Phosphorylation-Dependent Interactome of Ryanodine Receptor Type 2 in the Heart
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Lysate Preparation
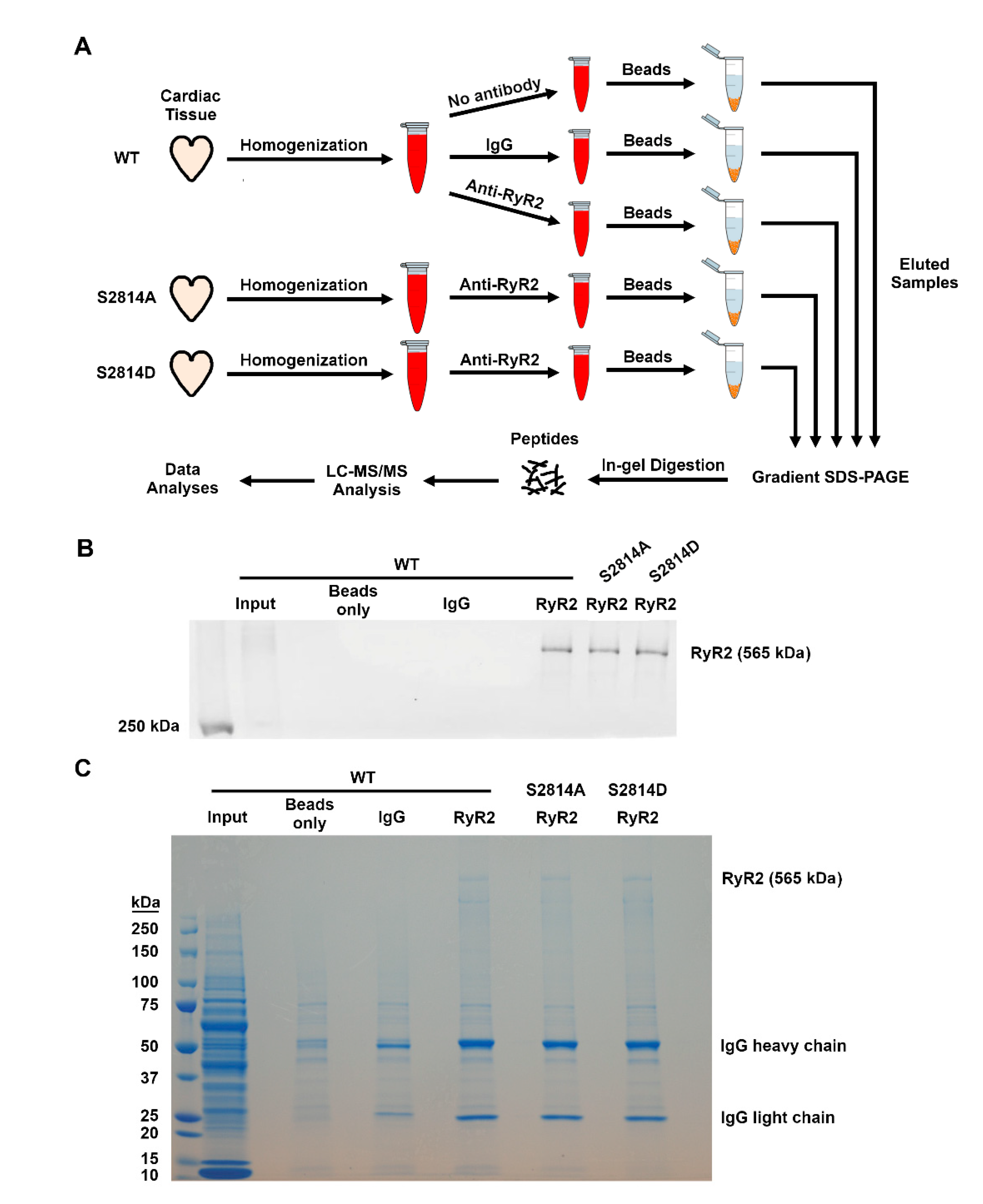
2.3. Co-Immunoprecipitation, Gel Electrophoresis, and Protein Digestion
2.4. Mass Spectrometry, Protein Identification, and Quantitation
2.5. Co-Immunoprecipitation and Western Blotting
2.6. Western Blot Analysis
2.7. Statistics
3. Results
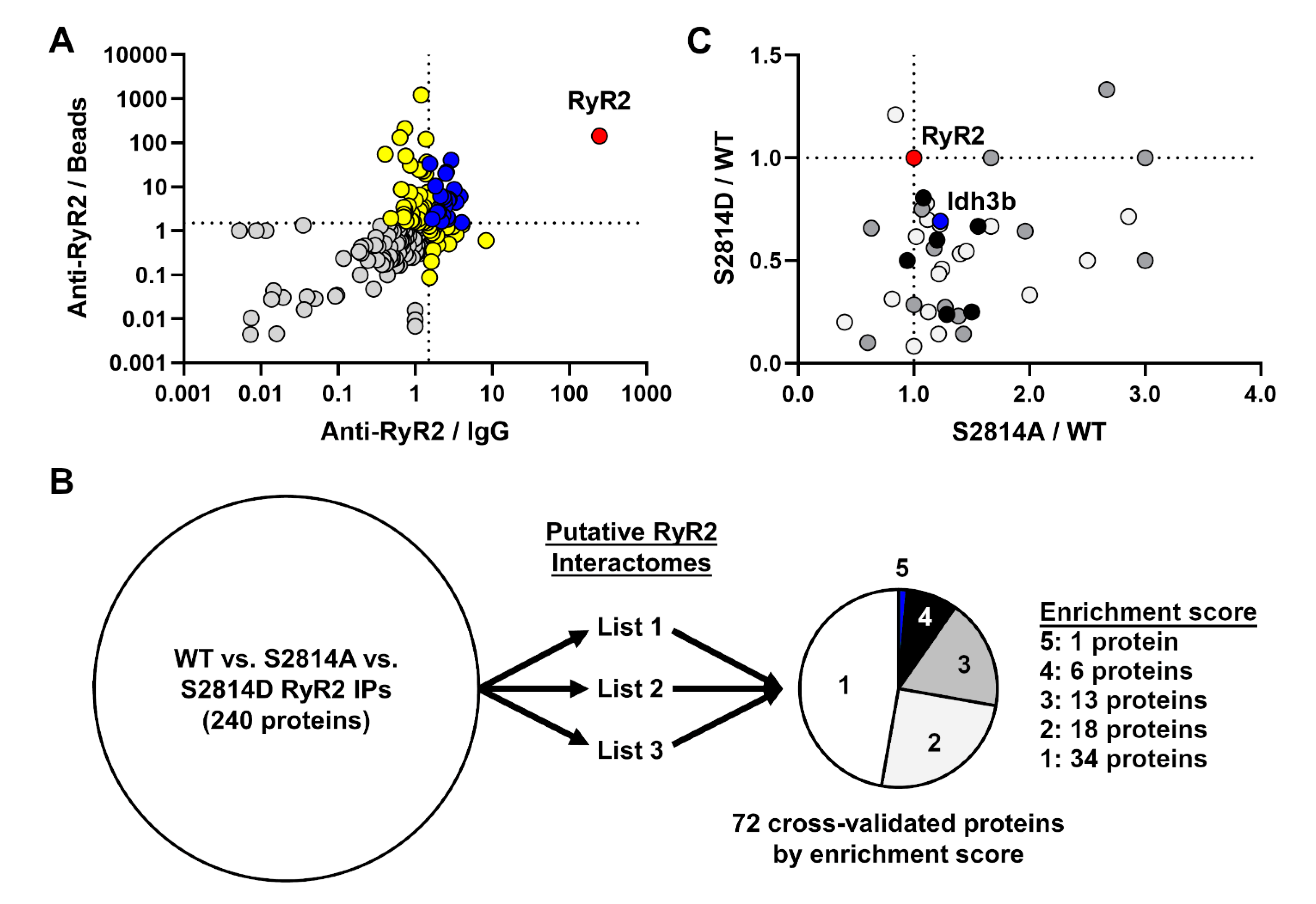
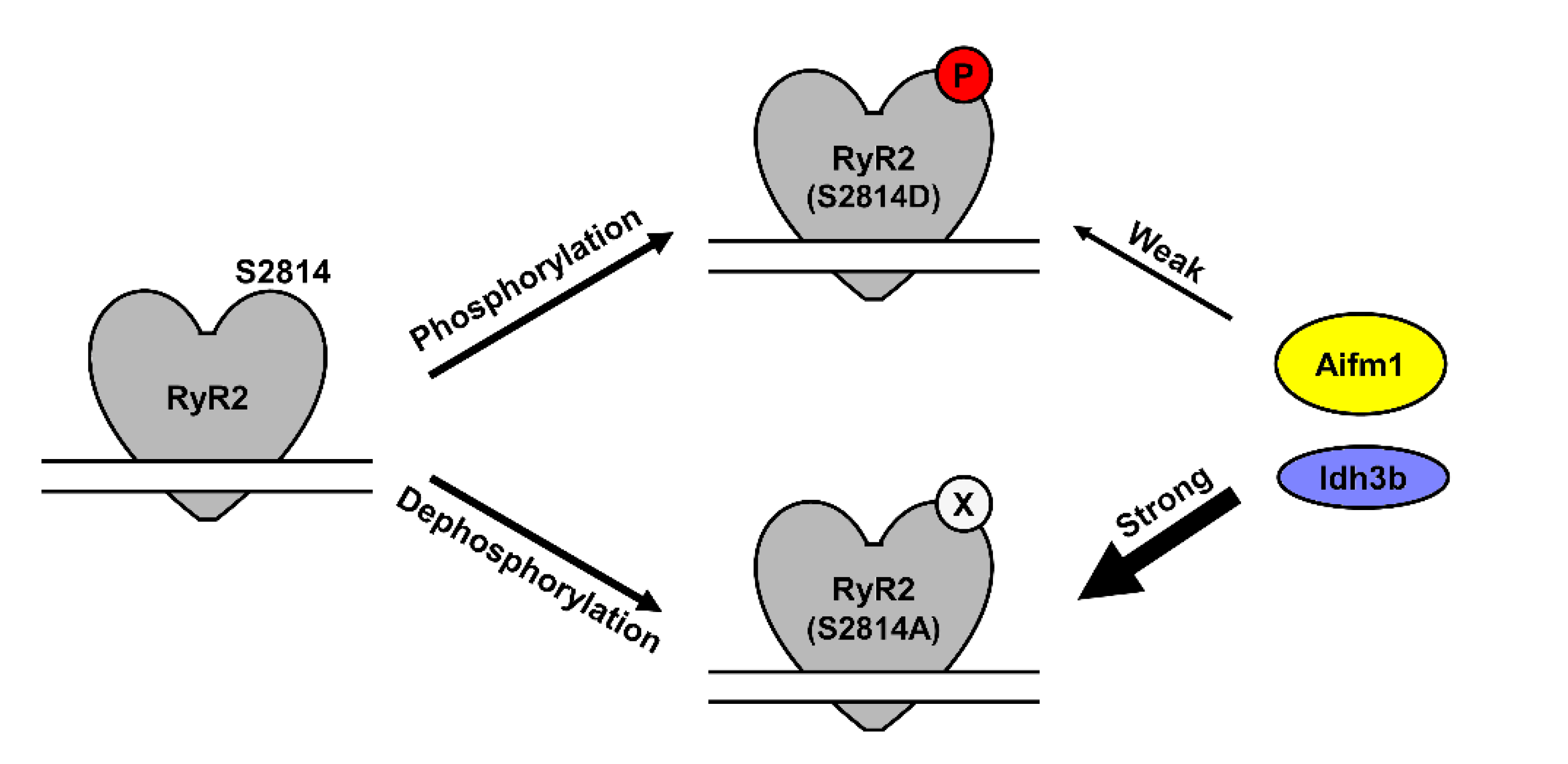
3.1. Identification of Novel RyR2 Interactors
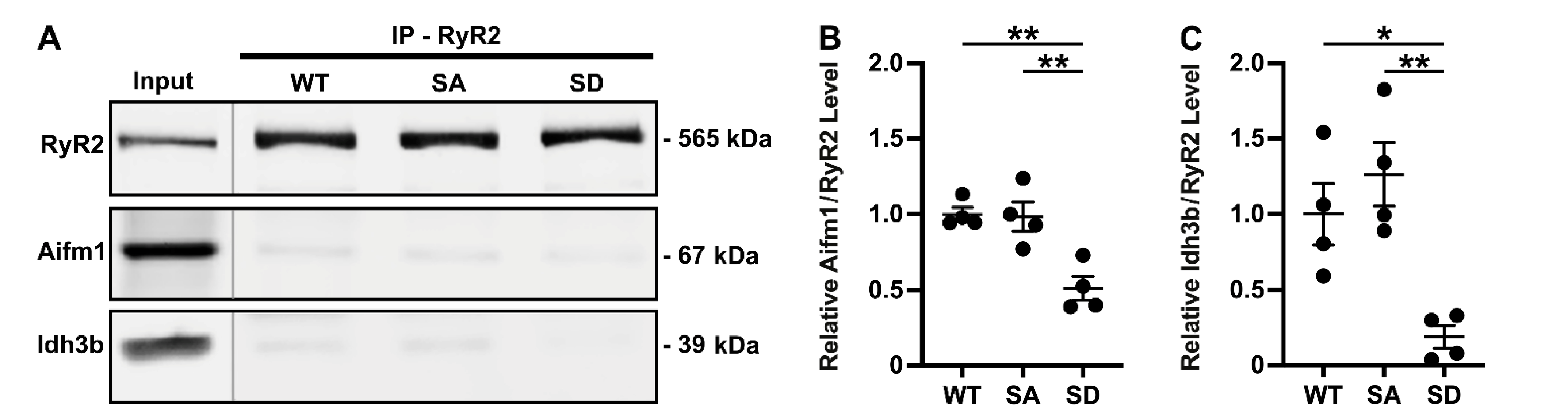
3.2. Effect of RyR2 S2814 Phosphorylation Status on Protein–Protein Interactions
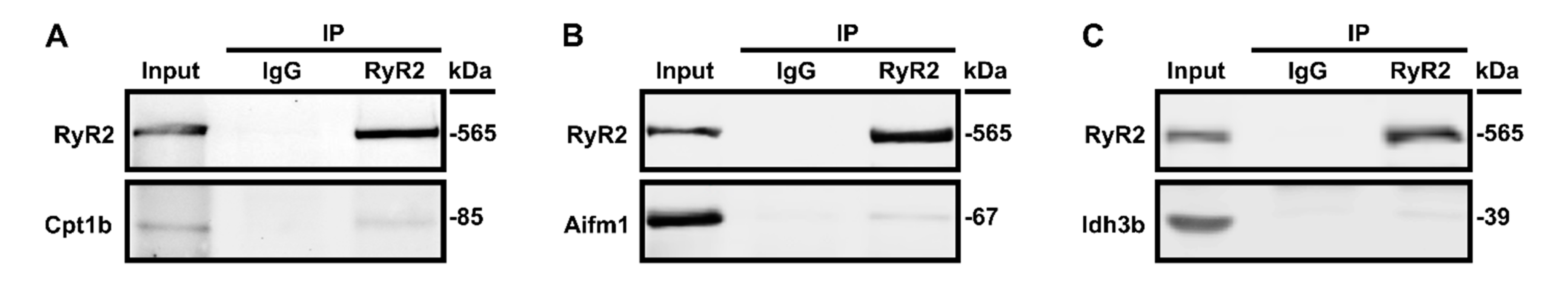
3.3. Validation of Select RyR2 Interactors
4. Discussion
4.1. Quantitative Affinity Purification Mass Spectrometry
4.2. Phosphorylation-Dependent Protein–Protein Interactions
4.3. CaMKII Phosphorylation-Dependent RyR2 Interactions
4.4. Novel Phosphorylation-Dependent RyR2 Interactors
4.5. Clinical Implications
5. Conclusions
Potential Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marx, S.; Reiken, S.; Hisamatsu, Y.; Jayaraman, T.; Burkhoff, D.; Rosemblit, N.; Marks, A.R. PKA Phosphorylation Dissociates FKBP12.6 from the Calcium Release Channel (Ryanodine Receptor). Cell 2000, 101, 365–376. [Google Scholar] [CrossRef]
- Connell, P.; Word, T.A.; Wehrens, X.H.T. Targeting pathological leak of ryanodine receptors: Preclinical progress and the potential impact on treatments for cardiac arrhythmias and heart failure. Expert Opin. Ther. Targets 2020, 24, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chiang, D.; Wang, S.; Wang, Q.; Sun, L.; Voigt, N.; Respress, J.L.; Ather, S.; Skapura, D.G.; Jordan, V.K.; et al. Ryanodine Receptor–Mediated Calcium Leak Drives Progressive Development of an Atrial Fibrillation Substrate in a Transgenic Mouse Model. Circulation 2014, 129, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.Y.; Li, N.; Wang, Q.; Alsina, K.M.; Quick, A.P.; Reynolds, J.O.; Wang, G.; Skapura, D.; Voigt, N.; Dobrev, D.; et al. Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc. Res. 2014, 103, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Respress, J.L.; Van Oort, R.J.; Li, N.; Rolim, N.; Dixit, S.S.; DeAlmeida, A.; Voigt, N.; Lawrence, W.S.; Skapura, D.G.; Skårdal, K.; et al. Role of RyR2 Phosphorylation at S2814 During Heart Failure Progression. Circ. Res. 2012, 110, 1474–1483. [Google Scholar] [CrossRef]
