A Simple Stability Analysis for a Mathematical Model of Migration Due to Noise and Resources
Abstract
1. Introduction
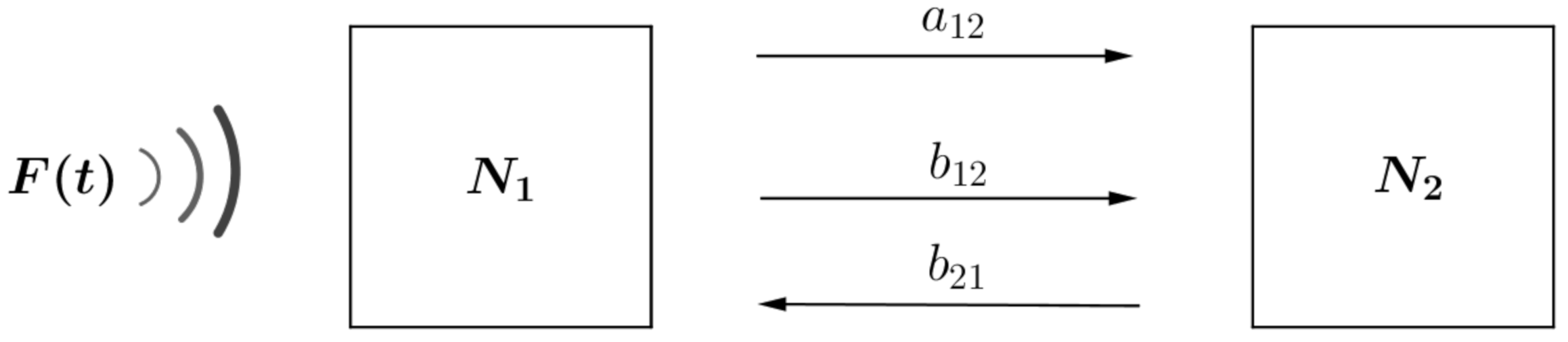
2. Model Formulation
- Unidirectional dependent on noise level. Individuals migrate towards patches with decreasing anthropogenic noise rate. For this, the migration rate is considered proportional to the noise aswhere, to simplify calculations, the value of is considered the same for all patches.
- Random dependent on the difference between occupation levels. Individuals migrate towards patch with lower occupancy level (independent of subpopulation sizes). For this, the migration rates is considered inversely proportional to the carrying capacity of each patch aswhere is the marginal rate of migration, which for simplicity, in the calculations, is considered the same value for all patches.
3. Model Analysis
3.1. The Existence of Equilibrium Points
- which represents the extinction of the population in both patches.
- , which represents the persistence of the population in both patches.
3.2. Local Stability Analysis
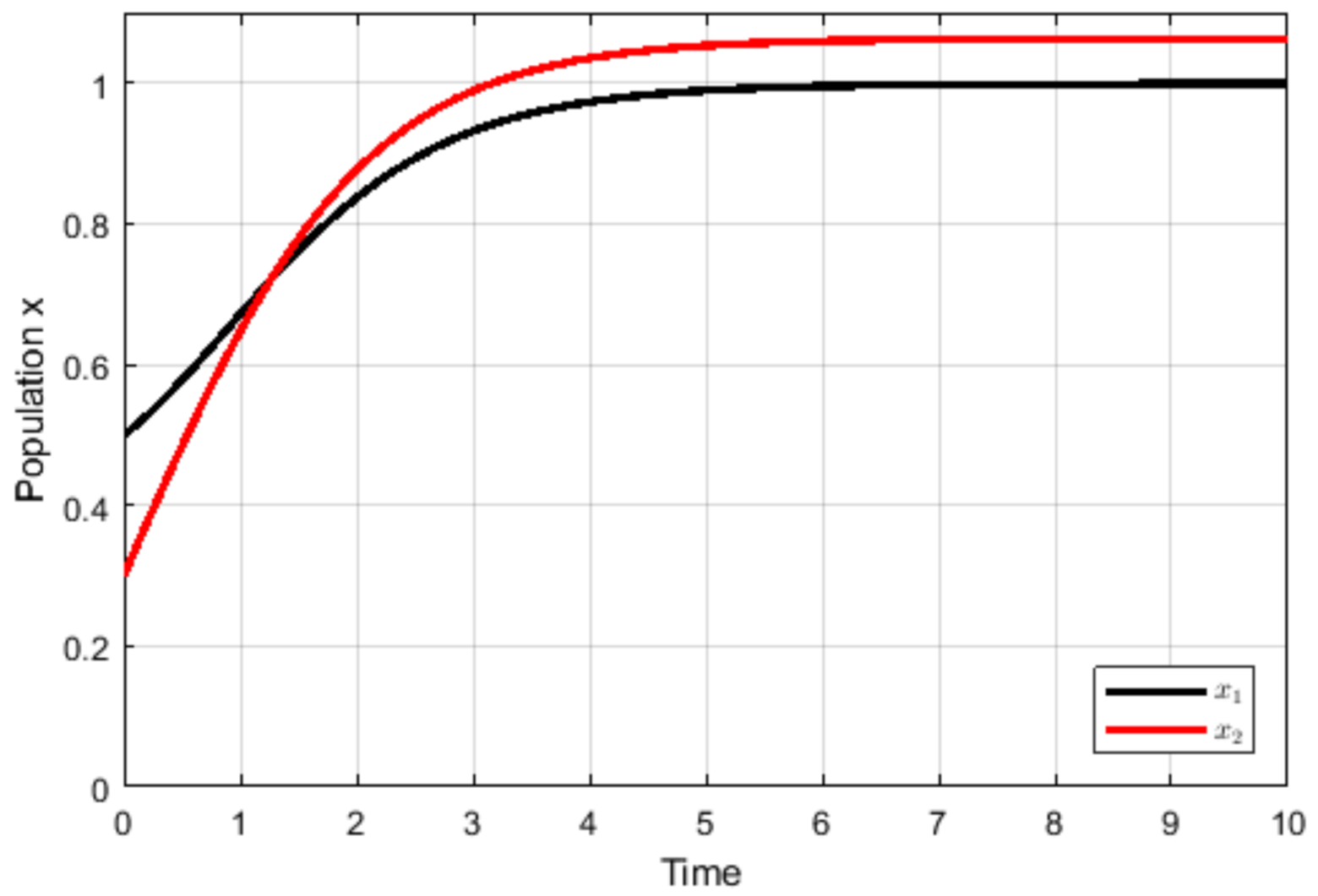
4. Numerical Simulations
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levins, R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Am. Entomol. 1969, 15, 237–240. [Google Scholar] [CrossRef]
- Skellam, J.G. Random dispersal in theoretical populations. Biometrika 1951, 38, 196–218. [Google Scholar] [CrossRef] [PubMed]
- McCullough, D.R. Metapopulations and Wildlife Conservation; Island Press: Washington, DC, USA, 1996. [Google Scholar]
- Dalpasso, A.; Ficetola, G.F.; Giachello, S.; Lo Parrino, E.; Manenti, R.; Muraro, M.; Falaschi, M. Similar species, different fates: Abundance dynamics in spatially structured populations of common and threatened frogs. Divers. Distrib. 2022, 28, 770–781. [Google Scholar] [CrossRef]
- Hanski, I.A.; Gaggiotti, O.E. (Eds.) Ecology, Genetics and Evolution of Metapopulations; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Keymer, J.E.; Marquet, P.A.; Velasco-Hernández, J.X.; Levin, S.A. Extinction thresholds and metapopulation persistence in dynamic landscapes. Am. Nat. 2000, 156, 478–494. [Google Scholar] [CrossRef]
- Bertassello, L.E.; Jawitz, J.W.; Bertuzzo, E.; Botter, G.; Rinaldo, A.; Aubeneau, A.F.; Rao, P.S.C. Persistence of amphibian metapopulation occupancy in dynamic wetlandscapes. Landsc. Ecol. 2022, 37, 695–711. [Google Scholar] [CrossRef]
- Holstein, D.M.; Smith, T.B.; van Hooidonk, R.; Paris, C.B. Predicting coral metapopulation decline in a changing thermal environment. Coral Reefs 2022, 41, 1–12. [Google Scholar] [CrossRef]
- Dingle, H.; Drake, V.A. What is migration? Bioscience 2007, 57, 113–121. [Google Scholar] [CrossRef]
