Exosome-Derived Mediators as Potential Biomarkers for Cardiovascular Diseases: A Network Approach
Abstract
1. Introduction
1.1. Biogenesis, Characteristics, and Functions of Exosomes
1.1.1. Exosomal Biogenesis
1.1.2. Sorting of Cargo into Exosomes
1.1.3. Characterization of Exosomes
1.1.4. Exosomal Functions
1.2. Exosomal Isolation Techniques
1.2.1. Ultracentrifugation-Based Isolation Techniques
1.2.2. Size-Based Isolation Techniques
1.2.3. Precipitation Methods
1.2.4. Immunoaffinity-Based Techniques
1.3. Exosomes in CVDs
2. Material and Methods
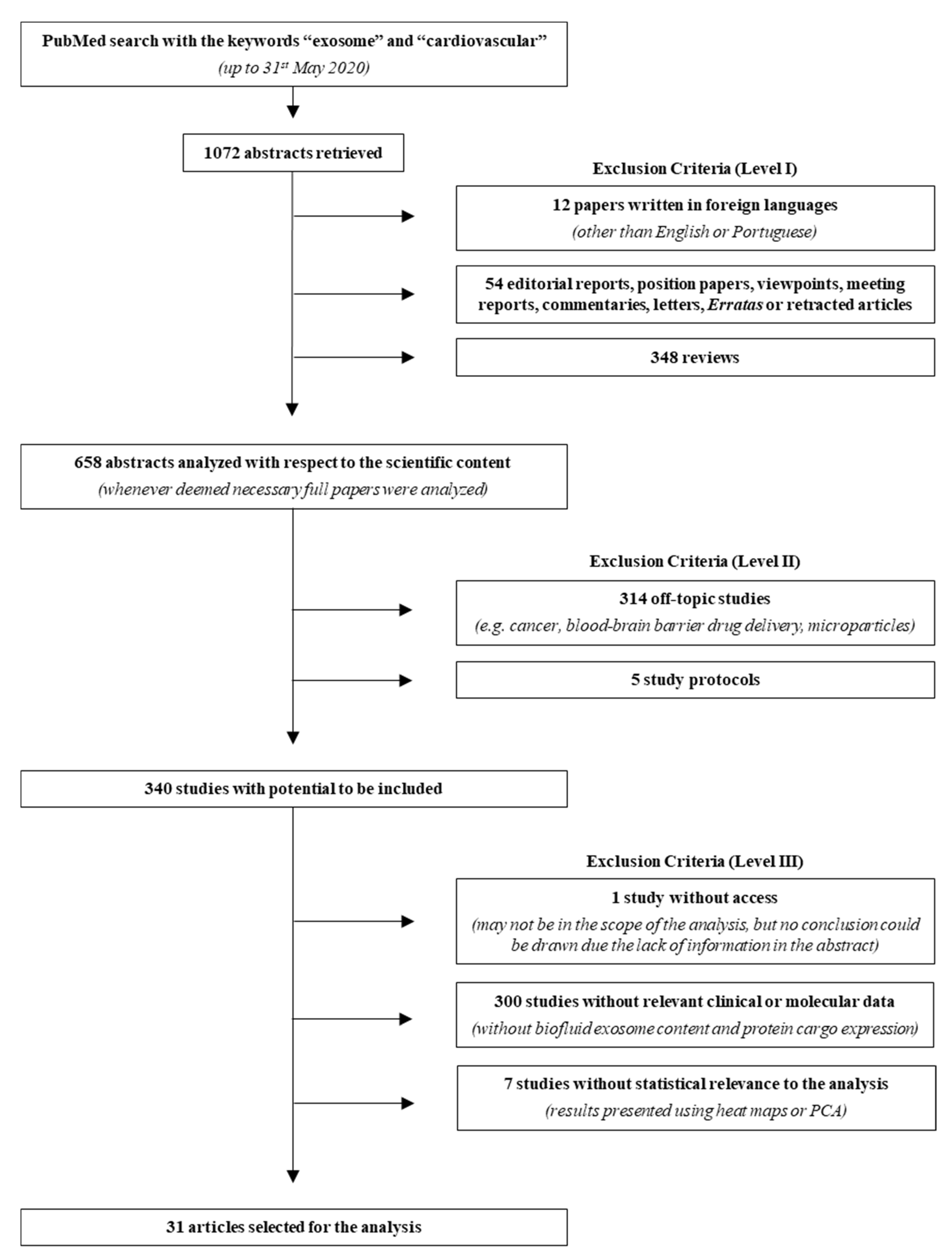
2.1. Literature Search
2.2. Bioinformatic Analysis
3. Results
3.1. Literature Search
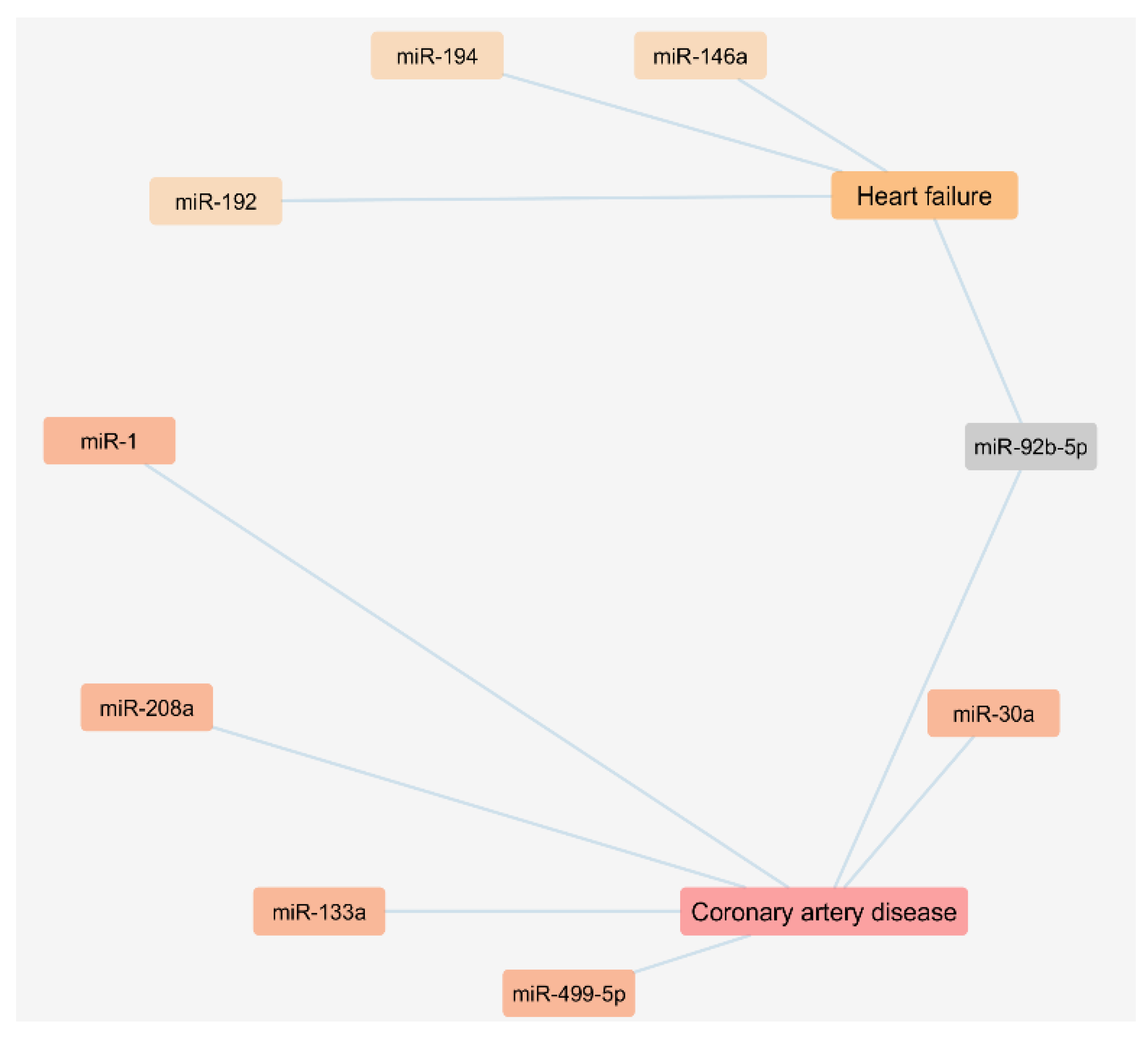
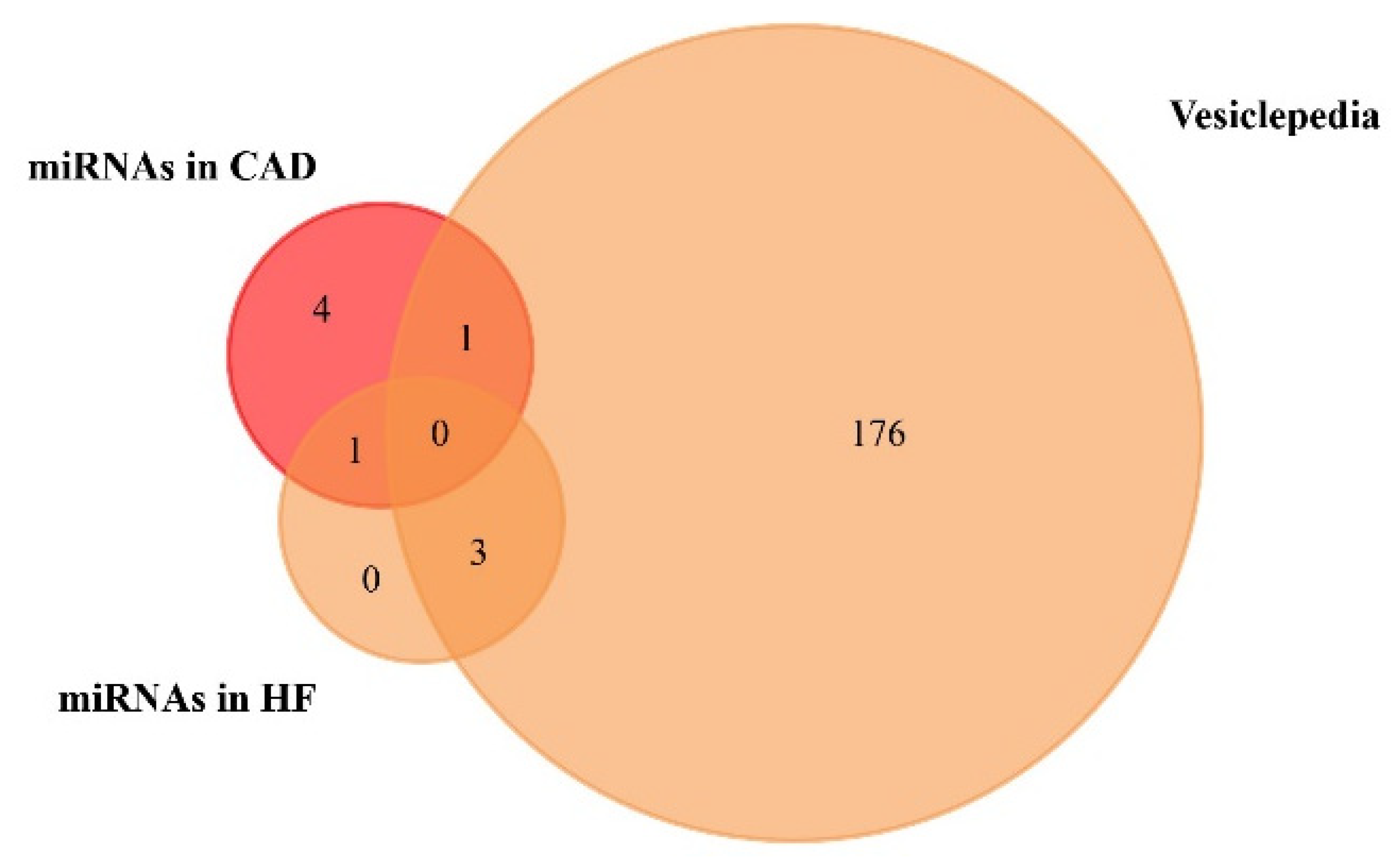
3.2. Circulating Exosomal miRNAs in Coronary Artery Disease and Heart Failure Patients
3.3. Circulating Exosomal Proteins in Coronary Artery Disease Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 16 July 2019).
- Zhao, Y.; Ponnusamy, M.; Zhang, L.; Zhang, Y.; Liu, C.; Yu, W.; Wang, K.; Li, P. The role of miR-214 in cardiovascular diseases. Eur. J. Pharm. 2017, 816, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Zhu, X.X.; Cai, W.W.; Qiu, L.Y. Functional roles of exosomes in cardiovascular disorders: A systematic review. Eur. Rev. Med. Pharm. Sci. 2017, 22, 5197–5206. [Google Scholar]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, S.; Wang, X.; Gu, H.; Fan, G.C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim. Biophys. Acta 2015, 1852, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aghabozorgi, A.S.; Ahangari, N.; Eftekhaari, T.E.; Torbati, P.N.; Bahiraee, A.; Ebrahimi, R.; Pasdar, A. Circulating exosomal miRNAs in cardiovascular disease? Pathogenesis: New emerging hopes. J. Cell Physiol. 2019, 234, 21796–21809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef]
- Gartz, M.; Strande, J.L. Examining the Paracrine Effects of Exosomes in Cardiovascular Disease and Repair. J. Am. Heart Assoc. 2018, 7, e007954. [Google Scholar] [CrossRef]
- Bellingham, S.A.; Guo, B.B.; Coleman, B.M.; Hill, A.F. Exosomes: Vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front. Physiol. 2012, 3, 124. [Google Scholar] [CrossRef]
- Arenaccio, C.; Federico, M. The Multifaceted Functions of Exosomes in Health and Disease: An Overview. Adv. Exp. Med. Biol. 2017, 998, 3–19. [Google Scholar]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Tanaka, N.; Nakano, T.; Kakazu, E.; Kondo, Y.; Inoue, J.; Shiina, M.; Fukushima, K.; Hoshino, T.; Sano, K.; et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem. Biophys. Res. Commun. 2010, 399, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012, 14, 1036–1045. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Laulagnier, K.; Grand, D.; Dujardin, A.; Hamdi, S.; Vincent-Schneider, H.; Lankar, D.; Salles, J.-P.; Bonnerot, C.; Perret, B.; Record, M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004, 572, 11–14. [Google Scholar] [CrossRef]
- Hirotami Matsuo, J.C. Nathalie Mayran, Isabelle Le Blanc, Charles Ferguson, Julien Fauré, Nathalie Sartori Blanc, Stefan Matile, Jacques Dubochet, Rémy Sadoul, Robert G Parton, Francis Vilbois, Jean Gruenberg. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 2004, 303, 531–534. [Google Scholar]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of beta-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Nkosi, D.; Conlon, M.M.; York, S.B.; Liu, X.; Tremblay, D.C.; Meckes, D.G., Jr. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-κB Signaling. J. Virol. 2017, 91, e02251-16. [Google Scholar] [CrossRef]
- Zhu, H.; Guariglia, S.; Yu, R.Y.; Li, W.; Brancho, D.; Peinado, H.; Lyden, D.; Salzer, J.; Bennett, C.; Chow, C.W. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol. Biol. Cell 2013, 24, 1619–1637. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernad, A.; Sanchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- van der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; van Leeuwen, T.G.; Nieuwland, R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef]
- Sharma, S.; Gillespie, B.M.; Palanisamy, V.; Gimzewski, J.K. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir 2011, 27, 14394–14400. [Google Scholar] [CrossRef]