- Van Oort, R.J.; McCauley, M.D.; Dixit, S.S.; Pereira, L.; Yang, Y.; Respress, J.L.; Wang, Q.; De Almeida, A.C.; Skapura, D.G.; Anderson, M.E.; et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation 2010, 122, 2669–2679. [Google Scholar] [CrossRef]
- Chelu, M.G.; Sarma, S.; Sood, S.; Wang, S.; Van Oort, R.J.; Skapura, D.G.; Li, N.; Santonastasi, M.; Müller, F.U.; Schmitz, W.; et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+leak promotes atrial fibrillation in mice. J. Clin. Investig. 2009, 119, 1940–1951. [Google Scholar] [CrossRef]
- Quick, A.P.; Wang, Q.; Philippen, L.E.; Barreto-Torres, G.; Chiang, D.; Beavers, D.; Wang, G.; Khalid, M.; Reynolds, J.O.; Campbell, H.M.; et al. SPEG (Striated Muscle Preferentially Expressed Protein Kinase) Is Essential for Cardiac Function by Regulating Junctional Membrane Complex Activity. Circ. Res. 2017, 120, 110–119. [Google Scholar] [CrossRef]
- Campbell, H.M.; Quick, A.P.; Abu-Taha, I.; Chiang, D.Y.; Kramm, C.F.; Word, T.A.; Brandenburg, S.; Hulsurkar, M.; Alsina, K.M.; Liu, H.-B.; et al. Loss of SPEG Inhibitory Phosphorylation of Ryanodine Receptor Type-2 Promotes Atrial Fibrillation. Circulation 2020, 142, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Alsina, K.M.; Hulsurkar, M.; Brandenburg, S.; Kownatzki-Danger, D.; Lenz, C.; Urlaub, H.; Abu-Taha, I.; Kamler, M.; Chiang, D.Y.; Lahiri, S.K.; et al. Loss of Protein Phosphatase 1 Regulatory Subunit PPP1R3A Promotes Atrial Fibrillation: SUPP. Circulation 2019, 140, 681–693. [Google Scholar] [CrossRef]
- Chiang, D.Y.; Lebesgue, N.; Beavers, D.L.; Alsina, K.M.; Damen, J.M.A.; Voigt, N.; Dobrev, D.; Wehrens, X.H.; Scholten, A. Alterations in the interactome of serine/threonine protein phosphatase type-1 in atrial fibrillation patients. J. Am. Coll. Cardiol. 2015, 65, 163–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cox, J.; Hein, M.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-wide Label-free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Dange, M.; McGee, T.L.; Berson, E.L.; Dryja, T.P.; Colman, R.F. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat. Genet. 2008, 40, 1230–1234. [Google Scholar] [CrossRef]