- Al-Darabsah, I.; Tang, X.; Yuan, Y. A prey-predator model with migrations and delays. Discrete Contin. Dyn. Syst.-B 2016, 21, 737–761. [Google Scholar] [CrossRef]
- Olli, K. Diel vertical migration of phytoplankton and heterotrophic flagellates in the Gulf of Riga. J. Mar. Syst. 1999, 23, 145–163. [Google Scholar] [CrossRef]
- Andersen, V.; Gubanova, A.; Nival, P.; Ruellet, T. Zooplankton community during the transition from spring bloom to oligotrophy in the open NW Mediterranean and effects of wind events. 2. Vertical distributions and migrations. J. Plankton Res. 2001, 23, 243–261. [Google Scholar] [CrossRef]
- El Abdllaoui, A.; Auger, P.; Kooi, B.W.; De la Parra, R.B.; Mchich, R. Effects of density-dependent migrations on stability of a two-patch predator–prey model. Math. Biosci. 2007, 210, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.N.; Tielens, J.T.; Estabrook, B.J.; Muirhead, C.A.; Rahaman, A.; Guerra, M.; Clark, C.W. Variation of ocean acoustic environmentsalong the western north Atlantic coast: A case study in context of the rightwhale migration route. Ecol. Inf. 2014, 21, 89–99. [Google Scholar] [CrossRef]
- Hildebrand, J.A. Anthropogenic and natural sources of ambient noisein the ocean. Mar. Ecol. Prog. Ser. 2009, 395, 5–20. [Google Scholar] [CrossRef]
- Pijanowski, B.C.; Villanueva-Rivera, L.J.; Dumyahn, S.L.; Farina, A.; Krause, B.L.; Napoletano, B.M.; Gage, S.H.; Pieretti, N. Soundscape ecology: The science of sound in the landscape. BioScience 2011, 61, 203–216. [Google Scholar] [CrossRef]
- Ware, H.E.; McClure, C.J.; Carlisle, J.D.; Barber, J.R. A phantom road experiment reveals traffic noise is an invisible source of habitat degradation. Proc. Natl. Acad. Sci. USA 2015, 112, 12105–12109. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Dooling, R.J.; Popper, A.N.; Fay, R.R. Effects of Anthropogenic Noise on Animals; Springer: New York, NY, USA, 2018. [Google Scholar]
- Dooling, R.J.; Leek, M.; West, E. Predicting the effects of masking noise on communication distance in birds. J. Acoust. Soc. Am. 2009, 125, 2517. [Google Scholar] [CrossRef]
- Erbe, C.; Reichmuth, C.; Cunningham, K.; Lucke, K.; Dooling, R. Communication masking in marine mammals: A review and research strategy. Mar. Pollut. Bull. 2016, 103, 15–38. [Google Scholar] [CrossRef]
- Römer, H. Masking by noise in acoustic insects: Problems and solutions. In Animal Communication and Noise; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–63. [Google Scholar]
- Sueur, J.; Farina, A. Ecoacoustics: The ecological investigation and interpretation of environmental sound. Biosemiotics 2015, 8, 493–502. [Google Scholar] [CrossRef]
