Profiling of Inflammatory Proteins in Plasma of HIV-1-Infected Children Receiving Antiretroviral Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Proteomic Plasma Profiling
2.3. Data Analysis
2.4. Ethical Statement
3. Results
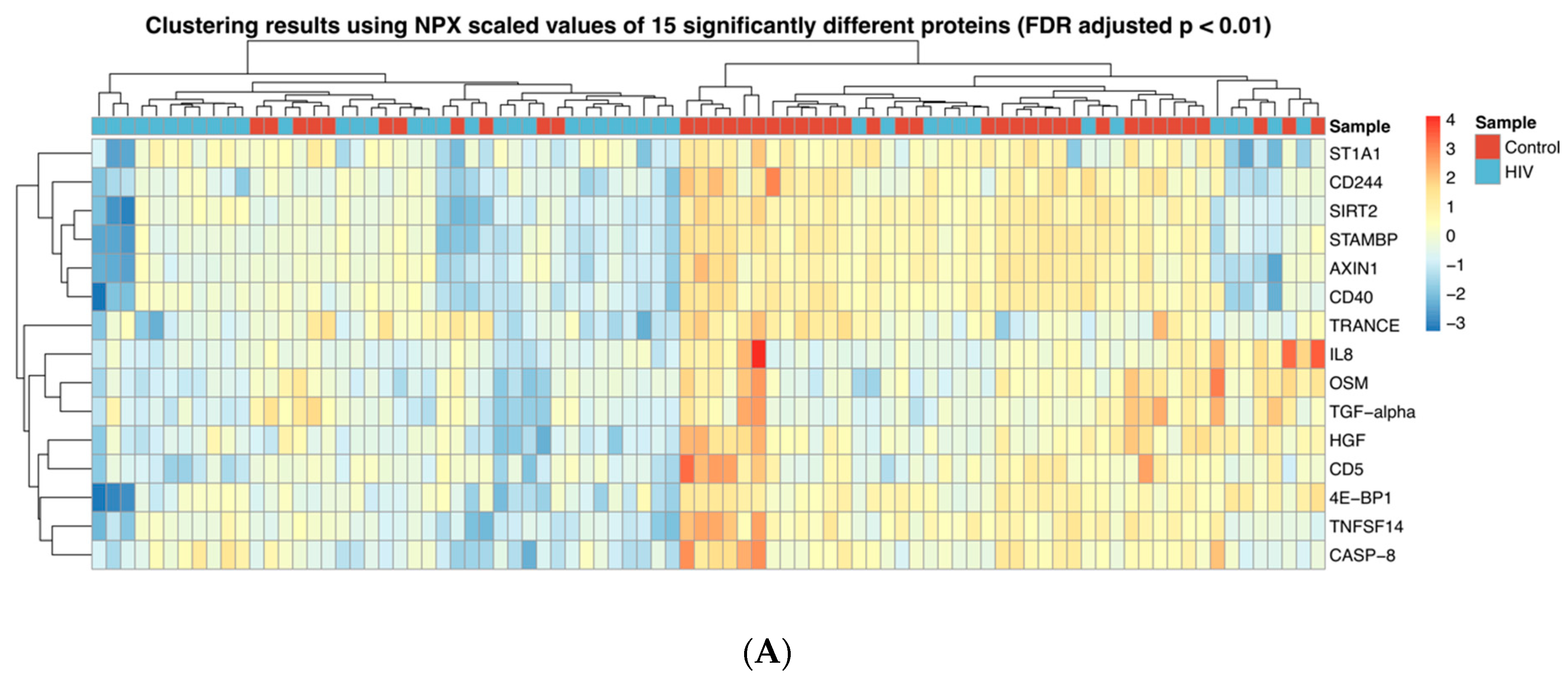
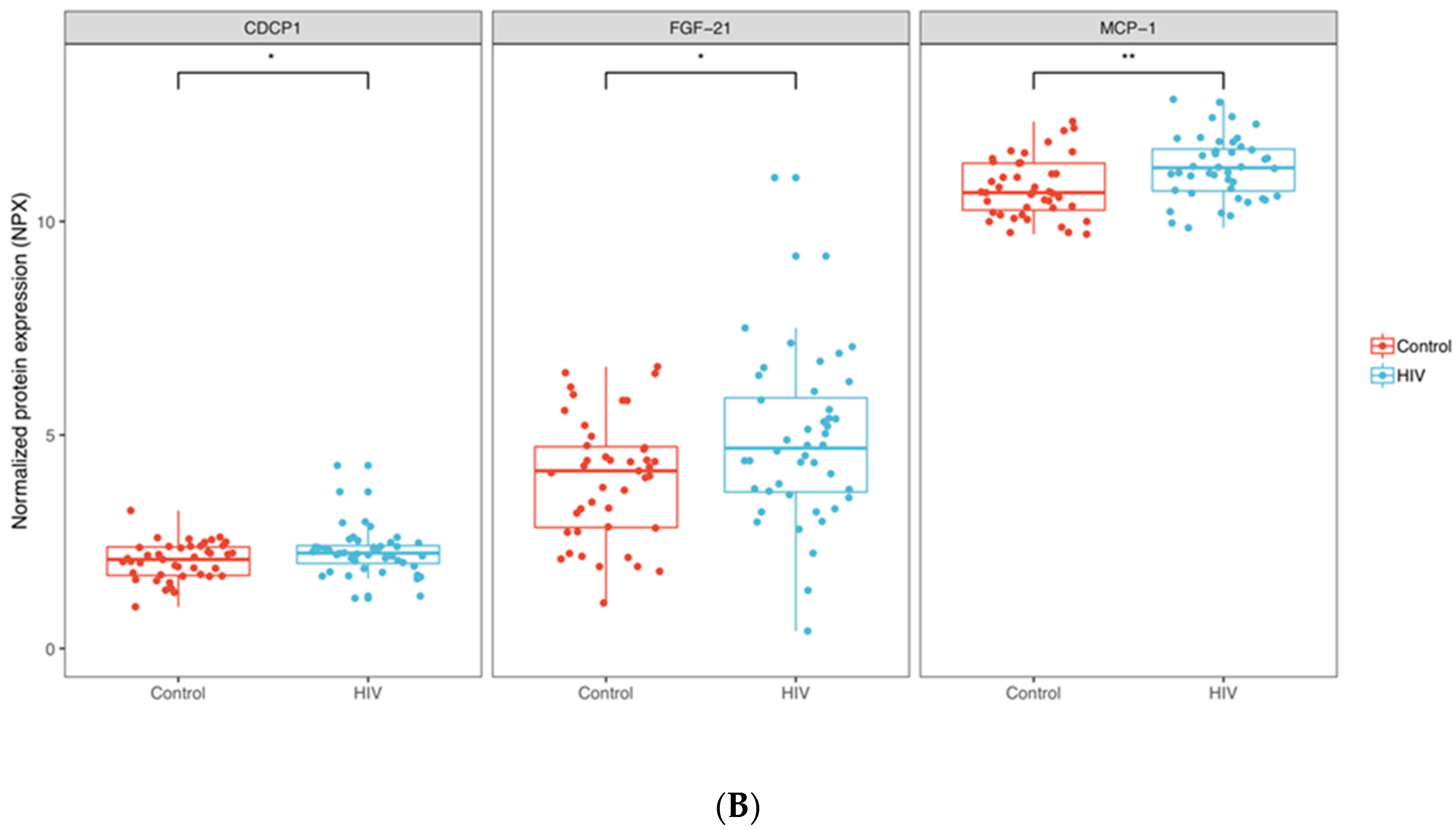
3.1. Inflammation-Related Factors in Plasma
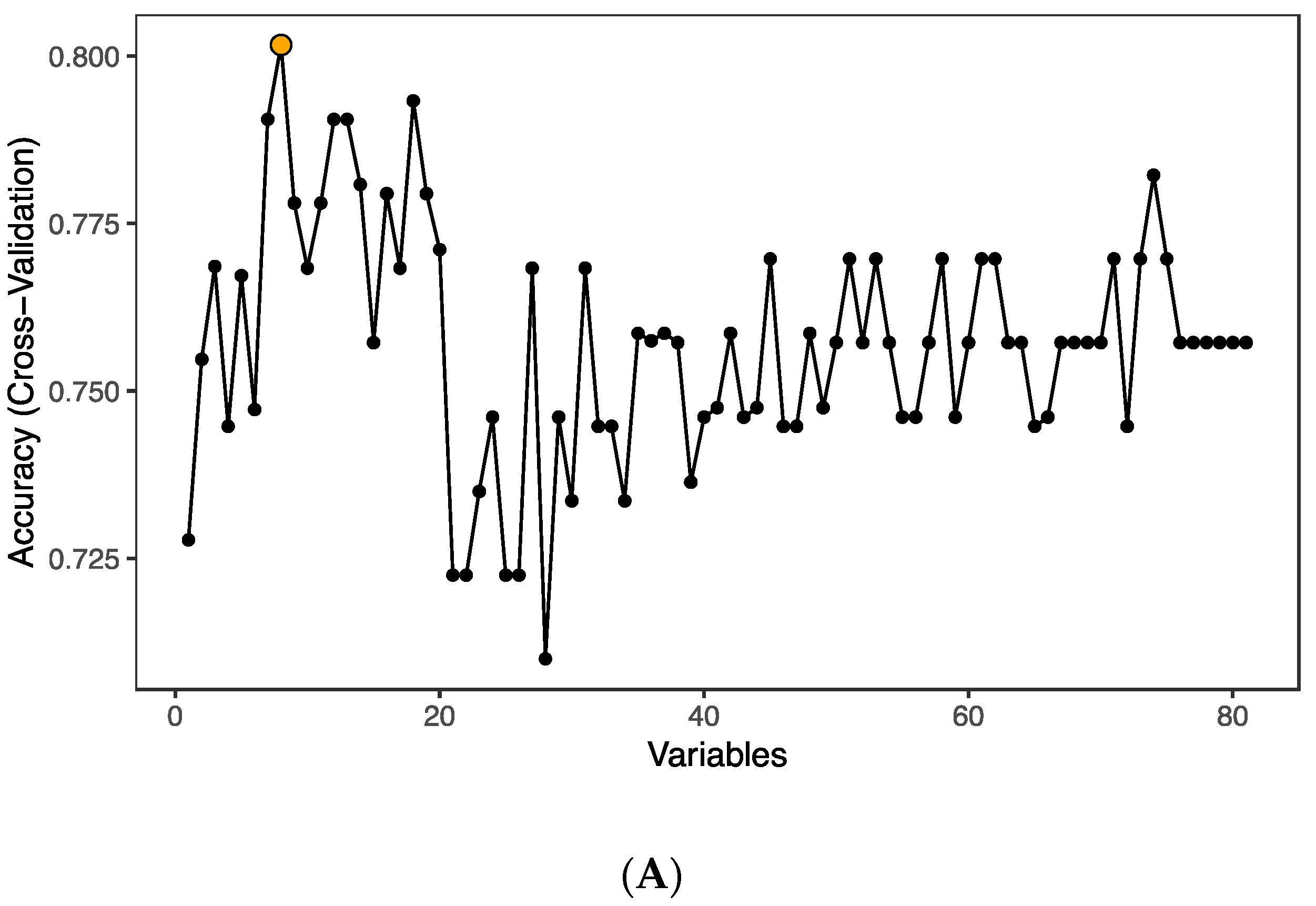
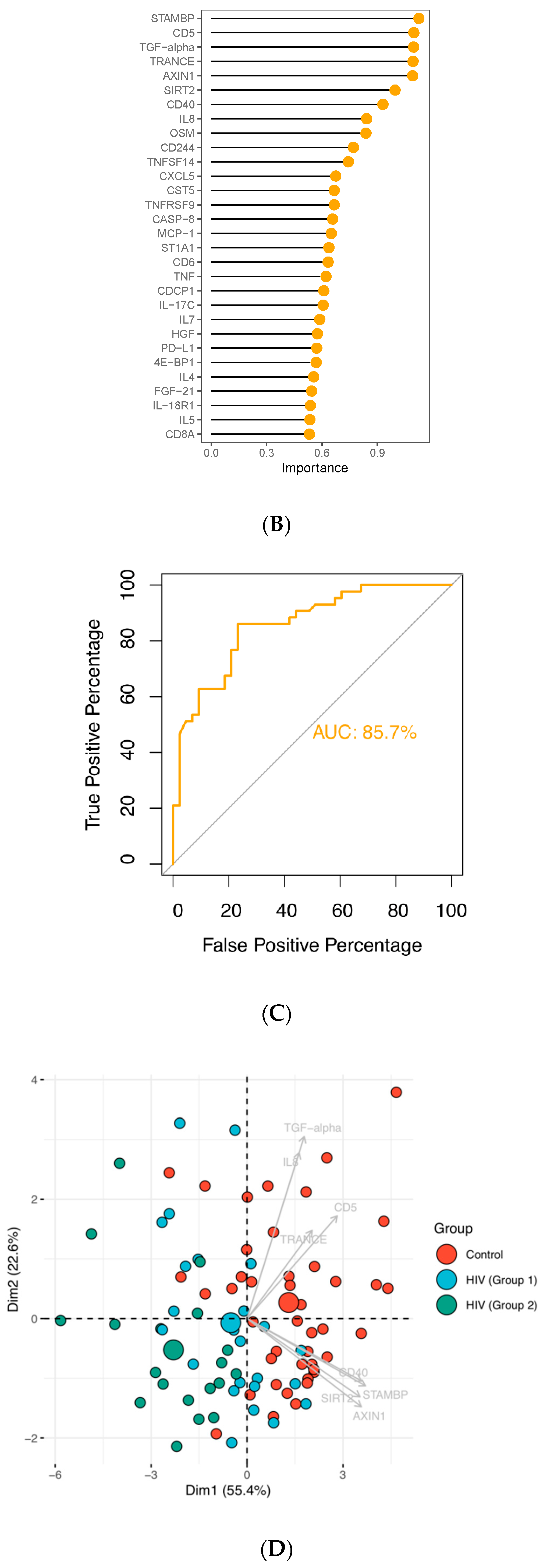
3.2. Age at ART Initiation and Inflammation Factors in Plasma
3.3. Enriched Pathways in HIV-Infected Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barlow-Mosha, L.; Musiime, V.; Davies, M.-A.; Prendergast, A.J.; Musoke, P.; Siberry, G.; Penazzato, M. Universal antiretroviral therapy for HIV-infected children: A review of the benefits and risks to consider during implementation. J. Int. AIDS Soc. 2017, 20, 21552. [Google Scholar] [CrossRef] [PubMed]
