Dynamic Analysis of Delayed Two-Species Interaction Model with Age Structure: An Application to Larch-Betula Platyphylla Forests in the Daxing’an Mountains, Northeast China
Abstract
1. Introduction
2. Mathematical Modeling
- (i)
- Since plant growth cannot be completed immediately, there will be a time delay during the shift of plants toward maturity. We use time delays to represent the mature time required for the immature Larch (x) to grow into mature Larch (y). Similarly, we define as the mature time required for the immature Betula platyphyll (u) to grow into mature Betula platyphyll (v). It is believed that the mature time, considered a plant trait, can affect population dynamics and the regeneration of plants after fires [31], which is consistent with the forest recovery background observed after the frequent fires of the last century in the Daxing’an Mountains. Based on the large mature time differences between Larch and Betula platyphylla, we consider the mature time as a population characteristic in our model.
- (ii)
- Considering the limited competitiveness of the immature population, we focus on the effect of the mature population on the immature population. We assume that the number of the immature population at t is related to the number of the mature population at [28] because some immature plants grow into mature plants at . Due to the loss of populations while plants grow, we let and denote the transition factor of the immature population growing into the mature population, for Larch and Betula platyphylla, respectively.
- (iii)
- To explore the interaction mechanism between Larch and Betula platyphylla in different interaction modes, we define interaction factors to vary the interaction mode. It has been shown that Betula platyphylla litter contributes to the decomposition of Larch litter, which accelerates the shift of nutrients from litter to soil and improves soil fertility. Moreover, Betula platyphylla improves the damaged ecological environments of the Daxing’an Mountains as a pioneer species. Therefore, we define as the interaction factor of Betula platyphylla on Larch. Then, considering the competition of Larch against Betula platyphylla, we define as the interaction factor of Larch on Betula platyphylla.
- (iv)
- We assume that the mature population has the ability to propagate, while the immature population does not. Thus, we believe that birth into the immature population with time is proportional to its mature population with a growth rate. Then, we discuss the expression of growth rates.Due to the restriction of environmental carrying capacity, the population size cannot continue to grow, and its growth rate will decrease with increasing population size. Therefore, we define and as the environmental carrying capacities of Larch and Betula platyphylla to constrain their growth rates, respectively. Based on the net growth rates and belonging to Larch and Betula platyphylla, respectively, we suggest that the growth rates are negatively proportional to their own population sizes, which are and , respectively. Interactions are the main driving forces of plant community organization and dynamics [5]. Considering the actual situation, we suggest that the Larch population can reproduce more rapidly with improvements in damaged environments provided by Betula platyphylla. Thus, different from the classical Lotka–Volterra model that simply focuses on competition, we consider the effects of interactions including facilitation and competition on the growth rate. We assume that the growth rate is related to the number of neighbor species with interaction factors of . Then, the growth rates of Larch and Betula platyphylla are as follows, and , respectively.
- (v)
- We assume that the immature death with time is proportional to the existing immature population with mortalities and for immature Larch and immature Betula platyphylla, respectively. In addition to the capacity of the population, mature death is also related to the intense competition among mature plants. With an increasing size of the mature population, the intraspecific competition among the mature members becomes increasingly intense, which increases the mature death. Thus, we suppose that the mature death with time is proportional to the square of the mature population with mortalities and [27,28,29,30] for mature Larch and mature Betula platyphylla, respectively. That is the reason we divide the population into immature and mature groups.
3. Stability of Equilibria and the Existence of Hopf Bifurcation
3.1. Analysis for Equilibrium
3.2. Analysis for Equilibrium
3.3. Analysis for Equilibrium
3.3.1. Case 1 for
3.3.2. Case 2 for
3.3.3. Case 3 for
- (1)
- Equilibrium is unstable, and system (1) has no fixed point bifurcation at when and .
- (2)
- When (H3) and (H4) hold, we have the following conclusions for equilibrium .
