3–10.5 years [42,43,44]; thus, vary from to . In addition, Dabo et al. [45] establish schistosomiasis infection in preschool children aged 1–4 years through both passive and active exposure to infected water bodies. On average, a child`s initial infection age is 2 years [43]; thus, days. Gryseels et al. [46] reported 2–10 weeks as the period a schistosomiasis patient takes to recover after chemotherapy treatment. Thus, we assume that the recovery period for a schistosomiasis patient varies between 14 and 70 days, which sets between and individuals per day. The Schistosoma parasite egg can stay for 7 days, with a 0.14286 per egg death rate, and miracidia survive 12 days in freshwater ( [46,47]. Cercaria can only spend 10–40 h in freshwater [48], which sets between and . Table 2 shows the numerical values (and potential ranges) of parameters set for the model system Equations (1)–(7) from the literature and estimations.

Table 2.
Model parameters, their definition, baseline values, potential ranges, and sources.
We performed a numerical simulation using the main R package ODE solver Version 1.10-4 for solving ordinary differential equations [53] in the R statistical environment version 4.0.3 [54]. The baseline values in Table 2 were used for all simulations and predictions. We conducted a sensitivity analysis of with respect to the baseline value using the Partial Rank Correlation Coefficients (PRCC) test to determine how robust our predictions are to changes in parameter values. In this way, we can determine quantitatively which key parameters can be targeted by control measures to reduce disease the most. For instance, increases when parameters with positive PRCC values are increased, increasing the likelihood of infection.
3. Results
The results show that the key parameters (bold in Table 3) that have the greatest influence on the transmission of the disease include the recruitment rate of human individuals , a portion of stool/urine per infected person ρ, number of miracidia per parasite egg , cercaria-to-human transmission per contact β1, rate of miracidia-to-snail transmission per contact β2, and rate of cercaria emergence from infected snails .

Table 3.
The partial rank correlation coefficients for the baseline parameter values for (without control) as a response function, with the most significant parameters that influence the dynamics of the model highlighted in bold.
The model system Equations (1)–(7) is solved numerically using the initial conditions , , , , , , , and , and the effects of different control strategies on the targetted human and snail populations are shown graphically in Figure 2. When a chemotherapy treatment of the infected humans is integrated with public literacy (two-tiered approach), the strategy is more successful in managing and reducing disease morbidity in the human population than when each strategy is carried out separately, i.e., the number of infected humans who recover, reduce, and join the susceptible humans, who grow in number (Figure 2a,b). Chemical/molluscicide control significantly increases IHs mortality, and when paired with a mechanical technique, this effect is essentially identical (Figure 2c,d).
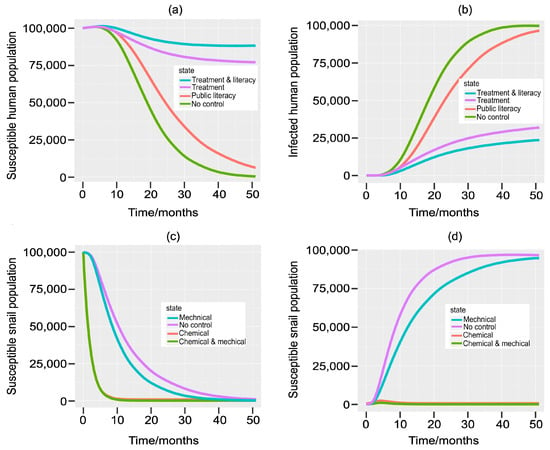
Figure 2.
Comparisons between treatment and public literacy targeting the populations of (a) susceptible humans, (b) infected humans, and molluscicide (chemical) and the mechanical strategies targeting (c) susceptible snails and (d) infected snails of schistosomiasis in contrast with no control measures.