- Resino, S.; Galán, I.; Bellón, J.M.; Navarro, M.L.; León, J.A.; Muñoz-Fernandez, M.A. Characterizing the immune system after long-term undetectable viral load in HIV-1-infected children. J. Clin. Immunol. 2003, 23, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Bekele, Y.; Graham, R.L.; Soeria-Atmadja, S.; Nasi, A.; Zazzi, M.; Vicenti, I.; Naver, L.; Nilsson, A.; Chiodi, F. Hepatitis B Virus vaccination in HIV-1-infected young adults: A tool to reduce the size of HIV-1 reservoirs? Front. Immunol. 2018, 8, 1966. [Google Scholar] [CrossRef] [PubMed]
- Bekele, Y.; Lemma, M.; Bobosha, K.; Yibeltal, D.; Nasi, A.; Gebre, M.; Nilssone, A.; Aseffab, A.; Howeb, R.; Chiodia, F. Homing defects of B cells in HIV-1 infected children impair vaccination responses. Vaccine 2019, 37, 2348–2355. [Google Scholar] [CrossRef]
- Smit, M.; Brinkman, K.; Geerlings, S.; Smit, C.; Thyagarajan, K.; Sighem, A.; Wolf, F.; Hallett, T.B. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect. Dis. 2015, 15, 810–818. [Google Scholar] [CrossRef]