- (a)
- When , the equilibrium is locally asymptotically stable.
- (b)
4. Parametric Analysis and Numerical Simulations
4.1. Parametric Analysis
- (i)
- Time delays
- (ii)
- Net growth rates and environmental carrying capacities
- (iii)
- Mortalities
- (iv)
- Transition factors
- (v)
- Interaction factors
4.2. Numerical Simulations
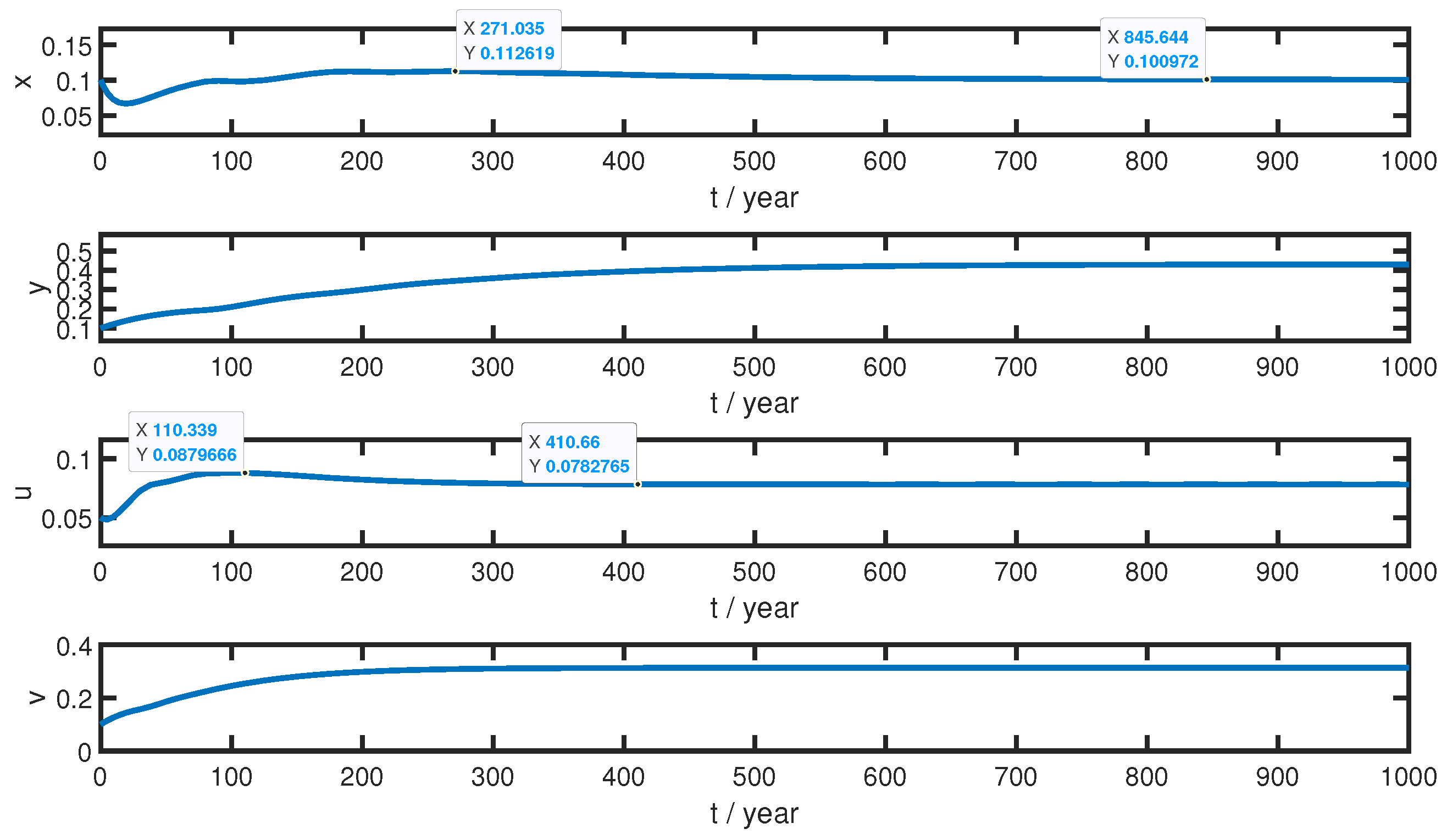
4.2.1. Exploration for the Evolution of Stand Structure
- (a)
- During the growing period, the number of immature Larch plants decreases and increases alternately, while the number of mature Larch plants continues to increase. The slopes of x and y with t are high at the beginning. Before 19 years, x drops rapidly. This indicates that the stand eliminates the uncompetitive immature Larch to ensure the quality of the stand. Next, after x drops to a minimum of 0.06675, x and y increase rapidly at . The reason for this is that trees grow and propagate fast at low stand densities with abundant ecological resources and weak intraspecific competition. Furthermore, immature Larch gradually grows into mature Larch. With the increase in maturity, the reproductive capacity of the forest is strengthened, which promotes the growth of immature plants in turn. In 80 years, the number of Larch reaches a threshold. When , x and the rising speed of y decrease significantly. This is because intraspecific competition forces the stand to adjust itself with the gradual occupation of ecological resources. Immature Larch, which has low competitiveness and avoids light, dies from intraspecific competition. Mature Larch is also slightly affected by intraspecific competition. Next, the number of immature Larch plants rises and falls alternately when . The forest stand dynamically adjusts and develops itself continually.
- (b)