- De Vincenzi, G.; Micarelli, P.; Viola, S.; Buffa, G.; Sciacca, V.; Maccarrone, V.; Corrias, V.; Reinero, F.R.; Giacoma, C.; Filiciotto, F. Biological Sound vs. Anthropogenic Noise: Assessment of Behavioural Changes in Scyliorhinus canicular Exposed to Boats Noise. Animals 2021, 11, 174. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Southall, B.L.; DeRuiter, S.L.; Calambokidis, J.; Friedlaender, A.S.; Hazen, E.L.; Tyack, P.L. Blue whales respond to simulated mid-frequency military sonar. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130657. [Google Scholar] [CrossRef]
- Goodwin, S.E.; Shriver, W.G. Effects of traffic noise on occupancy patterns of forest birds. Conserv. Biol. 2011, 25, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.N.; Hawkins, A. (Eds.) The Effects of Noise on Aquatic Life II; Springer: New York, NY, USA, 2016; p. 1292. [Google Scholar]
- McClure, C.J.; Ware, H.E.; Carlisle, J.; Kaltenecker, G.; Barber, J.R. An experimental investigation into the effects of traffic noise on distributions of birds: Avoiding the phantom road. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132290. [Google Scholar] [CrossRef] [PubMed]
- McClure, C.J.W.; Ware, H.E.; Carlisle, J.D.; Barber, J.R. Noise from a phantom road experiment alters the age structure of a community of migrating birds. Anim. Conserv. 2017, 20, 164–172. [Google Scholar] [CrossRef]
- Potvin, D.A.; MacDougall-Shackleton, S.A. Traffic noise affects embryo mortality and nestling growth rates in captive zebra finches. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2015, 323, 722–730. [Google Scholar] [CrossRef]
- Malme, C.I.; Miles, P.R.; Clark, C.W.; Tyack, P.; Bird, J.E. Investigations of the Potential Effects of Underwater Noise from Petroleum-Industry Activities on Migrating Gray-Whale Behavior. Phase 2: January 1984 Migration (No. PB-86-218377/XAB; BBN-5586); Bolt, Beranek and Newman, Inc.: Cambridge, MA, USA, 1984. [Google Scholar]
- Filadelfo, R.; Mintz, J.; Michlovich, E.; D’Amico, A.; Tyack, P.L.; Ketten, D.R. Correlating military sonar use with beaked whale mass strandings: What do the historical data show? Aquat. Mamm. 2009, 35, 435–444. [Google Scholar] [CrossRef]
- Simpson, S.D.; Radford, A.N.; Nedelec, S.L.; Ferrari, M.C.; Chivers, D.P.; McCormick, M.I.; Meekan, M.G. Anthropogenic noise increases fish mortality by predation. Nat. Commun. 2016, 7, 10544. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Radford, A.N.; Simpson, S.D.; Nedelec, B.; Lecchini, D.; Mills, S.C. Anthropogenic noise playback impairs embryonic development and increases mortality in a marine invertebrate. Sci. Rep. 2014, 4, 5891. [Google Scholar] [CrossRef]