- Ghezzi, D.; Sevrioukova, I.; Invernizzi, F.; Lamperti, C.; Mora, M.; D’Adamo, P.; Novara, F.; Zuffardi, O.; Uziel, G.; Zeviani, M. Severe X-Linked Mitochondrial Encephalomyopathy Associated with a Mutation in Apoptosis-Inducing Factor. Am. J. Hum. Genet. 2010, 86, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Wischhof, L.; Gioran, A.; Sonntag-Bensch, D.; Piazzesi, A.; Stork, M.; Nicotera, P.; Bano, D. A disease-associated Aifm1 variant induces severe myopathy in knockin mice. Mol. Metab. 2018, 13, 10–23. [Google Scholar] [CrossRef]
- He, L.; Kim, T.; Long, Q.; Liu, J.; Wang, P.; Zhou, Y.; Ding, Y.; Prasain, J.; Wood, P.A.; Yang, Q. Carnitine Palmitoyltransferase-1b Deficiency Aggravates Pressure Overload-Induced Cardiac Hypertrophy Caused by Lipotoxicity. Circulation 2012, 126, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Selbach, M. Quantitative affinity purification mass spectrometry: A versatile technology to study protein-protein interactions. Front. Genet. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Chiang, D.; Alsina, K.M.; Corradini, E.; Fitzpatrick, M.; Ni, L.; Lahiri, S.K.; Reynolds, J.O.; Pan, X.; Scott, L.; Heck, A.J.R.; et al. Rearrangement of the Protein Phosphatase 1 Interactome During Heart Failure Progression. Circulation 2018, 138, 1569–1581. [Google Scholar] [CrossRef]
- Watanabe, N.; Osada, H. Small molecules that target phosphorylation dependent protein–protein interaction. Bioorganic Med. Chem. 2016, 24, 3246–3254. [Google Scholar] [CrossRef]
- Dephoure, N.; Gould, K.; Gygi, S.P.; Kellogg, D.R. Mapping and analysis of phosphorylation sites: A quick guide for cell biologists. Mol. Biol. Cell 2013, 24, 535–542. [Google Scholar] [CrossRef]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef]