- At the beginning of the adjustment period, the number of Larch plants reaches a higher threshold. Then, the numbers of immature and mature plants decrease and increase slowly, respectively. This indicates that with high forest density and scarce ecological resources, competitive mature Larch has an advantage in the fierce intraspecific competition over immature Larch. Moreover, the slopes of x and y with time in the adjustment period are lower than those in the growth period. We suggest that mature Larch, with a large proportion in the population, enhances the stability of forests and makes adjustments of forests gentler. The stand structure is basically determined and gradually reaches stability.
- (c)
- During the stable period, the stand structure is determined and finally reaches its stable condition. At this time, the changing ranges of x and y with time are tiny. All curves become extraordinarily smooth.
4.2.2. Exploration of the Interaction Mechanism
- (a)
- The effect of interactions is directly proportional to density. The x and u differences between condition 1 and other conditions are insignificant in the preperiod, but increase over time and eventually stabilize, similar to the changing trends of the population size. Thus, we assume that this phenomenon is related to the stand density. With the total population continuously increasing, the stand density increases, and the average distance between trees decreases, which contributes to more frequent interactions between trees.
- (b)
- The mutualistic interaction mode is more conducive to the population reproduction and survival than other interaction modes. Under condition 2, the populations of Larch and Betula platyphylla have the highest rises and sizes. Compared with those of condition 1, x and u both increase, which suggests that the effects of interactions can outweigh the decrease caused by intraspecies competition in the later period.
- (c)
- In contrast to mature plants, interactions have a larger influence on immature plants. our results show that differences in x and u are all greater than the differences in y and v as the immature population is more susceptible to environmental stress.