- Aalberts, M.; van Dissel-Emiliani, F.M.; van Adrichem, N.P.; van Wijnen, M.; Wauben, M.H.; Stout, T.A.; Stoorvogel, W. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol. Reprod. 2012, 86, 82. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; Klinke, D.J., 2nd. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; Silva, O.A.B.D.C.E.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Palma, J.; Yaddanapudi, S.C.; Pigati, L.; Havens, M.A.; Jeong, S.; Weiner, G.A.; Weimer, K.M.; Stern, B.; Hastings, M.L.; Duelli, D.M. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012, 40, 9125–9138. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Mathew, A.; Mason, A.B.; Teng, K. Exosome formation during maturation of mammalian and avian reticulocytes: Evidence that exosome release is a major route for externalization of obsolete membrane proteins. J. Cell Physiol. 1991, 147, 27–36. [Google Scholar] [CrossRef]
- Zoller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Shao, K.; Liu, C.W.; Li, C.J.; Yu, B.T. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur. Rev. Med. Pharm. Sci. 2019, 23, 6691–6699. [Google Scholar]
- Mol, E.A.; Goumans, M.J.; Sluijter, J.P.G. Cardiac Progenitor-Cell Derived Exosomes as Cell-Free Therapeutic for Cardiac Repair. Adv. Exp. Med. Biol. 2017, 998, 207–219. [Google Scholar]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell Vesicles 2013, 2, 19861. [Google Scholar] [CrossRef]
- Caradec, J.; Kharmate, G.; Hosseini-Beheshti, E.; Adomat, H.; Gleave, M.; Guns, E. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin. Biochem. 2014, 47, 1286–1292. [Google Scholar] [CrossRef]
- Chen, B.Y.; Sung, C.W.; Chen, C.; Cheng, C.M.; Lin, D.P.; Huang, C.T.; Hsu, M.Y. Advances in exosomes technology. Clin. Chim. Acta 2019, 493, 14–19. [Google Scholar] [CrossRef]
- Abramowicz, A.; Widlak, P.; Pietrowska, M. Proteomic analysis of exosomal cargo: The challenge of high purity vesicle isolation. Mol. Biosyst. 2016, 12, 1407–1419. [Google Scholar] [CrossRef]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.B.; Knepper, M.A.; Yuen, P.S.; Star, R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Ren. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzas, E.I.; Bemis, L.T.; Bora, A.; Lasser, C.; Lotvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Jo, W.; Heo, Y.; Kang, J.Y.; Kwak, R.; Park, J. Isolation of extracellular vesicle from blood plasma using electrophoretic migration through porous membrane. Sens. Actuators B Chem. 2016, 233, 289–297. [Google Scholar] [CrossRef]
- Bei, Y.; Yu, P.; Cretoiu, D.; Cretoiu, S.M.; Xiao, J. Exosomes-Based Biomarkers for the Prognosis of Cardiovascular Diseases. Adv. Exp. Med. Biol. 2017, 998, 71–88. [Google Scholar]
- Bei, Y.; Yang, T.; Wang, L.; Holvoet, P.; Das, S.; Sluijter, J.P.G.; Monteiro, M.C.; Liu, Y.; Zhou, Q.; Xiao, J. Circular RNAs as Potential Theranostics in the Cardiovascular System. Mol. Ther. Nucleic Acids 2018, 13, 407–418. [Google Scholar] [CrossRef]
- Bellin, G.; Gardin, C.; Ferroni, L.; Chachques, J.C.; Rogante, M.; Mitrecic, D.; Ferrari, R.; Zavan, B. Exosome in Cardiovascular Diseases: A Complex World Full of Hope. Cells 2019, 8, 166. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Long, K.; Qiu, W.; Wang, Y.; Hu, Z.; Liu, C.; Luo, Y.; Jiang, A.; Jin, L.; et al. Overexpression of Exosomal Cardioprotective miRNAs Mitigates Hypoxia-Induced H9c2 Cells Apoptosis. Int. J. Mol. Sci. 2017, 18, 711. [Google Scholar] [CrossRef]
- Yu, X.; Deng, L.; Wang, D.; Li, N.; Chen, X.; Cheng, X.; Yuan, J.; Gao, X.; Liao, M.; Wang, M.; et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: Initiated by hypoxia inducible factor 1α, presented by exosomes. J. Mol. Cell Cardiol. 2012, 53, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.W.; Zheng, L.; Wang, Q. Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cell Biol. 2017, 36, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Fereydouni, N.; Butler, A.E.; Navashenaq, J.G.; Sahebkar, A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 2019, 29, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J. Extracell. Vesicles 2017, 6, 1321455. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, J.; Zhao, X.; Zhang, E.; Zeng, Q.; Yu, Y.; Yang, L.; Wu, B.; Yi, G.; Mao, X.; et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat. Commun. 2019, 10, 959. [Google Scholar] [CrossRef]