- Wehrens, X.H.T.; Lehnart, S.E.; Reiken, S.R.; Marks, A.R. Ca 2+/Calmodulin-Dependent Protein Kinase II Phosphorylation Regulates the Cardiac Ryanodine Receptor. Circ. Res. 2004, 94, e61–e70. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ikemoto, N. Peptide Probe Study of the Critical Regulatory Domain of the Cardiac Ryanodine Receptor. Biochem. Biophys. Res. Commun. 2002, 291, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Uchinoumi, H.; Yano, M.; Suetomi, T.; Ono, M.; Xu, X.; Tateishi, H.; Oda, T.; Okuda, S.; Doi, M.; Kobayashi, S.; et al. Catecholaminergic Polymorphic Ventricular Tachycardia Is Caused by Mutation-Linked Defective Conformational Regulation of the Ryanodine Receptor. Circ. Res. 2010, 106, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Yano, M.; Yamamoto, T.; Tokuhisa, T.; Okuda, S.; Doi, M.; Ohkusa, T.; Ikeda, Y.; Kobayashi, S.; Ikemoto, N.; et al. Defective Regulation of Interdomain Interactions Within the Ryanodine Receptor Plays a Key Role in the Pathogenesis of Heart Failure. Circulation 2005, 111, 3400–3410. [Google Scholar] [CrossRef]
- Oda, T.; Yang, Y.; Nitu, F.; Svensson, B.; Lu, X.; Fruen, B.R.; Cornea, R.L.; Bers, N.M. In cardiomyocytes, binding of unzipping peptide activates ryanodine receptor 2 and reciprocally inhibits calmodulin binding. Circ. Res. 2013, 112, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Uchinoumi, H.; Yang, Y.; Oda, T.; Li, N.; Alsina, K.M.; Puglisi, J.L.; Chen-Izu, Y.; Cornea, R.L.; Wehrens, X.H.T.; Bers, D.M. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J. Mol. Cell Cardiol. 2016, 98, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef]
- Hamilton, S.; Terentyeva, R.; Martin, B.; Perger, F.; Li, J.; Stepanov, A.; Bonilla, I.M.; Knollmann, B.C.; Radwański, P.B.; Györke, S.; et al. Increased RyR2 activity is exacerbated by calcium leak-induced mitochondrial ROS. Basic Res. Cardiol. 2020, 115, 1–20. [Google Scholar] [CrossRef]
- Di Carlo, M.N.; Said, M.; Ling, H.; Valverde, C.A.; De Giusti, V.C.; Sommese, L.; Palomeque, J.; Aiello, E.A.; Skapura, D.G.; Rinaldi, G.; et al. CaMKII-dependent phosphorylation of cardiac ryanodine receptors regulates cell death in cardiac ischemia/reperfusion injury. J. Mol. Cell Cardiol. 2014, 74, 274–283. [Google Scholar] [CrossRef]