The impact of individual control measures and various combinations of all control measures on disease transmission is determined by the value of , as shown in Table 4. The more successful a certain level of control is, the smaller the value of . We show that when a single control strategy is used, the chemical/molluscicides strategy () has a greater impact on reducing the value of followed by the chemotherapy treatment strategy (), public literacy (), and finally mechanical control (). However, a single intervention cannot eradicate the disease, : see Table 4. The results of integrating the various strategies show that a combination of two-tiered control measures, treatment strategies (), and molluscicides () have the greatest impact compared to any other pair of strategies (Table 4). Moreover, a combination of a three-tiered approach: treatment (), molluscicides (), and mechanical (), as well as another three-tiered approach: public literacy (, molluscicides and mechanical () have the potential to lower below 1, but only if the effectiveness of each separate control is greater than 90% (Table 4). However, schistosomiasis can be eradicated, , if a four-tiered approach (treatment, public literacy, molluscicides, and mechanical) is applied, with each method being more than 70% effective when combined (Table 4).

Table 4.
The variability of across rows for a single or different combination of control strategies, , , , . The lower the , the more successful the strategy(s) are in controlling schistosomiasis.
When considering mortality rate functions (Table 1), we show that temperature, the length of chemical exposure, and the chemical’s half-life affect the molluscicide performance on the death rates of targeted species. In general, chemical-induced mortality rates for targeted species (host snails and free-living Schistosoma cercaria and miracidia) decline as the temperature increases. However, the decline is more rapid when the half-life of molluscicides decreases, i.e., shorter half-lived molluscicides (e.g., non-persistent and persistent molluscicides) lose their toxicity sooner, leading to lower mortality rates. For instance, Figure 3a shows how temperature control impacts the half-life of molluscicides and the consequent mortality rate of susceptible snails. In the same context, chemical-induced mortality rates decrease with an increase in the duration of molluscicide exposure. Molluscicides with a longer half-life (very persistent molluscicides) remain in the environment longer, resulting in a higher mortality rate compared to molluscicides with a shorter half-life (Figure 3b). Thus, increasing the half-life of molluscicides increases the chemically induced mortality rates, especially for cercariae and infected snails (Figure 3c). As a result, increasing the temperature and days of chemical exposure decreases the mortality rate of snails (Figure 3d) associated with higher risks of disease transmission, thus increasing the value (Figure 3e).
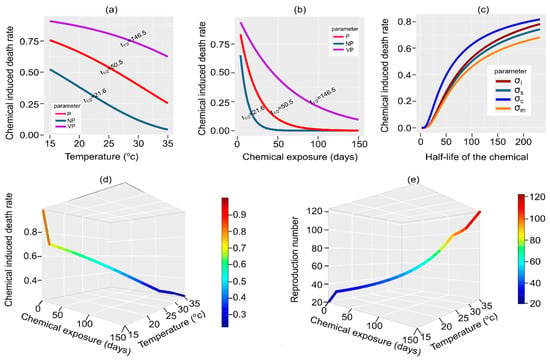
Figure 3.
Various molluscicides are regulated by temperature according to their half-lives and durations of chemical exposure to snails. A molluscicide is classified as a non-permanent molluscicide (NP) with a half-life of , a persistent molluscicide (P) with a half-life of , or a very-permanent molluscicide (VP) with a half-life of . (a) Shows variations in the temperature of the environment and the duration of chemical exposure (b) for different chemicals affecting the death rate of targeted host snails. (c) Shows the effect of chemicals with different half-lives on the death rate of different target species (susceptible snails , infected snails , cercaria , and miracidia ). At the same time, the effect of combined chemical exposure and temperature on the death rate of host snails and the subsequent reproduction number is shown in (d,e).