- Sharma, M.; Ravichandran, R.; Bansal, S.; Bremner, R.M.; Smith, M.A.; Mohanakumar, T. Tissue-associated self-antigens containing exosomes: Role in allograft rejection. Hum. Immunol. 2018, 79, 653–658. [Google Scholar] [CrossRef]
- Li, J.; Tan, M.; Xiang, Q.; Zhou, Z.; Yan, H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res. 2017, 154, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yan, H.B.; Li, J.N.; Li, W.K.; Fu, Y.Y.; Chen, W.; Zhou, Z. Thrombin stimulated platelet-derived exosomes inhibit platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Cell Physiol. Biochem. 2016, 38, 2348–2365. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, X.; Zhao, M.; Cai, T.; Liu, P.; Li, J.; Willard, B.; Zu, L.; Zhou, E.; Li, Y.; et al. Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J. Am. Heart Assoc. 2016, 5, e004099. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Chen, X.; Cheng, X.; Liao, Y.; Yu, X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J. Mol. Med. 2016, 94, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Foglio, E.; Puddighinu, G.; Fasanaro, P.; D’Arcangelo, D.; Perrone, G.A.; Mocini, D.; Campanella, C.; Coppola, L.; Logozzi, M.; Azzarito, T.; et al. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int. J. Cardiol. 2015, 197, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Cubedo, J.; Padro, T.; Garcia-Moll, X.; Pinto, X.; Cinca, J.; Badimon, L. Proteomic signature of Apolipoprotein J in the early phase of new-onset myocardial infarction. J. Proteome Res. 2011, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Bai, J.; Xu, Y.F.; Li, L.Q.; Hua, L.; Deng, L.; Jia, H.L. Proteomic analysis associated with coronary artery dilatation caused by Kawasaki disease using serum exosomes. Rev. Port. Cardiol. 2016, 35, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, Y.; Du, Y.; Tao, J.; Zhou, Z.; Yang, Z. Serum Exosomal MiR-92b-5p as a Potential Biomarker for Acute Heart Failure Caused by Dilated Cardiomyopathy. Cell Physiol. Biochem. 2018, 46, 1939–1950. [Google Scholar] [CrossRef]
- Wu, T.; Chen, Y.; Du, Y.; Tao, J.; Li, W.; Zhou, Z.; Yang, Z. Circulating exosomal miR-92b-5p is a promising diagnostic biomarker of heart failure with reduced ejection fraction patients hospitalized for acute heart failure. J. Thorac Dis. 2018, 10, 6211–6220. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sakata, Y.; Suna, S.; Nakatani, D.; Usami, M.; Hara, M.; Kitamura, T.; Hamasaki, T.; Nanto, S.; Kawahara, Y.; et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 2013, 113, 322–326. [Google Scholar] [CrossRef]
- Kuosmanen, S.M.; Hartikainen, J.; Hippelainen, M.; Kokki, H.; Levonen, A.L.; Tavi, P. MicroRNA profiling of pericardial fluid samples from patients with heart failure. PLoS ONE 2015, 10, e0119646. [Google Scholar] [CrossRef] [PubMed]
- Beg, F.; Wang, R.; Saeed, Z.; Devaraj, S.; Masoor, K.; Nakshatri, H. Inflammation-associated microRNA changes in circulating exosomes of heart failure patients. BMC Res. Notes 2017, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Luo, H.; Li, X.; Li, T.; He, J.; Qi, Q.; Liu, Y.; Yu, Z. Exosomes Derived from Human Pulmonary Artery Endothelial Cells Shift the Balance between Proliferation and Apoptosis of Smooth Muscle Cells. Cardiology 2017, 137, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, J.M.; Pereira, M.; Wen, S.; Dooner, M.S.; Del Tatto, M.; Papa, E.; Goldberg, L.R.; Baird, G.L.; Ventetuolo, C.E.; Quesenberry, P.J.; et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc. Res. 2016, 110, 319–330. [Google Scholar] [CrossRef]
- Kranendonk, M.E.; de Kleijn, D.P.; Kalkhoven, E.; Kanhai, D.A.; Uiterwaal, C.S.; van der Graaf, Y.; Pasterkamp, G.; Visseren, F.L.; Group, S.S. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc. Diabetol. 2014, 13, 37. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Yang, L.; Li, J.; Cai, J. miRNA Profiling of Exosomes from Spontaneous Hypertensive Rats Using Next-Generation Sequencing. J. Cardiovasc. Transl. Res. 2019, 12, 75–83. [Google Scholar] [CrossRef]
- Osada-Oka, M.; Shiota, M.; Izumi, Y.; Nishiyama, M.; Tanaka, M.; Yamaguchi, T.; Sakurai, E.; Miura, K.; Iwao, H. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hyperten Res. 2016, 40, 353–360. [Google Scholar] [CrossRef]
- Ivy, J.R.; Oosthuyzen, W.; Peltz, T.S.; Howarth, A.R.; Hunter, R.W.; Dhaun, N.; Al-Dujaili, E.A.S.; Webb, D.J.; Dear, J.W.; Flatman, P.W.; et al. Glucocorticoids Induce Nondipping Blood Pressure by Activating the Thiazide-Sensitive Cotransporter. Hypertension 2016, 67, 1029–1037. [Google Scholar] [CrossRef]