- Federico, M.; Portiansky, E.L.; Sommese, L.; Alvarado, F.J.; Blanco, P.G.; Zanuzzi, C.N.; Dedman, J.; Kaetzel, M.; Wehrens, X.H.T.; Mattiazzi, A.; et al. Calcium-calmodulin-dependent protein kinase mediates the intracellular signalling pathways of cardiac apoptosis in mice with impaired glucose tolerance. J. Physiol. 2017, 595, 4089–4108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, W.; Wang, G.; Rodney, G.G.; Wehrens, X.H. Crosstalk between RyR2 oxidation and phosphorylation contributes to cardiac dysfunction in mice with Duchenne muscular dystrophy. J. Mol. Cell. Cardiol. 2015, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Prehn, J.H. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Ferri, K.F.; Irinopoulou, T.; Larochette, N.; Prévost, M.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000, 14, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Vahsen, N.; Candé, C.; Brière, J.-J.; Bénit, P.; Joza, N.; Larochette, N.; Mastroberardino, P.G.; Pequignot, M.O.; Casares, N.; Lazar, V.; et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004, 23, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Ohsakaya, S.; Nagaura, Z.-I.; Ishihara, N.; Mihara, K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005, 24, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Polster, B.M.; Basañez, G.; Etxebarria, A.; Hardwick, J.M.; Nicholls, D.G. Calpain I Induces Cleavage and Release of Apoptosis-inducing Factor from Isolated Mitochondria. J. Biol. Chem. 2005, 280, 6447–6454. [Google Scholar] [CrossRef]
- Wang, Y.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) signals to mitochondrial AIF: A key event in parthanatos. Exp. Neurol. 2009, 218, 193–202. [Google Scholar] [CrossRef]
- Norberg, E.; Gogvadze, V.; Ott, M.; Horn, M.; Uhlén, P.; Orrenius, S.; Zhivotovsky, B. An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ. 2008, 15, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Overbaugh, J. Evidence that an IRES within the Notch2 coding region can direct expression of a nuclear form of the protein. Mol. Cell. 2000, 6, 939–945. [Google Scholar] [CrossRef]