- Shiau, S.; Broun, E.C.; Arpadi, S.M.; Yin, M.T. Incident fractures in HIV-infected individuals. AIDS 2013, 27, 1949–1957. [Google Scholar] [CrossRef]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature Age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126. [Google Scholar] [CrossRef]
- Scherzer, R.; Estrella, M.; Li, Y.; Choi, A.I.; Deeks, S.G.; Grunfeld, C.; Shlipak, M.G. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 2012, 26, 867–875. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Bahiru, E.; Achenbach, C.; Longenecker, C.T.; Hsue, P.; So-Armah, K.; Freiberg, M.S.; Lloyd-Jones, D.M. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am. J. Cardiol. 2016, 117, 214–220. [Google Scholar] [CrossRef]
- Sico, J.J.; Chang, C.-C.H.; So-Armah, K.; Justice, A.C.; Hylek, E.; Skanderson, M.; McGinnis, K.; Kuller, L.H.; Kraemer, K.L.; Rimland, D.; et al. HIV status and the risk of ischemic stroke among men. Neurology 2015, 84, 1933–1940. [Google Scholar] [CrossRef]
- Seider, T.R.; Luo, X.; Gongvatana, A.; Devlin, K.N.; de la Monte, S.M.; Chasman, J.D.; Yan, P.; Tashima, K.T.; Navia, B.; Cohen, R.A. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. J. Clin. Exp. Neuropsychol. 2014, 36, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ryom, L.; Weber, R.; Morlat, P.; Pradier, C.; Reiss, P.; Kowalska, J.D.; de Wit, S.; Law, M.; el Sadr, W.; et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet 2014, 384, 241–248. [Google Scholar] [CrossRef]
- Chan, A.W.; Patel, Y.A.; Choi, S. Aging of the liver: What this means for patients with HIV. Curr. HIV/AIDS Rep. 2016, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Cotton, M.F.; Violari, A.; Otwombe, K.; Panchia, R.; Dobbels, E.; Rabie, H.; Josipovic, D.; Liberty, A.; Lazarus, E.; Innes, S.; et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: Results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013, 382, 1555–1563. [Google Scholar] [CrossRef]
- The Joint United Nations Programme on HIV/AIDS (UNAIDS). Fact Sheet: Global HIV Statistics. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. (accessed on 1 September 2020).