- Tennessen, J.B.; Parks, S.E.; Langkilde, T. Traffic noise causes physiological stress and impairs breeding migration behaviour in frogs. Conserv. Physiol. 2014, 2, cou032. [Google Scholar] [CrossRef]
- Lombard, E. The signs of increasing the voice. Ann. Malad. l’Oreille Larynx, 37, 101-119. Biosemiotics 1911, 8, 493–502. [Google Scholar]
- Sinnott, J.M.; Stebbins, W.C.; Moody, D.B. Regulation of voice amplitude by the monkey. J. Acoust. Soc. Am. 1975, 58, 412–414. [Google Scholar] [CrossRef]
- Katti, M.; Warren, P.S. Tits, noise and urban bioacoustics. Trends Ecol. Evol. 2004, 19, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hage, S.R.; Moss, C.F. The Lombard effect: From acoustics to neural mechanisms. Trends Neurosci. 2018, 41, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.E.; Johnston, C.E. Evidence of the Lombard effect in fishes. Behav. Ecol. 2014, 25, 819–826. [Google Scholar] [CrossRef]
- Holt, M.M.; Noren, D.P.; Veirs, V.; Emmons, C.K.; Veirs, S. Speaking up: Killer whales (Orcinus orca) increase their call amplitude in response to vessel noise. J. Acoust. Soc. Am. 2009, 125, EL27–EL32. [Google Scholar] [CrossRef] [PubMed]
- Helble, T.A.; Guazzo, R.A.; Martin, C.R.; Durbach, I.N.; Alongi, G.C.; Martin, S.W.; Henderson, E.E. Lombard effect: Minke whale boing call source levels vary with natural variations in ocean noise. J. Acoust. Soc. Am. 2020, 147, 698–712. [Google Scholar] [CrossRef]
- Brackenbury, J.H. Power capabilities of the avian sound-producing system. J. Exp. Biol. 1979, 78, 163–166. [Google Scholar] [CrossRef]
- Calder, W.A., III. The scaling of sound output and territory size: Are they matched? Ecology 1990, 71, 1810–1816. [Google Scholar] [CrossRef]
- Oberweger, K.; Goller, F. The metabolic cost of birdsong production. J. Exp. Biol. 2001, 204, 3379–3388. [Google Scholar] [CrossRef]
- Warren, P.S.; Katti, M.; Ermann, M.; Brazel, A. Urban bioacoustics: It’s not just noise. Anim. Behav. 2006, 71, 491–502. [Google Scholar] [CrossRef]
- Barber, J.R.; Crooks, K.R.; Fristrup, K.M. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 2010, 25, 180–189. [Google Scholar] [CrossRef]
- Read, J.; Jones, G.; Radford, A.N. Fitness costs as well as benefits are important when considering responses to anthropogenic noise. Behav. Ecol. 2014, 25, 4–7. [Google Scholar] [CrossRef]
- Brown, N.A.; Halliday, W.D.; Balshine, S.; Juanes, F. Low-amplitude noise elicits the Lombard effect in plainfin midshipman mating vocalizations in the wild. Anim. Behav. 2021, 181, 29–39. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Francis, C.D.; Barber, J.R.; Cooper, C.B.; Kyba, C.C.; Dominoni, D.M.; Shannon, G.; Aschehoug, E.; Goodwin, S.E.; Kawahara, A.Y.; et al. A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 2015, 30, 550–560. [Google Scholar] [CrossRef]