- Li, T.; Li, K.; Zhang, S.; Wang, Y.; Xu, Y.; Cronin, S.J.F.; Sun, Y.; Zhang, Y.; Xie, C.; Rodriguez, J.I.; et al. Overexpression of apoptosis inducing factor aggravates hypoxic-ischemic brain injury in neonatal mice. Cell Death Dis. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Joza, N.; Oudit, G.Y.; Brown, D.; Bénit, P.; Kassiri, Z.; Vahsen, N.; Benoit, L.; Patel, M.M.; Nowikovsky, K.; Vassault, A.; et al. Muscle-Specific Loss of Apoptosis-Inducing Factor Leads to Mitochondrial Dysfunction, Skeletal Muscle Atrophy, and Dilated Cardiomyopathy. Mol. Cell. Biol. 2005, 25, 10261–10272. [Google Scholar] [CrossRef]
- van Empel, V.P.M.; Bertrand, A.T.; van der Nagel, R.; Kostin, S.; Doevendans, P.A.; Crijns, H.J.; de Wit, E.; Sluiter, W.; Ackerman, S.L.; De Windt, L.J. Downregulation of Apoptosis-Inducing Factor in Harlequin Mutant Mice Sensitizes the Myocardium to Oxidative Stress–Related Cell Death and Pressure Overload–Induced Decompensation. Circ. Res. 2005, 96, e92–e101. [Google Scholar] [CrossRef]
- Tscheschner, H.; Meinhardt, E.; Schlegel, P.; Jungmann, A.; Lehmann, L.H.; Müller, O.J.; Most, P.; Katus, H.A.; Raake, P.W. CaMKII activation participates in doxorubicin cardiotoxicity and is attenuated by moderate GRP78 overexpression. PLoS ONE 2019, 14, e0215992. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, X.; Hu, W.; Yu, D.; Shao, Z.; Li, W.; Huang, T.; Zhang, J.; Zhu, X.; Li, X.; et al. RIP3 facilitates necroptosis through CaMKII and AIF after intracerebral hemorrhage in mice. Neurosci. Lett. 2021, 749, 135699. [Google Scholar] [CrossRef]
- Zhu, H.; Shi, J.; Cregg, J.M.; Woldegiorgis, G. Reconstitution of Highly Expressed Human Heart Muscle Carnitine Palmitoyltransferase I. Biochem. Biophys. Res. Commun. 1997, 239, 498–502. [Google Scholar] [CrossRef]
- Jogl, G.; Tong, L. Crystal Structure of Carnitine Acetyltransferase and Implications for the Catalytic Mechanism and Fatty Acid Transport. Cell 2003, 112, 113–122. [Google Scholar] [CrossRef]
- Bonnefont, J.-P.; Djouadi, F.; Prip-Buus, C.; Gobin, S.; Munnich, A.; Bastin, J. Carnitine palmitoyltransferases 1 and 2, biochemical, molecular and medical aspects. Mol. Asp. Med. 2004, 25, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Schweda, S.; Holubarsch, C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin. Sci. 2000, 99, 27–35. [Google Scholar] [CrossRef]