- Frigati, L.J.; Ameyan, W.; Cotton, M.F.; Gregson, C.L.; Hoare, J.; Jao, J.; Majonga, E.D.; Myer, L.; Penazzato, M.; Rukuni, R.; et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-Saharan Africa in the era of antiretroviral therapy. Lancet Child Adolesc. Heal. 2020, 4, 688–698. [Google Scholar] [CrossRef]
- De Filippi, C.; Toribio, M.; Wong, L.P.; Sadreyev, R.; Grundberg, I.; Fitch, K.V.; Zanni, M.V.; Lo, J.; Sponseller, C.A.; Sprecher, E.; et al. Differential plasma protein regulation and statin effects in Human Immunodeficiency Virus (HIV)-infected and non-HIV-infected patients utilizing a proteomics approach. J. Infect. Dis. 2020, 222, 929–939. [Google Scholar] [CrossRef]
- Babu, H.; Ambikan, A.T.; Gabriel, E.E.; Svensson, A.S.; Palaniappan, A.N.; Sundaraj, V.; Mupanni, N.R.; Sperk, M.; Cheedarla, N.; Sridhar, R.; et al. Systemic inflammation and the increased risk of inflamm-Aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front. Immunol. 2019, 10, 1965. [Google Scholar] [CrossRef]
- Babu, H.; Sperk, M.; Ambikan, A.T.; Rachel, G.; Viswanathan, V.K.; Tripathy, S.P.; Nowak, P.; Hanna, L.E.; Neogi, U. Plasma metabolic signature and abnormalities in HIV-infected individuals on long-term successful antiretroviral therapy. Metabolites 2019, 9, 210. [Google Scholar] [CrossRef]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Bucht Thorsen, S.; Ekman, D.; Eriksson, A.; Rennel Dickens, E.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Packag. Version 1.0.8 2015. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 4 September 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 2011, 12, 35. [Google Scholar] [CrossRef]
- Pomaznoy, M.; Ha, B.; Peters, B. GOnet: A tool for interactive Gene Ontology analysis. BMC Bioinformatics 2018, 19, 470. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.gbif.org/zh/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 4 September 2020).