- Farina, A.; Gage, S.H. The ecological effects of noise on species and communities. Eco acoustics. In The Ecological Role of Sounds; Wiley: Oxford, UK, 2017; pp. 95–108. [Google Scholar]
- Zollinger, S.A.; Brumm, H. Why birds sing loud songs and why they sometimes don’t. Anim. Behav. 2015, 105, 289–295. [Google Scholar] [CrossRef]
- Brumm, H.; Todt, D. Noise-dependent song amplitude regulation in a territorial songbird. Anim. Behav. 2002, 63, 891–897. [Google Scholar] [CrossRef]
- Brumm, H. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 2004, 73, 434–440. [Google Scholar] [CrossRef]
- Luczkovich, J.J.; Krahforst, C.S.; Kelly, K.E.; Sprague, M.W. The Lombard effect in fishes: How boat noise impacts oyster toadfish vocalization amplitudes in natural experiments. Proc. Mtgs. Acoust. 2016, 27, 010035. [Google Scholar]
- Sementili-Cardoso, G.; Donatelli, R.J. Anthropogenic noise and atmospheric absorption of sound induce amplitude shifts in the songs of Southern House Wren (Troglodytes aedon musculus). Urban Ecosyst. 2021, 24, 1001–1009. [Google Scholar] [CrossRef]
- Ramirez-Carrasco, C.; Córdova-Lepe, F.; Moreno-Gómez, F.N.; Velásquez, N.A. A mathematical model for the impact of noise on population dynamics of a single species experiencing Lombard effect. Ecol. Model. 2022, 470, 110022. [Google Scholar] [CrossRef]
- Sanchirico, J.N.; Wilen, J.E. A bioeconomic model of marine reserve creation. J. Environ. Econ. Manag. 2001, 42, 257–276. [Google Scholar] [CrossRef]
- Howe, R.W.; Davis, G.J.; Mosca, V. The demographic significance of ‘sink’ populations. Biol. Conserv. 1991, 57, 239–255. [Google Scholar] [CrossRef]
- Saether, B.E.; Engen, S.; Lande, R. Finite metapopulation models with density–dependent migration and stochastic local dynamics. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1999, 266, 113–118. [Google Scholar] [CrossRef]
- Fonseca, D.M.; Hart, D.D. Density-dependent dispersal of black fly neonates is mediated by flow. Oikos 1996, 75, 49–58. [Google Scholar] [CrossRef]
- Veit, R.R.; Lewis, M.A. Dispersal, population growth, and the Allee effect: Dynamics of the house finch invasion of eastern North America. Am. Nat. 1996, 148, 255–274. [Google Scholar] [CrossRef]
- Aars, J.; Ims, R.A. Population dynamic and genetic consequences of spatial density-dependent dispersal in patchy populations. Am. Nat. 2000, 155, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Bass, H.E.; Sutherland, L.C.; Zuckerwar, A.J.; Blackstock, D.T.; Hester, D.M. Atmospheric absorption of sound: Further developments. J. Acoust. Soc. Am. 1995, 97, 680–683. [Google Scholar] [CrossRef]
- Wiley, R.; Richards, D.G. Physical constraints on acoustic communication in the atmosphere: Implications for the evolution of animal vocalizations. Behav. Ecol. Sociobiol. 1978, 3, 69–94. [Google Scholar] [CrossRef]