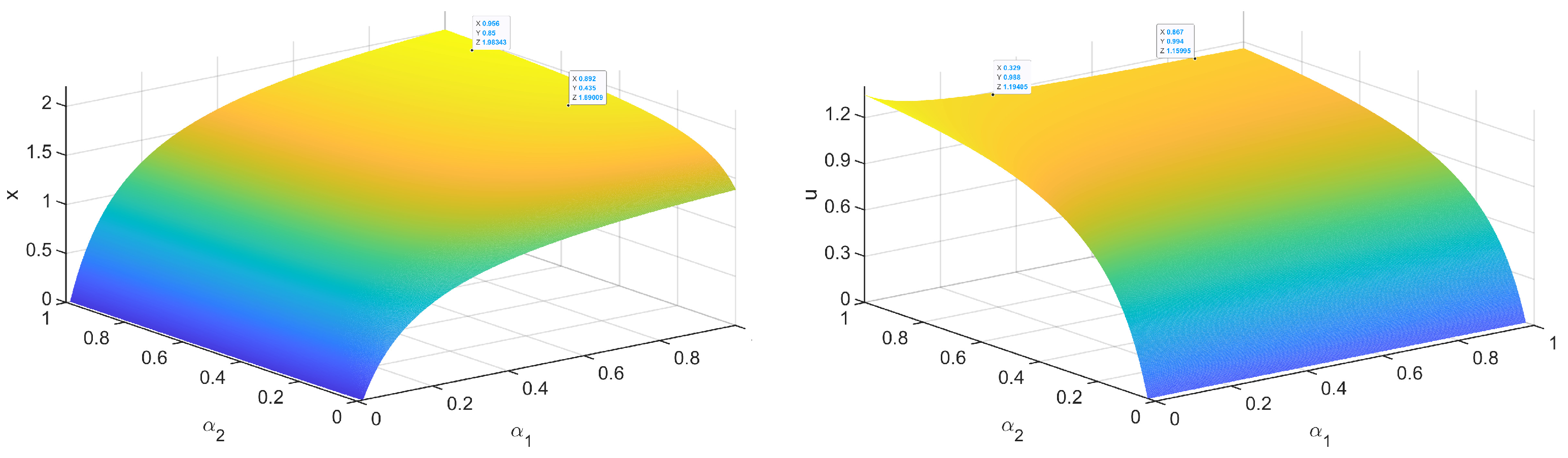
4.2.3. Study of the Influence of Each Parameter on Equilibrium
- (1)
- To discuss the reasonable ranges of the important parameters and for scientific forest management, we pay more attention to the values of equilibrium under stability with varied and , which contrasts with the simulation method used in Section 4.2.1 and Section 4.2.2.
- (a)
- Immature Larch is significantly correlated with its growth rate but weakly correlated with another population growth rate (). Similarly, Betula platyphylla is significantly correlated with its growth rate but weakly correlated with Larch’s growth rate.
- (b)
- The value changes observed with growth rate changes are apparent under certain thresholds but become insensitive to the parameters after the thresholds. When exceeds 0.46, the fluctuation of immature Larch does not exceed 0.5% of the environmental carrying capacity with every 0.01 increase in . Similarly, the influence of on immature Betula platyphylla decreases rapidly when is greater than 0.41. This illustrates that when the growth rates and exceed certain thresholds, the ecological system restricts the reproduction of Larch and Betula platyphylla. In addition to thresholds, we consider biological meanings to control the growth rates within reasonable ranges. We let x and u be the positive population sizes of Larch and Betula platyphylla under the preset environmental carrying capacity, respectively. We finally obtain reasonable values for the growth rates as follows, .
- (2)
- Since forest pests and diseases significantly hinder forestry developments, it is necessary to investigate their influences on populations and propose forest management strategies. For the Daxing’an Mountains, Larch and Betula platyphylla are both vulnerable to pests and disease during seedling growth. Therefore, we consider varying mortalities of immature populations and and study the dynamic properties of system (1) at equilibrium .