- Holubarsch, C.J.F.; Rohrbach, M.; Karrasch, M.; Boehm, E.; Polonski, L.; Ponikowski, P.; Rhein, S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: The ERGO (etomoxir for the recovery of glucose oxidation) study. Clin. Sci. 2007, 113, 205–212. [Google Scholar] [CrossRef]
- Schwarzer, M.; Faerber, G.; Rueckauer, T.; Blum, D.; Pytel, G.; Mohr, F.W.; Doenst, T. The metabolic modulators, Etomoxir and NVP-LAB121, fail to reverse pressure overload induced heart failure in vivo. Basic Res. Cardiol. 2009, 104, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Haynie, K.R.; Vandanmagsar, B.; Wicks, S.E.; Zhang, J.; Mynatt, R.L. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes, Obes. Metab. 2014, 16, 757–760. [Google Scholar] [CrossRef]
- Aitken-Buck, H.M.; Krause, J.; Zeller, T.; Jones, P.P.; Lamberts, R.R. Long-Chain Acylcarnitines and Cardiac Excitation-Contraction Coupling: Links to Arrhythmias. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Roussel, J.; Thireau, J.; Brenner, C.; Saint, N.; Scheuermann, V.; Lacampagne, A.; Le Guennec, J.-Y.; Fauconnier, J. Palmitoyl-carnitine increases RyR2 oxidation and sarcoplasmic reticulum Ca2+ leak in cardiomyocytes: Role of adenine nucleotide translocase. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Koh, H.J.; Kim, S.H.; Jo, S.H.; Huh, J.W.; Jeong, K.S.; Lee, I.J.; Song, B.J.; Huh, T.L. Identification and functional characterization of a novel, tissue-specific NAD(+)-dependent isocitrate dehydrogenase beta subunit isoform. J. Biol. Chem. 1999, 274, 36866–36875. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.O.; Park, S.H.; Kang, Y.J.; Koh, H.J.; Kim, S.H.; Park, S.Y.; Sohn, U.; Huh, T.L. Assignment of mitochondrial NAD(+)-specific isocitrate dehydrogenase beta subunit gene (IDH3B) to human chromosome band 20p13 by in situ hybridization and radiation hybrid mapping. Cytogenet. Cell Genet. 