- Damkjaer, M.; Jensen, P.H.; Schwämmle, V.; Sprenger, R.R.; Jacobsen, I.A.; Jensen, O.N.; Bie, P. Selective renal vasoconstriction, exaggerated natriuresis and excretion rates of exosomic proteins in essential hypertension. Acta Physiol. 2014, 212, 106–118. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, P.; Liu, J.; Xie, X. Exosomal microRNA-122 mediates obesity-related cardiomyopathy through suppressing mitochondrial ADP-ribosylation factor-like 2. Clin. Sci. 2019, 133, 1871–1881. [Google Scholar] [CrossRef]
- Kim, H.; Bae, Y.U.; Jeon, J.S.; Noh, H.; Park, H.K.; Byun, D.W.; Han, D.C.; Ryu, S.; Kwon, S.H. The circulating exosomal microRNAs related to albuminuria in patients with diabetic nephropathy. J. Transl. Med. 2019, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; De Nardis, V.; Marcantonio, P.; Mandolini, C.; Paganelli, C.; Vitale, E.; Buttitta, F.; Bucci, M.; Mezzetti, A.; Consoli, A.; et al. Plasma Exosome MicroRNA Profiling Unravels a New Potential Modulator of Adiponectin Pathway in Diabetes: Effect of Glycemic Control. J. Clin. Endocrinol. Metab. 2014, 99, E1681–E1685. [Google Scholar] [CrossRef] [PubMed]
- Escate, R.; Padro, T.; Suades, R.; Camino, S.; Muniz, O.; Diaz-Diaz, J.L.; Sionis, A.; Mata, P.; Badimon, L. High miR-133a levels in the circulation anticipates presentation of clinical events in familial hypercholesterolemia patients. Cardiovasc. Res. 2020, 117, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Khalyfa, A.; Khalyfa, A.A.; Mokhlesi, B.; Kheirandish-Gozal, L.; Almendros, I.; Peris, E.; Malhotra, A.; Gozal, D. Exosomal Cargo Properties, Endothelial Function and Treatment of Obesity Hypoventilation Syndrome: A Proof of Concept Study. J. Clin. Sleep Med. 2018, 14, 797–807. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Alvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Teran-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am. J. Respir Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef]
- Khalyfa, A.; Zhang, C.; Khalyfa, A.A.; Foster, G.E.; Beaudin, A.E.; Andrade, J.; Hanly, P.J.; Poulin, M.J.; Gozal, D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 2016, 39, 2077–2090. [Google Scholar] [CrossRef]
- Minghua, W.; Zhijian, G.; Chahua, H.; Qiang, L.; Minxuan, X.; Luqiao, W.; Weifang, Z.; Peng, L.; Biming, Z.; Lingling, Y.; et al. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell Death Dis. 2018, 9, 320. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Izumi, Y.; Nakamura, Y.; Yamazaki, T.; Shiota, M.; Sano, S.; Tanaka, M.; Osada-Oka, M.; Shimada, K.; Miura, K.; et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int. J. Cardiol. 2015, 178, 239–246. [Google Scholar] [CrossRef]
- Emanueli, C.; Shearn, A.I.; Laftah, A.; Fiorentino, F.; Reeves, B.C.; Beltrami, C.; Mumford, A.; Clayton, A.; Gurney, M.; Shantikumar, S.; et al. Coronary Artery-Bypass-Graft Surgery Increases the Plasma Concentration of Exosomes Carrying a Cargo of Cardiac MicroRNAs: An Example of Exosome Trafficking Out of the Human Heart with Potential for Cardiac Biomarker Discovery. PLoS ONE 2016, 11, e0154274. [Google Scholar] [CrossRef]
- Gao, X.F.; Wang, Z.M.; Wang, F.; Gu, Y.; Zhang, J.J.; Chen, S.L. Exosomes in Coronary Artery Disease. Int. J. Biol. Sci. 2019, 15, 2461–2470. [Google Scholar] [CrossRef]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029827. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Care, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef]
- Oliveira-Carvalho, V.; Carvalho, V.O.; Bocchi, E.A. The emerging role of miR-208a in the heart. DNA Cell Biol. 2013, 32, 8–12. [Google Scholar] [CrossRef]
- Satoh, M.; Minami, Y.; Takahashi, Y.; Tabuchi, T.; Nakamura, M. Expression of microRNA-208 is associated with adverse clinical outcomes in human dilated cardiomyopathy. J. Card Fail. 2010, 16, 404–410. [Google Scholar] [CrossRef]
- Hoffmann, S.; Clauss, S.; Berger, I.M.; Weiss, B.; Montalbano, A.; Roth, R.; Bucher, M.; Klier, I.; Wakili, R.; Seitz, H.; et al. Coding and non-coding variants in the SHOX2 gene in patients with early-onset atrial fibrillation. Basic Res. Cardiol. 2016, 111, 36. [Google Scholar] [CrossRef]