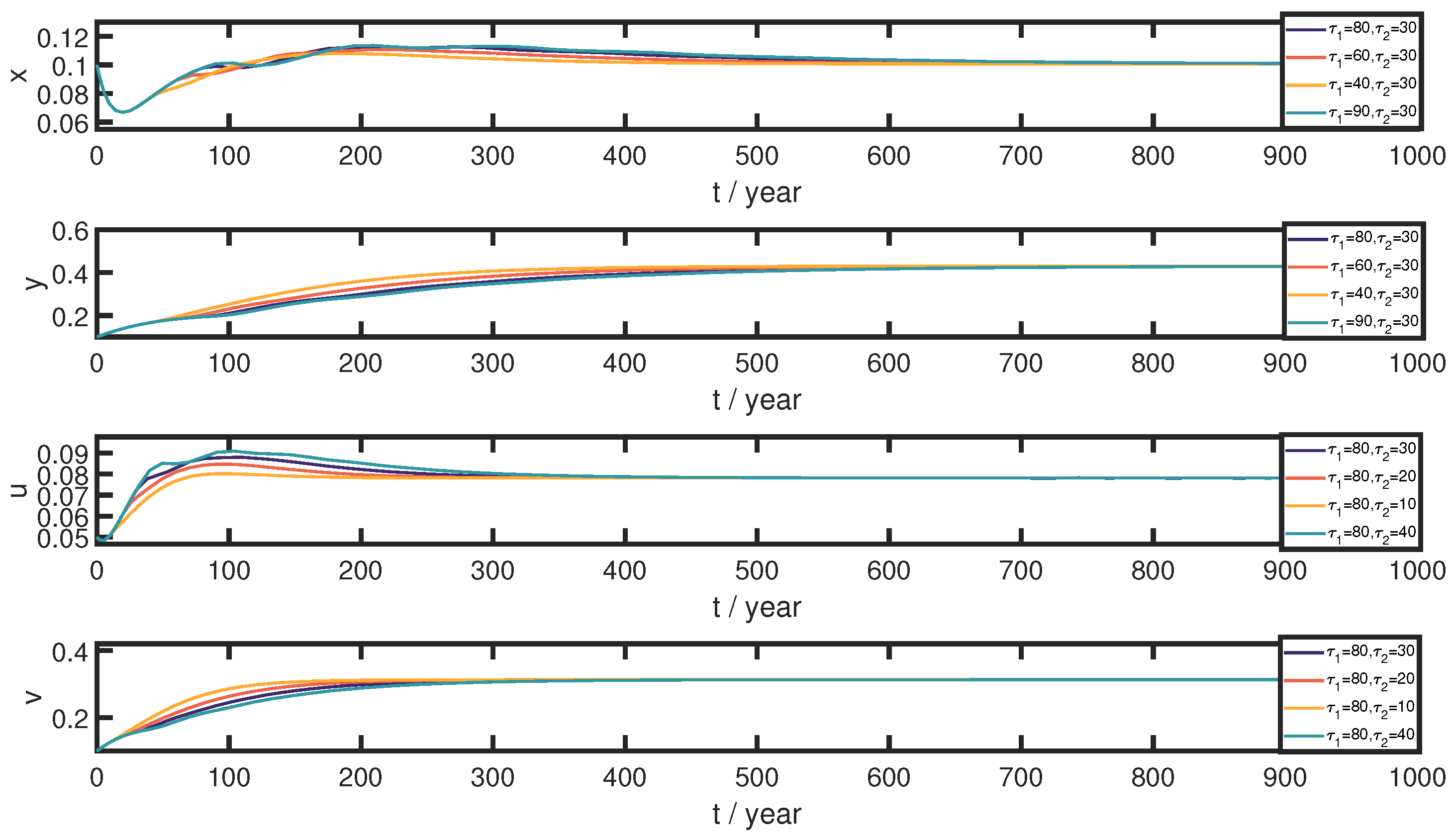
- (3)
- In this section, we explore the influence of time delays on the model. First, we select several sets of parameters, which are as follows:
4.3. Recommendations for China
- (i)
- Our results show that the populations of Larch and Betula platyphylla will both increase in the future, which indicates that the Larch-Betula platyphylla forest in the Daxing’an Mountains has enormous ecological value and carbon sink potential. Furthermore, when the structure of Larch-Betula platyphylla stands becomes stable, the proportion of Larch will be greater than that of Betula platyphylla and the mature population will account for a substantial part of the whole population. Thus, we predict that mature Larch will play a major role in the forest community organization and forest dynamics.