4. Discussion
We formulate a mathematical model to assess the effectiveness of methods that target humans and those targeting IHs in the control of schistosomiasis. The results of the PRCC test suggest that control strategies focusing on the key parameters , β1, β2, and in the transmission dynamics may be more effective in reducing and may result in disease-free conditions of , indicating disease control. Our results show that the chemotherapy treatment strategy (reducing ) and application of molluscicides (reducing β1, β2, and ) are the most successful methods for reducing and disease morbidity among the human population and increasing snail mortality, respectively, when applied independently and compared to mechanical control (reducing ) and public literacy (reducing β1 and β2) with limited impact. But each strategy cannot eradicate schistosomiasis individually, which complements the finding by Mangal et al. [52], King and Bertsch [9], and Zacharia et al. [10]. Thus, a single approach may reduce morbidity [2,55] but often without reducing the local and environmental transmission of parasites [6], which contributes to the development of the disease. In addition, chemotherapy has shown ineffectiveness against juvenile worms within the body. This is the reason why worms inside infected and treated children can continue to release eggs, observed in their feces three weeks after the drug has been administered [56,57,58]. This thus perpetuates local transmission, and residents who stay in endemic areas run the risk of re-infection [6,59]. The fragility of drug control alone is demonstrated by this.
On the other hand, mechanical control practices like the physical picking of snails are considered old-fashioned but prove to be a good complementary strategy to drugs, molluscicides, and public literacy [17]. However, its use may be limited, especially in large water bodies, such as lakes, rivers, and streams, or where a larger number of aquatic inhabitants must be controlled. In addition, public literacy advocates for significant behavior change in water use and contact. However, this ultimately requires the provision of safe and hygienic alternative water supplies, latrines, and washing facilities. When compared to using individual control strategies, integrated strategies are far more effective. Our study further shows that when a four-tiered control strategy is used, the results are much better than using one-, two- or three-tiered controls. Although a three-tiered strategy would be effective as well, it is practically difficult to achieve 90% effectiveness from each method. These findings support, to a certain extent, the findings of Mangal et al. [52], Thétiot-Laurent et al. [56], Lo et al. [58], and Zheng et al. [13], which suggest that the integrated intervention of chemotherapy and snail management has a greater impact on schistosomiasis control than a single method like chemotherapy treatment alone.
However, in such integrated efforts to control schistosomiasis, the time and season of application of molluscicides are crucial. This is relevant in providing a realistic assessment of the spread of schistosomiasis under the current and projected temperature increases in areas like sub-Saharan Africa, where the disease is most prevalent. Our results corroborate with the reports by Feng et al. [60], Fishel [25], Ziska [26], and Zheng et al. [13] that the temperature of the environment, chemical degradation, and half-life of the chemical are important factors in the chemical control of snails. Regardless of the chemical type used, non-persistent (NP), persistent (P), or very persistent (VP), the results show that the chemical strategy may be more effective in areas with low temperatures (15–25 °C). In the first two weeks (14 days) of application, the chemically induced mortality rate of snails is significantly higher, especially for cercariae and infected snails, and consequently, is less than at unity, indicating disease control. This corroborates the finding by Montanari et al. [33] that, at a standard temperature of 25 °C, molluscicides proved to be effective against adult snails; the snails reduced their food intake and stopped eating, whereas the same effects were significantly stronger at 18 °C. No effects on survival or feeding rate have been recorded at incubations of 30 °C. In regions with higher temperatures, an even longer treatment period using NP chemicals is associated with low rates of induced mortality and is, therefore, less effective in reducing snails, free-living parasite populations, and . The results also show that the molluscicides NP, P, and VP with increasing half-lives reduce species mortality by more than 50% only up to 2 weeks, 3 weeks, and 6 weeks, respectively, suggesting that the chemical should be reapplied regularly thereafter. Thus, the half-life of the molluscicide is directly proportional to the active length of the molluscicides in the water. This result supports the finding of Seligman et al. [61], who observed that the half-lives/chemical degradation of different chemicals ranges from 4 to 19 days and up to several months under specific conditions. For efficient snail management, integration efforts, and schistosomiasis control, different areas with varying weather patterns and seasonal variations may require different molluscicide-type and variable time intervals of reapplication.
Therefore, it is critical to quantify the use of chemical control agents in response to climatic conditions to determine alternative changes in the target intermediate host population and the best season (temperature) for control. This result complements the efforts to investigate molluscicidal action, the structure-activity relationship, and the potential mechanisms of several molluscicides [13,14,62] toward developing suitable chemicals.