- Deftu, A.T.; Radu, B.M.; Cretoiu, D.; Deftu, A.F.; Cretoiu, S.M.; Xiao, J. Exosomes as intercellular communication messengers for cardiovascular and cerebrovascular diseases. In Exosomes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–238. [Google Scholar] [CrossRef]
- Evans, S.; Mann, D.L. Circulating p53-responsive microRNAs as predictive biomarkers in heart failure after acute myocardial infarction: The long and arduous road from scientific discovery to clinical utility. Circ. Res. 2013, 113, 242–244. [Google Scholar] [CrossRef]
- Nukala, S.B.; Regazzoni, L.; Aldini, G.; Zodda, E.; Tura-Ceide, O.; Mills, N.L.; Cascante, M.; Carini, M.; D’Amato, A. Differentially Expressed Proteins in Primary Endothelial Cells Derived from Patients With Acute Myocardial Infarction. Hypertension 2019, 74, 947–956. [Google Scholar] [CrossRef]
- Priovolos, A.; Neerman-Arbez, M.; Morris, M.; Angelillo-Scherrer, A.; Notzli, J. Dysfibrinogenaemia associated with a novel heterozygous mutation in FGB (c.680delG) and a mild clinical history of bleeding. Blood Coagul. Fibrinolysis. 2015, 26, 231–232. [Google Scholar] [CrossRef][Green Version]
- Kuang, M.; Tao, X.; Peng, Y.; Zhang, W.; Pan, Y.; Cheng, L.; Yuan, C.; Zhao, Y.; Mao, H.; Zhuge, L.; et al. Proteomic analysis of plasma exosomes to differentiate malignant from benign pulmonary nodules. Clin. Proteom. 2019, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Gourgari, E.; Ma, J.; Playford, M.P.; Mehta, N.N.; Goldman, R.; Remaley, A.T.; Gordon, S.M. Proteomic alterations of HDL in youth with type 1 diabetes and their associations with glycemic control: A case-control study. Cardiovasc. Diabetol. 2019, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Gilutz, H.; Siegel, Y.; Paran, E.; Cristal, N.; Quastel, M.R. Alpha1-antitrypsin in acute myocardial infarction. Br. Heart J. 1983, 49, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rojas, T.; Mourino-Alvarez, L.; Gil-Dones, F.; de la Cuesta, F.; Rosello-Lleti, E.; Laborde, C.M.; Rivera, M.; Lopez-Almodovar, L.F.; Lopez, J.A.; Akerstrom, F.; et al. A clinical perspective on the utility of alpha 1 antichymotrypsin for the early diagnosis of calcific aortic stenosis. Clin. Proteom. 2017, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Chen, G.H.; Yang, Y.J. Exosomes: A Rising Star in Falling Hearts. Front. Physiol. 2017, 8, 494. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, C.Y.; Lin, C.F.; Yeh, H.I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31. [Google Scholar] [CrossRef]
- Schalk, B.W.; Visser, M.; Bremmer, M.A.; Penninx, B.W.; Bouter, L.M.; Deeg, D.J. Change of serum albumin and risk of cardiovascular disease and all-cause mortality: Longitudinal Aging Study Amsterdam. Am. J. Epidemiol. 2006, 164, 969–977. [Google Scholar] [CrossRef]
- Gopal, D.M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Tang, W.W.; Methvin, A.; Smith, A.L.; Bauer, D.C.; Newman, A.B.; Kim, L.; Harris, T.B.; et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am. Heart J. 2010, 160, 279–285. [Google Scholar] [CrossRef]
- Suzuki, S.; Hashizume, N.; Kanzaki, Y.; Maruyama, T.; Kozuka, A.; Yahikozawa, K. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PLoS ONE 2019, 14, e0219044. [Google Scholar] [CrossRef]
- Ku, E.J.; Cho, K.C.; Lim, C.; Kang, J.W.; Oh, J.W.; Choi, Y.R.; Park, J.M.; Han, N.Y.; Oh, J.J.; Oh, T.J.; et al. Discovery of Plasma Biomarkers for Predicting the Severity of Coronary Artery Atherosclerosis by Quantitative Proteomics. BMJ Open Diabetes Res. Care 2020, 8, e001152. [Google Scholar] [CrossRef]
- Karasu, E.; Eisenhardt, S.U.; Harant, J.; Huber-Lang, M. Extracellular Vesicles: Packages Sent with Complement. Front. Immunol. 2018, 9, 721. [Google Scholar] [CrossRef] [PubMed]
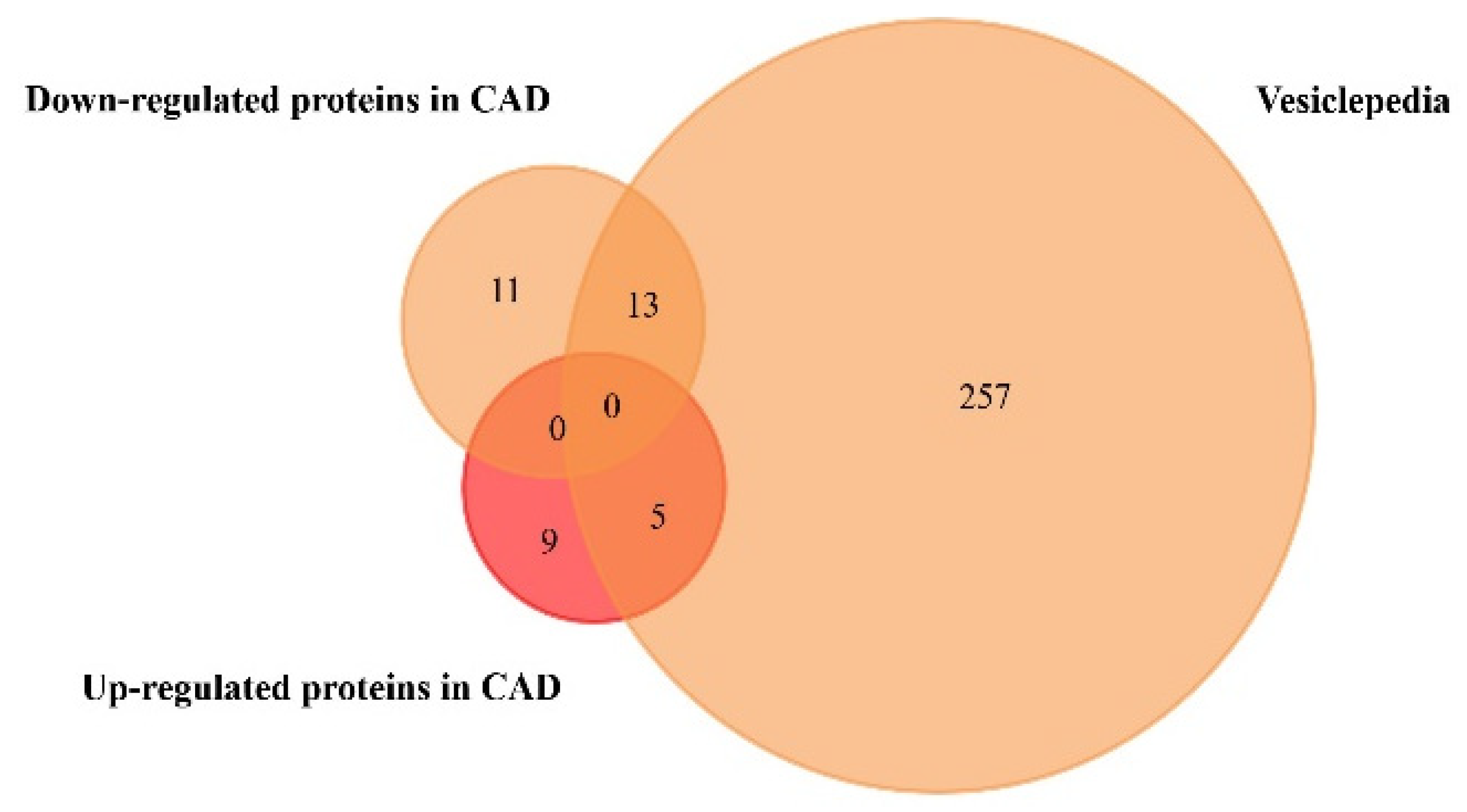
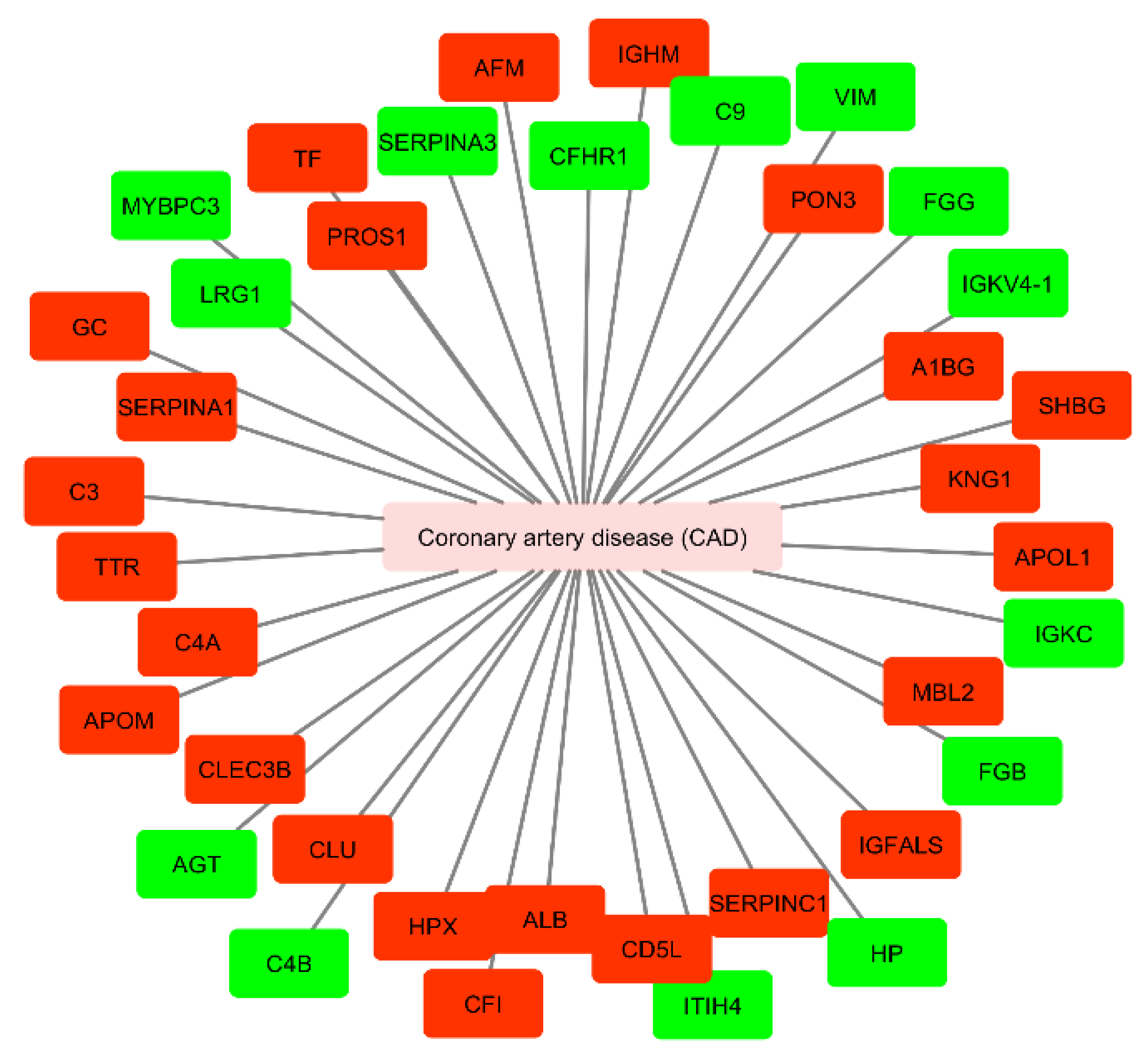
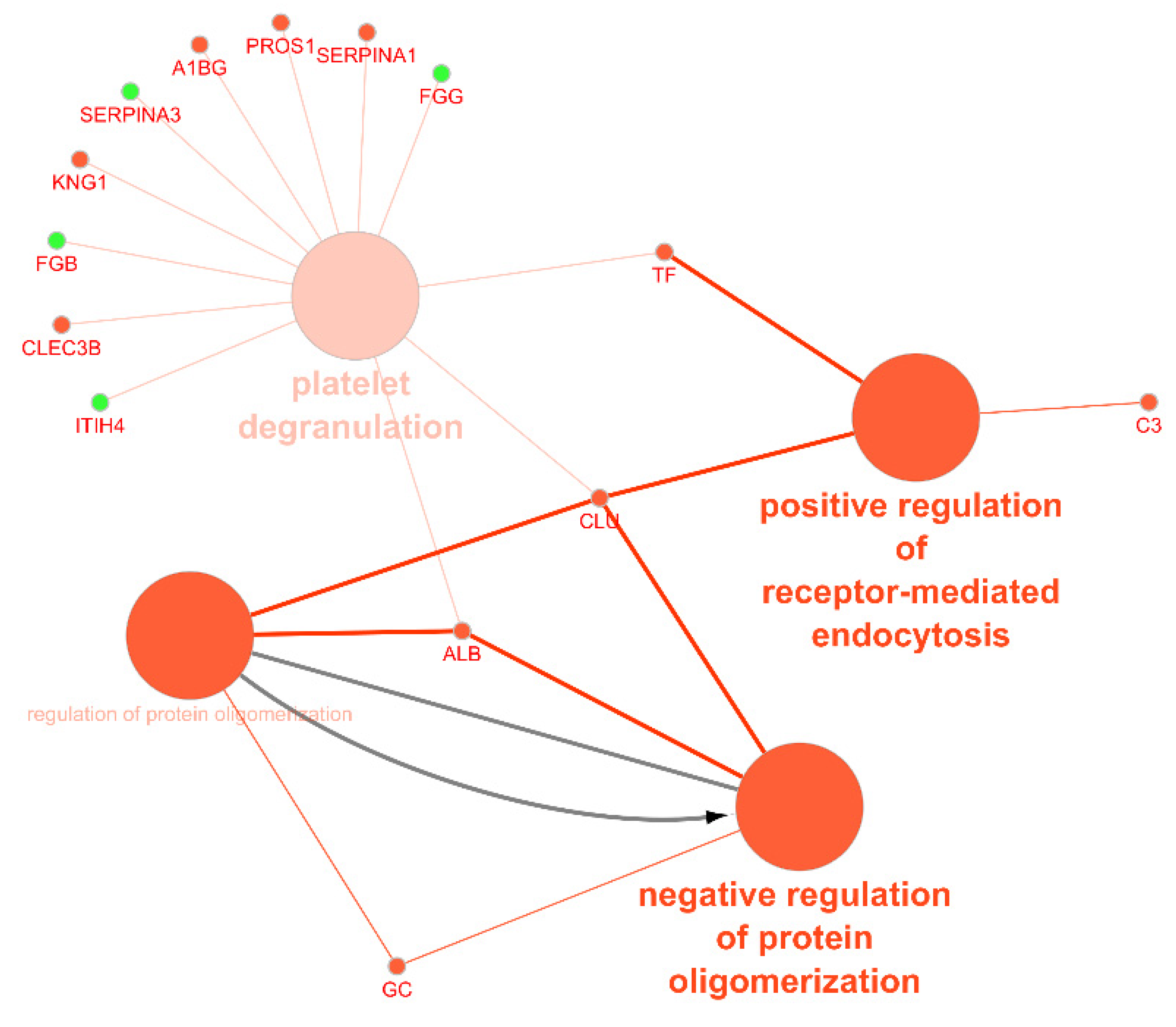

| Isolation Method | Main Advantages | Main Disadvantages | Ref. |
|---|---|---|---|
| Sucrose density gradient ultracentrifugation | Easy to perform, requires little technical expertise. | Time-consuming, risk of exosomal rupture and loss, requires a large volume of samples. | [47,48,49] |
Size-based methods
| Fast method with no requirement for special equipment. | Risk of exosomal rupture and loss. | [50,51,52] |
| Preserves exosomal structure with high purity and good reproducibility. | Laborious, possible contamination with lipoproteins. | [50,53] |