- (ii)
- The numbers of Larch and Betula platyphylla plants in mixed forests are greater than those in pure forests. We should develop mixed-species forests to achieve improved forest utilization efficiency and increase the capacity of forest carbon sinks.
- (iii)
- The increase in and increase the number of Larch and Betula platyphylla plants, respectively. However, when the net growth rate is higher than a certain threshold, the resulting population increase is not significant. Therefore, we should control the net growth rates of Larch and Betula platyphylla within and , respectively, through intermediate cuttings, afforestation, and reforestation.
- (iv)
- The increase in and can cause a decrease in the number of Larch and Betula platyphylla plants, respectively. Moreover, when the mortality of immature plants is higher than a certain threshold, the population markedly decreases to a low level. Therefore, serious forest diseases and pests disturb forest ecosystems and hinder China’s green development. It is necessary to reduce the mortality of immature Larch and immature Betula platyphylla under 0.2 and 0.19, respectively, by regulating site conditions, resisting exotic forest insect pests, and developing mixed-species forests.
5. Conclusions
- (i)
- Without interactions, the evolution of a stand structure roughly experiences three periods, the growing period, the adjustment period, and the stable period. The characteristics of those periods are fluctuating rises, adjustments along one trend, and a stable stand structure. At the beginning of the growth period, the stand eliminates the uncompetitive immature Larch to ensure the quality of the stand. During the growth period, the population rises and fluctuates with frequent self-adjustments. Then, when the number of the population reaches a certain threshold, the stand enters the adjustment period. At this time, the stand structure is basically established and stable. The numbers of immature and mature plants rise or decline slowly with one trend. Finally, the stand reaches the stable period, and the stand structure is entirely established.
- (ii)
- With interactions, we find that age structure and density influence the responses of the population to interactions with magnitudes and patterns. First, the interaction effect is directly proportional to the stand density. Second, interactions have a larger influence on immature individuals than on mature individuals. Third, the mutualistic interaction mode is most conducive to the population reproduction across the five interaction modes. Furthermore, the interaction mechanism between Larch and Betula platyphylla is elaborated herein.
- (iii)
- Among the parameters under certain thresholds, the number of the population is more sensitive. Based on threshold control, we consider the biological meanings of these parameters and obtain that .
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kagawa, A.; Sugimoto, A.; Maximov, T.C. Seasonal course of translocation, storage and remobilization of 13C pulse-labeled photoassimilate in naturally growing Larix gmelinii saplings. New Phytol. 2006, 171, 793–804. [Google Scholar] [CrossRef]
- Harley, C.D. Climate change, keystone predation, and biodiversity loss. Science 2011, 334, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Sun, T.; Xue, S.F.; Yang, W.; Shao, D.D.; Martinez-Lopez, J. Competitive ability, stress tolerance and plant interactions along stress gradients. Ecology 2018, 99, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dai, X.F.; Shen, Z.X.; Zhang, J.Y.; Wang, G.X. Facilitation can maintain clustered spatial pattern of plant populations during density-dependent mortality: Insights from a zone-of-influence model. Oikos 2011, 120, 472–480. [Google Scholar] [CrossRef]