Our findings, thus, demonstrate that effective schistosomiasis control programs necessitate a co-ordinated effort and comprehensive integration of preventative treatment, public literacy, and mechanical and molluscicide controls. Despite the promising results, we believe that controlling schistosomiasis with fewer than three different combinations of control strategies will be challenging and may not be worth the effort to eradicate schistosomiasis compared to when the four strategies are integrated and carried out simultaneously. However, achieving the schistosomiasis eradication goal may require a large investment of resources (high cost, which severely limits the use of the molluscicide on a big scale), time, and commitment, and also has associated ecological risks. For instance, chemical management has several negative effects, including the destruction of aquatic biodiversity due to toxicity [63] and the emergence of snail resistance to the chemicals are very likely. If chemicals are released into the environment, local people will not be able to use the water [13,64]. Several molluscicides have a half-life that ranges from 3.17 to 223 days [65], while others have a half-life of up to 2 years. This means that the presence of molluscicides in water would continue to pose a health risk to people for more than 30 years before it decays completely [61].
Furthermore, the development and use of molluscicide need to be blended with an increase in health education and an assessment of their levels of toxicity when ingested by humans and domestic animals through agricultural products and their effect on non-target aquatic fauna and flora [13]. However, our results suggest that chemicals with shorter half-lives, such as an extract from C. viminalis fruits, or natural insecticides, such as Nereistoxin, are effective in killing snails at low concentrations in a shorter amount of time [13,66]. They could provide an alternative to long-term exposures to common molluscicides with less effects on biodiversity. Furthermore, specific molluscicides, such as nicotinanilide molluscicides and silver (Ag) nanopowder (Ag-NPs) molluscicides [13], have been shown to exhibit strong molluscicidal effects with less toxicity to humans, animals, fish, and plants, can be applied in small waterbodies to manage snail populations despite their high cost and strong human skin irritant effects. The effect of change in temperature on the environment on molluscicide performance greatly affects the mortality rate of the targeted organism (IHs, cercariae, and miracidia) and general disease transmission. Therefore, better molluscicide performance should be achieved by developing harmless, low-toxicity, and temperature-dependent molluscicides, with low environmental impact to control the targeted species holistically.
Our study has some limitations given that the parameters that best reflect the biological representation and real-life situations of schistosomiasis disease and transmission had to be used based on the published record. Since neither the Biomphalaria-S. mansoni nor the Bulinus-S. haematobium infection system had a complete list of genus-specific model parameters, we used published parameter values for both infection systems. Due to the absence of specifically published data relating to the two systems, two parameter values were estimated, and two others were assumed based on expert knowledge. Furthermore, the literature data are not always consistent and are collected under different conditions. Nonetheless, the baseline values of such parameters provide a good first approximation of the parameter values, and the model can be used generally to develop a better understanding of the effects of interventions. This model can be improved to incorporate more features, such as the effect of temperature and molluscicides on the development of eggs into miracidia, snail recruitment, and biodiversity of nontarget species.
5. Conclusions
We conclude that the proposed model provides valuable mechanistic insights into how the impact of schistosomiasis and future control programs will depend on the integration of chemotherapy, public literacy, and mechanical and molluscicide types in different endemic regions. In addition, molluscicide performances in different regions with changing temperatures will vary depending on their type and half-life, degradation rate, and reapplication frequency.
Author Contributions
Conceptualization, Z.T.; Methodology, Z.T. and C.K.; Formal analysis, Z.T.; Funding acquisition, L.B. and C.A.; Supervision, C.A. and L.B.; Visualization, Z.T.; Writing—original draft, Z.T.; Writing—review & editing, Z.T., C.K., L.B. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
The German Academic Exchange Service (DAAD) supported Z.T with a Ph.D. scholarship (Grant No. 57507871). The Alexander von Humboldt Foundation supported C.K as a Georg Forster Research Fellow with a scholarship for his research visit and stay at Justus Liebig University Giessen. (Grant No. ZMB1217528GF-P). The funders had no role in study design, data collection or analysis, the decision to publish, or the preparation of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
It is with great gratitude that the authors acknowledge the German Academic Exchange Service (DAAD) for providing Z.T. with a Ph.D. scholarship, as well as Alexander von Humboldt Foundation for providing C.K. with a scholarship.