- Vilar, J.M.; Solé, R.V. Effects of noise in symmetric two-species competition. Phys. Rev. Lett. 1998, 80, 4099. [Google Scholar] [CrossRef]
- Spagnolo, B.; Fiasconaro, A.; Valenti, D. Noise induced phenomena in LotkaVolterra systems. Fluct. Noise Lett. 2003, 3, L177–L185. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Mukhopadhyay, A.; Iyengar, S.R.K. Influence of environmental noise on the dynamics of a realistic ecological model. Fluct. Noise Lett. 2007, 7, L61–L77. [Google Scholar] [CrossRef]
- Das, A.; Samanta, G.P. Stochastic prey–predator model with additional food for predator. Phys. A Stat. Mech. Its Appl. 2018, 512, 121–141. [Google Scholar] [CrossRef]
- Watts, G.; Chinn, L.; Godfrey, N. The effects of vegetation on the perception of traffic noise. Appl. Acoust. 1999, 56, 39–56. [Google Scholar] [CrossRef]
- Ow, L.F.; Ghosh, S. Urban cities and road traffic noise: Reduction through vegetation. Appl. Acoust. 2017, 120, 15–20. [Google Scholar] [CrossRef]
- Klingberg, J.; Broberg, M.; Strandberg, B.; Thorsson, P.; Pleijel, H. Influence of urban vegetation on air pollution and noise exposure—A case study in Gothenburg, Sweden. Sci. Total Environ. 2017, 599, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E. International Regulation of Underwater Sound: Establishing Rules and Standards to Address Ocean Noise Pollution; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- McCarthy, E.; Lichtman, F. The origin and evolution of ocean noise regulation under the US Marine Mammal Protection Act. Ocean Coast. LJ 2007, 13. Available online: https://digitalcommons.mainelaw.maine.edu/oclj/vol13/iss1/2 (accessed on 8 September 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Carrasco, C.; Córdova-Lepe, F.; Velásquez, N. A Simple Stability Analysis for a Mathematical Model of Migration Due to Noise and Resources. Mathematics 2022, 10, 3485. https://doi.org/10.3390/math10193485
Ramirez-Carrasco C, Córdova-Lepe F, Velásquez N. A Simple Stability Analysis for a Mathematical Model of Migration Due to Noise and Resources. Mathematics. 2022; 10(19):3485. https://doi.org/10.3390/math10193485
Chicago/Turabian StyleRamirez-Carrasco, Carlos, Fernando Córdova-Lepe, and Nelson Velásquez. 2022. "A Simple Stability Analysis for a Mathematical Model of Migration Due to Noise and Resources" Mathematics 10, no. 19: 3485. https://doi.org/10.3390/math10193485
APA StyleRamirez-Carrasco, C., Córdova-Lepe, F., & Velásquez, N. (2022). A Simple Stability Analysis for a Mathematical Model of Migration Due to Noise and Resources. Mathematics, 10(19), 3485. https://doi.org/10.3390/math10193485