| Precipitation | Easy to perform, minimal cost with no requirement for special equipment. | Risk of contamination with lipoproteins. | [54,55] |
| Immunoaffinity-based methods | Preserves exosomal structure with high purity, requires a small volume of samples with a low experiment time. | Low yield, exosomal tags need to be established. | [52,56] |
| Microfluidics-based methods | Preserves exosomal structure and compositions, requires a small volume of samples and reagent consumption at a low cost. | Lack of method validation, standardization and large-scale tests on clinical samples. | [49,52,57] |
| Cardiovascular Disease | Isolation Method | Biofluid | Discovery Cohort Size | Exosome Validation Method | Exosomes Characteristics | Biomarker Candidate(s) | Ref. |
|---|---|---|---|---|---|---|---|
| Coronary artery disease (CAD) | ExoQuick Exosome Precipitation Solution | Plasma | C57BL/6 mice, n = 5 | Electron microscopy Nanoparticle tracking analysis Western Blotting | n. d. | miR-1, miR-208a, miR-133a, miR-499-5p | [70] |
| Ultracentrifugation (sucrose) | Serum | Human, n = 5 | Electron microscopy CD9 staining | n. d. | MYBPC3, VIM | [71] | |
| n. d. | Plasma | Tandem stenosis group, mice n = 4 | n. d. | n. d. | miR-223, miR-339, miR-21 | [72] | |
| Ultracentrifugation | Plasma | Tandem stenosis group, mice n = 4 | Electron microscopy Western blotting | n. d. | miR-223, miR-339, miR-21 | [73] | |
| Ultracentrifugation | Plasma | Human, n = 25 | Mass spectrometry for proteome analysis, using nano–liquid chromatography LTQ Orbitrap XL mass spectrometer | n. d. | n. d. | [74] | |
| Acute myocardial infarction (AMI) | ExoQuick Exosome Precipitation Solution | Plasma | C57BL/6 mice, n = 5 | Electron microscopy Nanoparticle tracking analysis Western Blotting | n. d. | miR-1, miR-208a, miR-133a, miR-499-5p | [70] |
| ExoQuick Exosome Precipitation Solution | Serum | Human, n = 28 | Transmission electron microscopy Flow cytometry Immunoblotting | CD63 expression | miR-30a | [75] | |
| Ultracentrifugation | Plasma; Pericardial fluid | Human, n = 12 | Western Blotting | Rab 5B and CD81 expression | n. d. | [76] | |
| Ultracentrifugation | Serum | Human, n = 10 | n. d. | n. d. | Apo-J | [77] | |
| Coronary artery dilatation due to Kawasaki disease (KD) | ExoQuick Exosome Precipitation Solution | Serum | Human, n = 6 | Transmission electron microscopy Western blotting | CD9, CD81, and flotillin expression | ITIH4, PROS1, C9, AFM, A1BG IGFALS, C4A, HPX, SERPINC 1, Inter-alpha (Globulin) inhibitor H4 (Plasma Kallikrein-sensitive glycoprotein) variant, AGT, DBP, KNG1, SERPINA 3, LRG1, C4B, HP, CLU, PON3, C3, CD5L, SHBG, FGG, APOL1, CFHR1, FGB, TF, ALB, CFI, IGHM, ALB isoform CRA_k, IGKVA-1, MBL2, TTR, IGKC, APOM, SERPINA 1, CLEC3B | [78] |
| Acute heart failure (HF) | Exosome isolation kit | Serum | Human, n = 43 | Electron microscopy Nanoparticle tracking analysis Western Blotting | CD63 and Hsp70 expression | miR-92b-5p, miR-192-5p, miR-320a | [79] |
| Exosome isolation kit | Serum | Human, n = 28 | Electron microscopy Nanoparticle tracking analysis Western blotting | Size: 40–150 nm (average 80 nm); CD63 and Hsp70 expression | miR-92b-5p, miR-192-5p, miR-320a | [80] | |
| HF | ExoQuick Exosome Precipitation Solution | Serum | Human, n = 4 | Western Blotting | CD63 expression | miR-192, miR-194, miR-34a | [81] |
| Ultracentrifugation | Pericardial fluid | Human, n = 51 | n. d. | n. d. | miR-210, let-7b-3p, let-7d-3p, miR-1, miR-125a-5p, miR-126-3p, miR-129-5p, miR-132-3p, miR-133a, miR-135a-5p, miR-135b-5p, miR-138-5p, miR-139-5p, miR-140-5p, miR-143-3p, miR-145-5p, miR-146a-3p, miR-146a-5p, miR-17-5p, miR-181a-5p, miR-181b-5p, miR-181c-5p, miR-208a, miR-20a-5p, miR-21-3p, miR-214-3p, miR-23a-3p, miR-23b-3p, miR-25-3p, miR-30a-3p, miR-30c-5p, miR-30e-3p, miR-320a, miR-330-5p, miR-339-3p, miR-346, miR-34c-3p, miR-365a-3p, miR-375, miR-499a-5p, miR-505-3p, miR-532-3p, miR-671-5p, miR-92b-3p, miR-9-3p | [82] | |
| Exosome Isolation kit | Plasma | Human, n = 40 | n. d. | n. d. | miR-486, miR-146a | [83] | |
| Idiopathic pulmonary arterial hypertension (IPAH) | Ultracentrifugation | Plasma | Human, n = 5 | Nanoparticle tracking analysis BCA Protein assay Immunoblotting | CD31, CD63 and TSG101 expression | n. d. | [84] |