Conflicts of Interest
The authors declare that they do not have any competing interests.
Appendix A
Invariant region
In model system Equations (1)–(7), we prove that non-negative and bounded solutions exist in Ω, as follows:
Let be the solution set of the model equations Equations (1)–(7) from which are the non-negative initial conditions.
Lemma A1.
is non-negative in with for .
Proof.
It is evident from model equation Equation (1) that
, where , .
Thus, is non-negative and, in the same way, it can be shown that and . Therefore, in the region is positively invariant. □
Lemma A2.
remains bounded in .
Proof.
Let the total human population , and the total snail population , such that and and Thus, can be expressed analytically as follows:
Assuming , and , we conclude that , and , and therefore, both human () and snail () populations are biologically feasible. Similarly , shows that the egg, miracidia, and cercaria populations are biologically feasible. Thus, with initial conditions remains bounded for all . □
As a result of Lemmas A1 and A2, our model is epidemiologically and mathematically well-posed.
References
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef]
- WHO, World Health Organization. Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 3 February 2023).
- Wang, L.; Utzinger, J.; Zhou, X.-N. Schistosomiasis control: Experiences and lessons from China. Lancet 2008, 372, 1793–1795. [Google Scholar] [CrossRef]
- Rosenberg, M.; Utzinger, J.; Addiss, D.G. Preventive chemotherapy versus innovative and intensified disease management in neglected tropical diseases: A distinction whose shelf life has expired. PLoS Negl. Trop. Dis. 2016, 10, e0004521. [Google Scholar] [CrossRef] [PubMed]
- WHO, World Health Organization. Elimination of Schistosomiasis. Sixty-Fifth World Health Assembly, WHA65. 21, Agenda item 13.11. 26 May 2012. Available online: http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_R21-en.pdf (accessed on 23 February 2023).
- French, M.D.; Churcher, T.S.; Gambhir, M.; Fenwick, A.; Webster, J.P.; Kabatereine, N.B.; Basáñez, M.-G. Observed reductions in Schistosoma mansoni transmission from large-scale administration of praziquantel in Uganda: A mathematical modelling study. PLoS Negl. Trop. Dis. 2010, 4, e897. [Google Scholar] [CrossRef] [PubMed]
- Stothard, J.R.; Chitsulo, L.; Kristensen, T.K.; Utzinger, J. Control of schistosomiasis in sub-Saharan Africa: Progress made, new opportunities and remaining challenges. Parasitology 2009, 136, 1665–1675. [Google Scholar] [CrossRef]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Bertsch, D. Historical perspective: Snail control to prevent schistosomiasis. PLoS Negl. Trop. Dis. 2015, 9, e0003657. [Google Scholar] [CrossRef]
- Zacharia, A.; Mushi, V.; Makene, T. A systematic review and meta-analysis on the rate of human schistosomiasis reinfection. PLoS ONE 2020, 15, e0243224. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H. Biological methods for the control of freshwater snails. Parasitol. Today 1990, 6, 237–241. [Google Scholar] [CrossRef]
- Sokolow, S.H.; Huttinger, E.; Jouanard, N.; Hsieh, M.H.; Lafferty, K.D.; Kuris, A.M.; Riveau, G.; Senghor, S.; Thiam, C.; N’Diaye, A.; et al. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc. Natl. Acad. Sci. USA 2015, 112, 9650–9655. [Google Scholar] [CrossRef]
- Zheng, L.; Deng, L.; Zhong, Y.; Wang, Y.; Guo, W.; Fan, X. Molluscicides against the snail-intermediate host of Schistosoma: A review. Parasitol. Res. 2021, 120, 3355–3393. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Sutherland, L.J.; Bertsch, D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Negl. Trop. Dis. 2015, 9, e0004290. [Google Scholar] [CrossRef]
- Lo, N.C.; Gurarie, D.; Yoon, N.; Coulibaly, J.T.; Bendavid, E.; Andrews, J.R.; King, C.H. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc. Natl. Acad. Sci. USA 2018, 115, E584–E591. [Google Scholar] [CrossRef]