- Michalet, R.; Xiao, S.; Touzard, B.; Smith, D.S.; Cavieres, L.A.; Callaway, R.M.; Whitham, T.G. Phenotypic variation in nurse traits and community feedbacks define an alpine community. Ecol. Lett. 2011, 14, 433–443. [Google Scholar] [CrossRef]
- Kikvidze, Z.; Pugnaire, F.I.; Brooker, R.W.; Choler, P.; Lortie, C.J.; Michalet, R.; Callaway, R.M. Linking patterns and processes in alpine plant communities: A global study. Ecology 2005, 86, 1395–1400. [Google Scholar] [CrossRef][Green Version]
- McIntire, E.J.; Fajardo, A. Facilitation within Species: A possible origin of group-selected superorganisms. Am. Nat. 2011, 178, 88–97. [Google Scholar] [CrossRef]
- Armas, C.; Rodriguez-Echeverria, S.; Pugnaire, F.I. A field test of the stress-gradient hypothesis along an aridity gradient. J. Veg. Sci. 2011, 22, 818–827. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Hernandez-Fuentes, C.; Sierra-Almeida, A.; Kikvidze, Z. Facilitation among plants as an insurance policy for diversity in Alpine communities. Funct. Ecol. 2016, 30, 52–59. [Google Scholar] [CrossRef]
- Bastolla, U.; Fortuna, M.A.; Pascual-Garcia, A.; Ferrera, A.; Luque, B.; Bascompte, J. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 2009, 458, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Kefi, S.; van Baalen, M.; Rietkerk, M.; Loreau, M. Evolution of local facilitation in arid ecosystems. Am. Nat. 2008, 172, E1–E17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Filotas, E.; Grant, M.; Parrott, L.; Rikvold, P.A. The effect of positive interactions on community structure in a multi-species metacommunity model along an environmental gradient. Ecol. Model. 2010, 221, 885–894. [Google Scholar] [CrossRef][Green Version]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.C.; Tielborger, K. Facilitation from an intraspecific perspective-stress tolerance determines facilitative effect and response in plants. New Phytol. 2019, 221, 2203–2212. [Google Scholar] [CrossRef]
- Lin, Y.; Berger, U.; Grimm, V.; Ji, Q.R. Differences between symmetric and asymmetric facilitation matter: Exploring the interplay between modes of positive and negative plant interactions. J. Ecol. 2012, 100, 1482–1491. [Google Scholar] [CrossRef][Green Version]
- Stoll, P.; Bergius, E. Pattern and process: Competition causes regular spacing of individuals within plant populations. J. Ecol. 2005, 93, 395–403. [Google Scholar] [CrossRef]
- Weiner, J.; Stoll, P.; Muller-Landau, H.; Jasentuliyana, A. The effects of density, spatial pattern, and competitive symmetry on size variation in simulated plant populations. Am. Nat. 2001, 158, 438–450. [Google Scholar] [CrossRef]
- Berger, U.; Hildenbrandt, H.; Grimm, V. Towards a standard for the individual-based modeling of plant populations: Self-thinning and the field-of-neighborhood approach. Nat. Resour. Model. 2002, 15, 39–54. [Google Scholar] [CrossRef]
- Berger, U.; Hildenbrandt, H.; Grimm, V. Age-related decline in forest production: Modelling the effects of growth limitation, neighbourhood competition and self-thinning. J. Ecol. 2004, 92, 846–853. [Google Scholar] [CrossRef]
- Chu, C.J.; Weiner, J.; Maestre, F.T.; Xiao, S.; Wang, Y.; Li, Q.; Yuan, J.; Zhao, L.; Ren, Z.; Wang, G. Positive interactions can increase size inequality in plant populations. J. Ecol. 2009, 97, 1401–1407. [Google Scholar] [CrossRef]
- Zhang, B.; DeAngelis, D.L. An overview of agent-based models in plant biology and ecology. Ann. Bot. 2020, 126, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.K.; Sidhu, H.S.; Sidhu, L.A.; Mercer, G.N. Extended Lotka-Volterra equations incorporating population heterogeneity: Derivation and analysis of the predator-prey case. Ecol. Model. 2015, 297, 187–195. [Google Scholar] [CrossRef]