| Ultracentrifugation | Plasma | Human, n = n. d. | Nanoparticle tracking analysis Transmission electron microscopy Western blotting | n. d. | miR-let-7c, miR-let-7d, miR-16, miR-18a, miR-19b, miR-20a, miR-20b, miR-27b, miR-30b, miR-30c, miR-125a-5p, miR-145, miR-146b. miR-148a, miR-195, miR-200b, miR-215, miR-218, miR-221, miR-339-3p, miR-365 | [85] | |
| Arterial disease/ cardiovascular risk factors | ExoQuick Exosome Precipitation Solution | Plasma | Human, n = 1012 | BCA Protein assay | n. d. | n. d. | [86] |
| (i) Hypertension | Exosome isolation kit | Plasma | Spontaneous hypertensive rats (SHRs), n = n. d. | Dynamic light scattering Western blotting | Size: 10–200 nm diameter (those ranging 30–150 nm accounted for 80%); CD63 and Hsp70 expression | rno-miR-148a-3p, rno-miR-122-5p, rno-miR-143-3p, rno-miR-192-5p, rno-let-7i-5p, rno-miR-215, rno-miR-140-3p, rno-miR-99a-5p, rno-miR-6329, rno-miR-378a-3p, rno-miR-486, rno-miR-378a-5p, rno-miR-6328, rno-miR-187-3p, rno-miR-383-5p, rno-miR-206-3p, rno-miR-425-5p, rno-miR-128-3p, rno-miR-181c-3p, rno-let-7d-5p, rno-miR-191a-3p, rno-miR-185-5p, rno-miR-218a-5p, rno-let-7f-5p, rno-miR-148a-5p, rno-miR-322-3p, rno-miR-181d-5p, rno-miR-223-5p, rno-miR-191a-5p, rno-miR-17-5p, rno-miR-3559-5p, rno-let-7a-5p, rno-miR-15b-5p, rno-miR-223-3p, rno-miR-872-5p, rno-miR-3068-3p | [87] |
| Ultracentrifugation | Serum | Cardiac hypertrophic Wistar rats, n = 6 | Electron microscopy Western blotting | n. d. | HSP90, HSC70, CD63, CD9, GAPDH, CD68, miR-17-3p, miR-145-5p, miR-221-3p, miR-222-5p | [88] | |
| n. d. | Urine | C57BL6J/Ola mice, n = n. d. | Nanoparticle tracking analysis Western blotting | n. d. | NCC | [89] | |
| Ultracentrifugation | Urine | Human, n = 11 | n. d. | n. d. | RAIG-2, SDCBP, NKCC2, TSC, ACTB, RAIG-3, ANPEP, GAPDH, MME, EZR, KRT1, ENO1, LDHB, HSPA8, ANXA2 | [90] | |
| (ii) Obesity | n. d. | Plasma | Human, n = 23 | n. d. | n. d. | miR-122 | [91] |
| (iii) Type 2 diabetes (T2D) | ExoQuick Exosome Precipitation Solution | Serum | Human, n = 33 | n. d. | n. d. | miR-122-5p, let-7a-3p, miR-26b-3p, miR-193b-5p, miR-4532, miR-432-5p, let-7f-1-3p, miR-183-5p, miR-3656, miR-340-3p, miR-6751-3p, miR-1273a, miR-4484, miR-8485, miR-4644, miR-1273g-3p, miR-4271, miR-7847-3p, miR-4461, miR-6885-5p | [92] |
| ExoQuick Exosome Precipitation Solution | Plasma | Human, n = 18 | n. d. | n. d. | miR-326, miR-532-5p, miR-186, miR-127-3p, let-7g, let-7d, miR-126, miR-101, miR-18b, miR-21, miR-199a-3p, miR-502-3p, miR-495, miR-132, miR-15b, miR-200c, miR-223-5p, miR-16, miR-543, miR-195, let-7a, miR-26b, miR-374a, miR-26a, let-7f, ADIPOR1, ADIPOR2, APPL1 | [93] | |
| (iv) Diabetic nephropathy | ExoQuick Exosome Precipitation Solution | Serum | Human, n = 33 | n. d. | n. d. | miR-122-5p, miR-432-5p, miR-3656, miR-193b-5p, miR-6087, miR-4488, miR-26b-3p, miR-8485, miR-23a-5p, miR-4532, let-7a-3p, miR-6739-5p, miR-1273a, miR-7641, miR-4461, miR-6751-3p, miR-4484, miR-7847-3p, miR-1273g-3p, miR-140-5p | [92] |
| (v) Familial hypercholesterolemia (with a CV event) | Filtration | Plasma | Human, n = 42 | Nanoparticle tracking analysis Flow cytometry (CD63 and CD81) | n. d. | miR-130b, miR-133a, miR-142-3p, miR-200c, miR-324-5p, miR-339-3p, miR-425-5p, miR-660, miR-744, miR-122 | [94] |
| (vi) Obstructive sleep apnea (OSA) | Exosome isolation kit | Plasma | Human, n = 8 | Electron microscopy Western blotting | n. d. | hsa-miR-16-5p, hsa-miR-4459, hsa-miR-451a, hsa-miR-6510-5p | [95] |
| Exosome isolation kit | Plasma | Human, n = n. d. | Electron microscopy Flow cytometry Western blotting | CD63 expression | n. d. | [96] | |
| Exosome isolation kit | Plasma | Human, n = 10 | Electron microscopy | n. d. | n. d. | [97] | |
| Myocardial ischemia/reperfusion (IR) | ExoQuick Exosome Precipitation Solution | Plasma | Human, n = 4 | Transmission electron microscopy BCA Protein assay Flow cytometry Western blotting | Cup-shaped membrane-bound vesicles;size: ~100 nm diameter; CD63, CD9 and CD81 expression | miR-24, miR-21, miR-214, miR-132, miR-195, miR-210, miR-144, miR-150, miR-34a | [98] |
| Ultracentrifugation | Serum | MI Wistar rats, n = 3 | BCA Protein assay Western blotting | CD9 and Hsp90 expression | miR-21, miR-29a, miR-30a, miR-133a | [99] | |
| Coronary artery bypass graft (CABG) | Column-based system | Plasma | Human, n = 21 | Nanoparticle tracking analysis Transmission electron microscopy Western blotting | n. d. | miR-1, miR-23a, miR-24, miR-92a, miR-126, miR-133a, miR-133b, miR-208a, miR-208b, miR-210, miR-223, miR-451 | [100] |