- WHO, World Health Organization. Field Use of Molluscicides in Schistosomiasis Control Programmes: An Operational Manual for Programme Managers; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Lafferty, K.D.; Kuris, A.M.; Hsieh, M.H.; De Leo, G.A. To reduce the global burden of human schistosomiasis, use ‘old fashioned’ snail control. Trends Parasitol. 2017, 34, 23–40. [Google Scholar] [CrossRef]
- Chiyaka, E.T.; Garira, W. Mathematical analysis of the transmission dynamics of schistosomiasis in the human-snail hosts. J. Biol. Syst. 2009, 17, 397–423. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Luo, Y.; Xie, D. Control problems of a mathematical model for schistosomiasis transmission dynamics. Nonlinear Dyn. 2010, 63, 503–512. [Google Scholar] [CrossRef]
- Abokwara, A.; Madubueze, C.E. The Role of Non-pharmacological Interventions on the Dynamics of Schistosomiasis. J. Math. Fundam. Sci. 2021, 53, 243–260. [Google Scholar] [CrossRef]
- Nur, W.; Trisilowati; Suryanto, A.; Kusumawinahyu, W.M. Mathematical model of schistosomiasis with health education and molluscicide intervention. J. Phys. Conf. Ser. 2021, 1821, 012033. [Google Scholar] [CrossRef]
- Stensgaard, A.-S.; Booth, M.; Nikulin, G.; McCreesh, N. Combining process-based and correlative models improves predictions of climate change effects on Schistosoma mansoni transmission in eastern Africa. Geospat. Health 2016, 11, 406. [Google Scholar] [CrossRef]
- De Leo, G.A.; Stensgaard, A.-S.; Sokolow, S.H.; N’goran, E.K.; Chamberlin, A.J.; Yang, G.-J.; Utzinger, J. Schistosomiasis and climate change. BMJ 2020, 371, m4324. [Google Scholar] [CrossRef]
- Tabo, Z.; Neubauer, T.A.; Tumwebaze, I.; Stelbrink, B.; Breuer, L.; Hammoud, C.; Albrecht, C. Factors controlling the distribution of intermediate host snails of Schistosoma in crater lakes in Uganda: A machine learning approach. Front. Environ. Sci. 2022, 10, 871735. [Google Scholar] [CrossRef]
- Fishel, F.M. Storage Limitation Statements: Temperature–Herbicides: PI123/PI160, 4/2013. EDIS 2013, 4, 123–160. [Google Scholar]
- Ziska, L.H. Increasing minimum daily temperatures are associated with enhanced pesticide use in cultivated soybean along a latitudinal gradient in the mid-western United States. PLoS ONE 2014, 9, e98516. [Google Scholar] [CrossRef] [PubMed]
- Matthies, M.; Beulke, S. Considerations of temperature in the context of the persistence classification in the EU. Environ. Sci. Eur. 2017, 29, 15. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil-Scientific Opinion of the Panel on Plant Protection Products and their Residues (PPR Panel). EFSA J. 2008, 6, 622. [Google Scholar] [CrossRef]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment; Chapter R.11: PBT/vPvB Assessment; Version 2; Technical Report ECHA-14-G-07-EN; European Chemicals Agency: Helsinki, Finland, 2014. [Google Scholar]
- ECHA. Guidance for Information Requirements and Chemical Safety Assessment; Chapter R.7b: Endpoint Specific Guidance; Draft Version 4.0 (Public); European Chemicals Agency: Helsinki, Finland, 2016. [Google Scholar]
- Ronoh, M.; Chirove, F.; Pedro, S.A.; Tchamga, M.S.S.; Madubueze, C.E.; Madubueze, S.C.; Addawe, J.; Mwamtobe, P.M.; Mbra, K.R. Modelling the spread of schistosomiasis in humans with environmental transmission. Appl. Math. Model. 2021, 95, 159–175. [Google Scholar] [CrossRef]
- Grimes, J.E.T.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasites Vectors 2015, 8, 156. [Google Scholar] [CrossRef]
- Montanari, A.L.; Accorsi, A.; Nasi, M.; Malagoli, D. Effects of a nematode-based molluscicide on survival and antimicrobial peptide expression in Pomacea canaliculata. Invertebr. Surviv. J. 2019, 16, 37. [Google Scholar]
- Laidler, K.J.; Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S.; Abyazisani, M.; et al. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Lewis, K.; Tzilivakis, J.; Green, A.; Warner, D. Pesticide Properties DataBase (PPDB). 2006. Available online: http://hdl.handle.net/2299/15375 (accessed on 14 February 2023).