- Yan, S.L.; Du, Z.J. Hopf bifurcation in a Lotka-Volterra competition-diffusion-advection model with time delay. J. Differ. Equ. 2023, 344, 74–101. [Google Scholar] [CrossRef]
- Baden, H.M.; Roach, D.; Schweingruber, F.H.; Reitzel, K.; Lundgreen, K.; Dahlgren, J.P. The effects of age on the demography of a perennial plant depend on interactions with size and environment. J. Ecol. 2021, 109, 1068–1077. [Google Scholar] [CrossRef]
- Zaghrout, A.A.S.; Attalah, S.H. Analysis of a model of stage-structured population dynamics growth with time state-dependent time delay. Appl. Math. Comput. 1996, 77, 2–3. [Google Scholar] [CrossRef]
- Aiello, W.G.; Roach, D.; Freedman, H.I. A time-delay model of single-species growth with stage structure. Math. Biosci. 1990, 101, 139–153. [Google Scholar] [CrossRef]
- Aiello, W.G.; Freedman, H.I.; Wu, J. Analysis of a model representing stage-structured population growth with state-dependent time delay. SIAM J. Appl. Math. 1992, 52, 855–869. [Google Scholar] [CrossRef]
- Lin, Z.G. Time delayed parabolic system in a two-species competitive model with stage structure. J. Math. Anal. Appl. 2006, 315, 202–215. [Google Scholar] [CrossRef][Green Version]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Hu, H.Q.; Luo, B.Z.; Wei, S.J.; Wei, S.; Wen, Z.; Sun, L.; Luo, S.; Wang, L.; Ma, H. Estimating biological carbon storage of five typical forest types in the Daxing’ anling Mountains, Heilongjiang, China. Acta Ecol. Sin. 2015, 35, 5745–5760. [Google Scholar]




| Symbol | Description | Unit |
|---|---|---|
| x | Amount of immature Larch | |
| y | Amount of mature Larch | |
| u | Amount of immature Betula platyphylla | |
| v | Amount of mature Betula platyphylla | |
| Environmental carrying capacity of Larch | ||
| Environmental carrying capacity of Betula platyphylla | ||
| Net growth rate of Larch | - | |
| Net growth rate of Betula platyphylla | - | |
| Interaction factor of Betula platyphylla on Larch | - | |
| Interaction factor of Larch on Betula platyphylla | - | |
| Transition factor of immature Larch growing into mature Larch | - | |
| Transition factor of immature Betula platyphylla growing into mature Betula platyphylla | - | |
| Mortality of immature Larch | - | |
| Mortality of mature Larch | - | |
| Mortality of immature Betula platyphylla | - | |
| Mortality of mature Betula platyphylla | - | |
| Time delay for immature Larch to grow into mature Larch | year | |
| Time delay for immature Betula platyphylla to grow into mature Betula platyphylla | year |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Ding, Y.; Pan, N. Dynamic Analysis of Delayed Two-Species Interaction Model with Age Structure: An Application to Larch-Betula Platyphylla Forests in the Daxing’an Mountains, Northeast China. Mathematics 2023, 11, 2485. https://doi.org/10.3390/math11112485
Huang X, Ding Y, Pan N. Dynamic Analysis of Delayed Two-Species Interaction Model with Age Structure: An Application to Larch-Betula Platyphylla Forests in the Daxing’an Mountains, Northeast China. Mathematics. 2023; 11(11):2485. https://doi.org/10.3390/math11112485
Chicago/Turabian StyleHuang, Xuan, Yuting Ding, and Ning Pan. 2023. "Dynamic Analysis of Delayed Two-Species Interaction Model with Age Structure: An Application to Larch-Betula Platyphylla Forests in the Daxing’an Mountains, Northeast China" Mathematics 11, no. 11: 2485. https://doi.org/10.3390/math11112485
APA StyleHuang, X., Ding, Y., & Pan, N. (2023). Dynamic Analysis of Delayed Two-Species Interaction Model with Age Structure: An Application to Larch-Betula Platyphylla Forests in the Daxing’an Mountains, Northeast China. Mathematics, 11(11), 2485. https://doi.org/10.3390/math11112485