1999, 86, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Cardin, S.; Libby, E.; Pelletier, P.; Le Bouter, S.; Shiroshita-Takeshita, A.; Le Meur, N.; Léger, J.; Demolombe, S.; Ponton, A.; Glass, L.; et al. Contrasting Gene Expression Profiles in Two Canine Models of Atrial Fibrillation. Circ. Res. 2007, 100, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Chung, M.K.; Smith, J.D.; Hsu, J.; Serre, D.; Newton, D.W.; Castel, L.; Soltesz, E.G.; Pettersson, G.B.; Gillinov, A.M.; et al. Weighted Gene Coexpression Network Analysis of Human Left Atrial Tissue Identifies Gene Modules Associated With Atrial Fibrillation. Circ. Cardiovasc. Genet. 2013, 6, 362–371. [Google Scholar] [CrossRef]
- Wehrens, X.H.T.; Lehnart, S.E.; Reiken, S.R.; Deng, S.-X.; Vest, J.A.; Cervantes, D.; Coromilas, J.; Landry, D.W.; Marks, A.R. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 2004, 304, 292–296. [Google Scholar] [CrossRef]
- Hino, A.; Yano, M.; Kato, T.; Fukuda, M.; Suetomi, T.; Ono, M.; Murakami, W.; Susa, T.; Okuda, S.; Doi, M.; et al. Enhanced binding of calmodulin to the ryanodine receptor corrects contractile dysfunction in failing hearts. Cardiovasc. Res. 2012, 96, 433–443. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, D.Y.; Lahiri, S.; Wang, G.; Karch, J.; Wang, M.C.; Jung, S.Y.; Heck, A.J.R.; Scholten, A.; Wehrens, X.H.T. Phosphorylation-Dependent Interactome of Ryanodine Receptor Type 2 in the Heart. Proteomes 2021, 9, 27. https://doi.org/10.3390/proteomes9020027
Chiang DY, Lahiri S, Wang G, Karch J, Wang MC, Jung SY, Heck AJR, Scholten A, Wehrens XHT. Phosphorylation-Dependent Interactome of Ryanodine Receptor Type 2 in the Heart. Proteomes. 2021; 9(2):27. https://doi.org/10.3390/proteomes9020027
Chicago/Turabian StyleChiang, David Y., Satadru Lahiri, Guoliang Wang, Jason Karch, Meng C. Wang, Sung Y. Jung, Albert J. R. Heck, Arjen Scholten, and Xander H. T. Wehrens. 2021. "Phosphorylation-Dependent Interactome of Ryanodine Receptor Type 2 in the Heart" Proteomes 9, no. 2: 27. https://doi.org/10.3390/proteomes9020027
APA StyleChiang, D. Y., Lahiri, S., Wang, G., Karch, J., Wang, M. C., Jung, S. Y., Heck, A. J. R., Scholten, A., & Wehrens, X. H. T. (2021). Phosphorylation-Dependent Interactome of Ryanodine Receptor Type 2 in the Heart. Proteomes, 9(2), 27. https://doi.org/10.3390/proteomes9020027