- Carvalho, S.A.; da Silva, S.O.; Charret, I.D.C. Mathematical modeling of dengue epidemic: Control methods and vaccination strategies. Theory Biosci. 2019, 138, 223–239. [Google Scholar] [CrossRef]
- European Commission EC. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances Part II. In Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances and of Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market; Technical Report EUR 20418 EN/2; European Commission EC: Luxembourg, 2003. [Google Scholar]
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A.J. On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Chavez, C.C.; Feng, Z.; Huang, W. On the computation of R0 and its role on global stability. In Mathematical Approaches for Emerging and Re-Emerging Infection Diseases: An Introduction; Springer: Berlin/Heidelberg, Germany, 2002; Volume IMA 125, pp. 229–250. [Google Scholar]
- Driessche, P.V.D.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Jackson, J.; Sitharam, M. Descartes’ rule of signs revisited. Am. Math. Mon. 1998, 105, 447. [Google Scholar] [CrossRef]
- Fulford, A.J.C.; Butterworth, A.E.; Ouma, J.H.; Sturrock, R.F. A statistical approach to schistosome population dynamics and estimation of the life-span of Schistosoma mansoni in man. Parasitology 1995, 110, 307–316. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Anderson, R.; Turner, H.; Farrell, S.; Truscott, J. Studies of the transmission dynamics, mathematical model development and the control of schistosome parasites by mass drug administration in human communities. Adv. Parasitol. 2016, 94, 199–246. [Google Scholar] [CrossRef]
- Dabo, A.; Badawi, H.M.; Bary, B.; Doumbo, O.K. Urinary schistosomiasis among preschool-aged children in Sahelian rural communities in Mali. Parasites Vectors 2011, 4, 21. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Michaels, R.M.; Prata, A. Evolution and characteristics of Schistosoma mansoni eggs laid in vitro. J. Parasitol. 1968, 54, 921. [Google Scholar] [CrossRef]
- Braun, L.; Grimes, J.E.T.; Templeton, M.R. The effectiveness of water treatment processes against schistosome cercariae: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006364. [Google Scholar] [CrossRef]
- Kanyi, E.; Afolabi, A.S.; Onyango, N.O. Mathematical modelling and analysis of transmission dynamics and control of schistosomiasis. J. Appl. Math. 2021, 2021, 1–20. [Google Scholar] [CrossRef]
- Feng, Z.; Eppert, A.; Milner, F.; Minchella, D. Estimation of parameters governing the transmission dynamics of schistosomes. Appl. Math. Lett. 2004, 17, 1105–1112. [Google Scholar] [CrossRef]
- Liang, S.; Spear, R.C.; Seto, E.; Hubbard, A.; Qiu, D. A multi-group model of Schistosoma japonicum transmission dynamics and control: Model calibration and control prediction. Trop. Med. Int. Health 2005, 10, 263–278. [Google Scholar] [CrossRef]
- Mangal, T.D.; Paterson, S.; Fenton, A. Predicting the impact of long-term temperature changes on the epidemiology and control of schistosomiasis: A mechanistic model. PLoS ONE 2008, 3, e1438. [Google Scholar] [CrossRef]
- Soetaert, K.; Petzoldt, T.; Setzer, R.W. Solving differential equations in R: Package deSolve. J. Stat. Softw. 2010, 33, 1–25. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. Available online: http://www.R-project.org/ (accessed on 6 March 2021).