| miRNA | Cardiovascular Disease | Variation | Included/Not Included in the Vesiclepedia |
|---|---|---|---|
| miR-133a | Coronary artery disease (CAD) | + | Included |
| miR-208a | Coronary artery disease (CAD) | + | Not included |
| miR-1 | Coronary artery disease (CAD) | + | Not included |
| miR-499-5p | Coronary artery disease (CAD) | + | Not included |
| miR-92b-5p | Coronary artery disease (CAD) | + | Not included |
| miR-30a | Coronary artery disease (CAD) | + | Not included |
| miR-192 | Heart failure (HF) | + | Included |
| miR-194 | Heart failure (HF) | + | Included |
| miR-146a | Heart failure (HF) | + | Included |
| miR-92b-5p | Heart failure (HF) | + | Not included |
| Gene Name | Cardiovascular Disease | Variation | Included/Not Included in the Vesiclepedia |
|---|---|---|---|
| AGT | Coronary artery disease (CAD) | + | Included |
| C4B | Coronary artery disease (CAD) | + | Included |
| HP | Coronary artery disease (CAD) | + | Included |
| FGG | Coronary artery disease (CAD) | + | Included |
| FGB | Coronary artery disease (CAD) | + | Included |
| VIM | Coronary artery disease (CAD) | + | Not included |
| ITIH4 | Coronary artery disease (CAD) | + | Not included |
| C9 | Coronary artery disease (CAD) | + | Not included |
| IGKC | Coronary artery disease (CAD) | + | Not included |
| MYBPC3 | Coronary artery disease (CAD) | + | Not included |
| SERPINA3 | Coronary artery disease (CAD) | + | Not included |
| LRG1 | Coronary artery disease (CAD) | + | Not included |
| CFHR1 | Coronary artery disease (CAD) | + | Not included |
| IGKVA-1 | Coronary artery disease (CAD) | + | Not included |
| PROS1 | Coronary artery disease (CAD) | − | Included |
| C4A | Coronary artery disease (CAD) | − | Included |
| A1BG | Coronary artery disease (CAD) | − | Included |
| KNG1 | Coronary artery disease (CAD) | − | Included |
| CLU | Coronary artery disease (CAD) | − | Included |
| C3 | Coronary artery disease (CAD) | − | Included |
| CD5L | Coronary artery disease (CAD) | − | Included |
| APOL1 | Coronary artery disease (CAD) | − | Included |
| TF | Coronary artery disease (CAD) | − | Included |
| ALB | Coronary artery disease (CAD) | − | Included |
| MBL2 | Coronary artery disease (CAD) | − | Included |
| TTR | Coronary artery disease (CAD) | − | Included |
| SERPINA1 | Coronary artery disease (CAD) | − | Included |
| AFM | Coronary artery disease (CAD) | − | Not included |
| IGFALS | Coronary artery disease (CAD) | − | Not included |
| HPX | Coronary artery disease (CAD) | − | Not included |
| SERPINC1 | Coronary artery disease (CAD) | − | Not included |
| DBP | Coronary artery disease (CAD) | − | Not included |
| CFI | Coronary artery disease (CAD) | − | Not included |
| IGHM | Coronary artery disease (CAD) | − | Not included |
| APOM | Coronary artery disease (CAD) | − | Not included |
| CLEC3B | Coronary artery disease (CAD) | − | Not included |
| PON3 | Coronary artery disease (CAD) | − | Not included |
| SHBG | Coronary artery disease (CAD) | − | Not included |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira-Costa, L.; Barros, A.S.; Lourenço, A.P.; Leite-Moreira, A.F.; Nogueira-Ferreira, R.; Thongboonkerd, V.; Vitorino, R. Exosome-Derived Mediators as Potential Biomarkers for Cardiovascular Diseases: A Network Approach. Proteomes 2021, 9, 8. https://doi.org/10.3390/proteomes9010008
Moreira-Costa L, Barros AS, Lourenço AP, Leite-Moreira AF, Nogueira-Ferreira R, Thongboonkerd V, Vitorino R. Exosome-Derived Mediators as Potential Biomarkers for Cardiovascular Diseases: A Network Approach. Proteomes. 2021; 9(1):8. https://doi.org/10.3390/proteomes9010008
Chicago/Turabian StyleMoreira-Costa, Liliana, António S. Barros, André P. Lourenço, Adelino F. Leite-Moreira, Rita Nogueira-Ferreira, Visith Thongboonkerd, and Rui Vitorino. 2021. "Exosome-Derived Mediators as Potential Biomarkers for Cardiovascular Diseases: A Network Approach" Proteomes 9, no. 1: 8. https://doi.org/10.3390/proteomes9010008
APA StyleMoreira-Costa, L., Barros, A. S., Lourenço, A. P., Leite-Moreira, A. F., Nogueira-Ferreira, R., Thongboonkerd, V., & Vitorino, R. (2021). Exosome-Derived Mediators as Potential Biomarkers for Cardiovascular Diseases: A Network Approach. Proteomes, 9(1), 8. https://doi.org/10.3390/proteomes9010008