- Andrade, G.; Bertsch, D.J.; Gazzinelli, A.; King, C.H. Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005372. [Google Scholar] [CrossRef] [PubMed]
- Thétiot-Laurent, S.A.-L.; Boissier, J.; Robert, A.; Meunier, B. Schistosomiasis chemotherapy. Angew. Chem. Int. Ed. 2013, 52, 7936–7956. [Google Scholar] [CrossRef]
- Faust, C.L.; Osakunor, D.N.; Downs, J.A.; Kayuni, S.; Stothard, J.R.; Lamberton, P.H.; Reinhard-Rupp, J.; Rollinson, D. Schistosomiasis control: Leave no age group behind. Trends Parasitol. 2020, 36, 582–591. [Google Scholar] [CrossRef]
- Lo, N.C.; Bezerra, F.S.M.; Colley, D.G.; Fleming, F.M.; Homeida, M.; Kabatereine, N.; Kabole, F.M.; King, C.H.; Mafe, M.A.; Midzi, N.; et al. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect. Dis. 2022, 22, e327–e335. [Google Scholar] [CrossRef]
- Satayathum, S.A.; King, C.; Muchiri, E.M.; Ouma, J.H.; Whalen, C.C. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: Survival analysis during a nine-year, school-based treatment program. Am. J. Trop. Med. Hyg. 2006, 75, 83–92. [Google Scholar] [CrossRef]
- Feng, W. Progress of research on molluscicidal effect of nereistoxin pesticide. Chin. J. Schistosomiasis Control 2012, 19, 482. [Google Scholar]
- Seligman, P.F.; Maguire, R.J.; Lee, R.F.; Hinga, K.R.; Valkirs, A.O.; Stang, P.M. Persistence and fate of tributyltin in aquatic ecosystems. In Organotin; Springer: Dordrecht, The Netherlands, 1996; Volume 20, pp. 429–457. [Google Scholar] [CrossRef]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Swartz, S.J.; Lopez, M.; Hsieh, M.H.; Lafferty, K.D.; Kuris, A.M.; Rickards, C.; De Leo, G.A. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl. Trop. Dis. 2016, 10, e0004794. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, S.L.; Getsinger, K.D. Zebra Mussel Chemical Control Guide; ERDC/EL TR-00-1; U.S. Army Engineer Research and Development Center: Vicksburg, MS, USA, 2000; 114p. [Google Scholar]
- Zayed, K.M.; Guo, Y.-H.; Lv, S.; Zhang, Y.; Zhou, X.-N. Molluscicidal and antioxidant activities of silver nanoparticles on the multi-species of snail intermediate hosts of schistosomiasis. PLoS Negl. Trop. Dis. 2022, 16, e0010667. [Google Scholar] [CrossRef]
- Castle, G.D.; Mills, G.A.; Gravell, A.; Jones, L.; Townsend, I.; Cameron, D.G.; Fones, G.R. Review of the molluscicide metaldehyde in the environment. Environ. Sci. Water Res. Technol. 2017, 3, 415–428. [Google Scholar] [CrossRef]
- Gohar, A.A.; Maatooq, G.T.; Gadara, S.R.; Aboelmaaty, W.S.; El-Shazly, A.M. Molluscicidal activity of the methanol extract of Callistemon viminalis (Sol. ex Gaertner) G. Don ex Loudon fruits, bark and leaves against Biomphalaria alexandrina snails. Iran. J. Pharm. Res. 2014, 13, 505. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).