- Chen, J.; Tan, L.; Liao, Y.; Long, J.; Zhou, Y.; Wei, J.; Zhou, Y. Chemokine CCL2 impairs spatial memory and cognition in rats via influencing inflammation, glutamate metabolism and apoptosis-associated genes expression- a potential mechanism for HIV-associated neurocognitive disorder. Life Sci. 2020, 255, 117828. [Google Scholar] [CrossRef] [PubMed]
- Voisinne, G.; Gonzalez de Peredo, A.; Roncagalli, R. CD5, an undercover regulator of TCR signaling. Front. Immunol. 2018, 9, 2900. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Places, L.; Espinosa, G.; Padilla, O.; Vila, J.M.; Villamor, N.; Ingelmo, M.; Gallart, T.; Vives, J.; Font, J.; et al. Identification of a natural soluble form of human CD5. Tissue Antigens 1999, 54, 128–137. [Google Scholar] [CrossRef]
- Simões, I.T.; Aranda, F.; Carreras, E.; Andrés, M.V.; Casadó-Llombart, S.; Martinez, V.G.; Lozano, F. Immunomodulatory effects of soluble CD5 on experimental tumor models. Oncotarget 2017, 8, 108156–108169. [Google Scholar] [CrossRef]
- Fallon, J.; Reid, S.; Kinyamu, R.; Opole, I.; Opole, R.; Baratta, J.; Korc, M.; Endo, T.L.; Duong, A.; Nguyen, G.; et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 2000, 97, 14686–14691. [Google Scholar] [CrossRef]
- Stern, P.H.; Krieger, N.S.; Nissenson, R.A.; Williams, R.D.; Winkler, M.E.; Derynck, R.; Strewler, G.J. Human transforming growth factor-alpha stimulates bone resorption in vitro. J. Clin. Invest. 1985, 76, 2016–2019. [Google Scholar] [CrossRef]
- Arpadi, S.M. Bone Health in HIV-Infected Children, Adolescents and Young Adults: A Systematic Review. J. AIDS Clin. Res. 2014, 5, 374. [Google Scholar] [CrossRef]
- Yin, M.T.; Brown, T.T. HIV and bone complications: Understudied populations and new management strategies. Curr. HIV/AIDS Rep. 2016, 13, 349–358. [Google Scholar] [CrossRef]
- Yin, M.T.; Lund, E.; Shah, J.; Zhang, C.A.; Foca, M.; Neu, N.; Nishiyama, K.K.; Zhou, B.; Guo, X.E.; Nelson, J.; et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 2014, 28, 345–353. [Google Scholar] [CrossRef]
- Titanji, K. Beyond antibodies: B cells and the OPG/RANK-RANKL pathway in health, non-HIV disease and HIV-induced bone loss. Front. Immunol. 2017, 8, 1851. [Google Scholar] [CrossRef] [PubMed]
- Titanji, K.; Vunnava, A.; Sheth, A.N.; Delille, C.; Lennox, J.L.; Sanford, S.E.; Foster, A.; Knezevic, A.; Easley, K.A.; Weitzmann, M.N.; et al. Dysregulated B cell expression of RANKL and OPG correlates with loss of bone mineral density in HIV infection. PLoS Pathog. 2014, 10, e1004497. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Soluble RANKL and risk of nontraumatic fracture. JAMA 2004, 291, 1108. [Google Scholar] [CrossRef]
- Itoh, F. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory smads. EMBO J. 2001, 20, 4132–4142. [Google Scholar] [CrossRef]
- Zhu, X.-B.; Lin, W.-J.; Lv, C.; Wang, L.; Huang, Z.-X.; Yang, S.-W.; Chen, X. MicroRNA-539 promotes osteoblast proliferation and differentiation and osteoclast apoptosis through the AXNA-dependent Wnt signaling pathway in osteoporotic rats. J. Cell. Biochem. 2018, 119, 8346–8358. [Google Scholar] [CrossRef] [PubMed]
- Pensieroso, S.; Cagigi, A.; Palma, P.; Nilsson, A.; Capponi, C.; Freda, E.; Bernardi, S.; Thorstensson, R.; Chiodi, F.; Rossi, P. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc. Natl. Acad. Sci. USA 2009, 106, 7939–7944. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.F.; Cocchi, F.; Bagley, K.C.; Kamin-Lewis, R.; Gallo, R.C.; DeVico, A.; Lewis, G.K. HIV-1-suppressive factors are secreted by CD4+ T cells during primary immune responses. Proc. Natl. Acad. Sci. USA 2003, 100, 15006–15010. [Google Scholar] [CrossRef]
- Koye, D.N.; Zeleke, B.M. Mother-to-child transmission of HIV and its predictors among HIV-exposed infants at a PMTCT clinic in northwest Ethiopia. BMC Public Health 2013, 13, 398. [Google Scholar] [CrossRef]
- Yitayew, Y.A.; Bekele, D.M.; Demissie, B.W.; Argaw Menji, Z. Mother to child transmission of HIV and associated factors among HIV exposed infants at public health facilities, Dessie Town, Ethiopia. HIV/AIDS Res. Palliat. Care 2019, 11, 343–350. [Google Scholar] [CrossRef]
- Wudineh, F.; Damtew, B. Mother-to-child transmission of HIV infection and its determinants among exposed infants on care and follow-up in Dire Dawa City, Eastern Ethiopia. AIDS Res. Treat. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
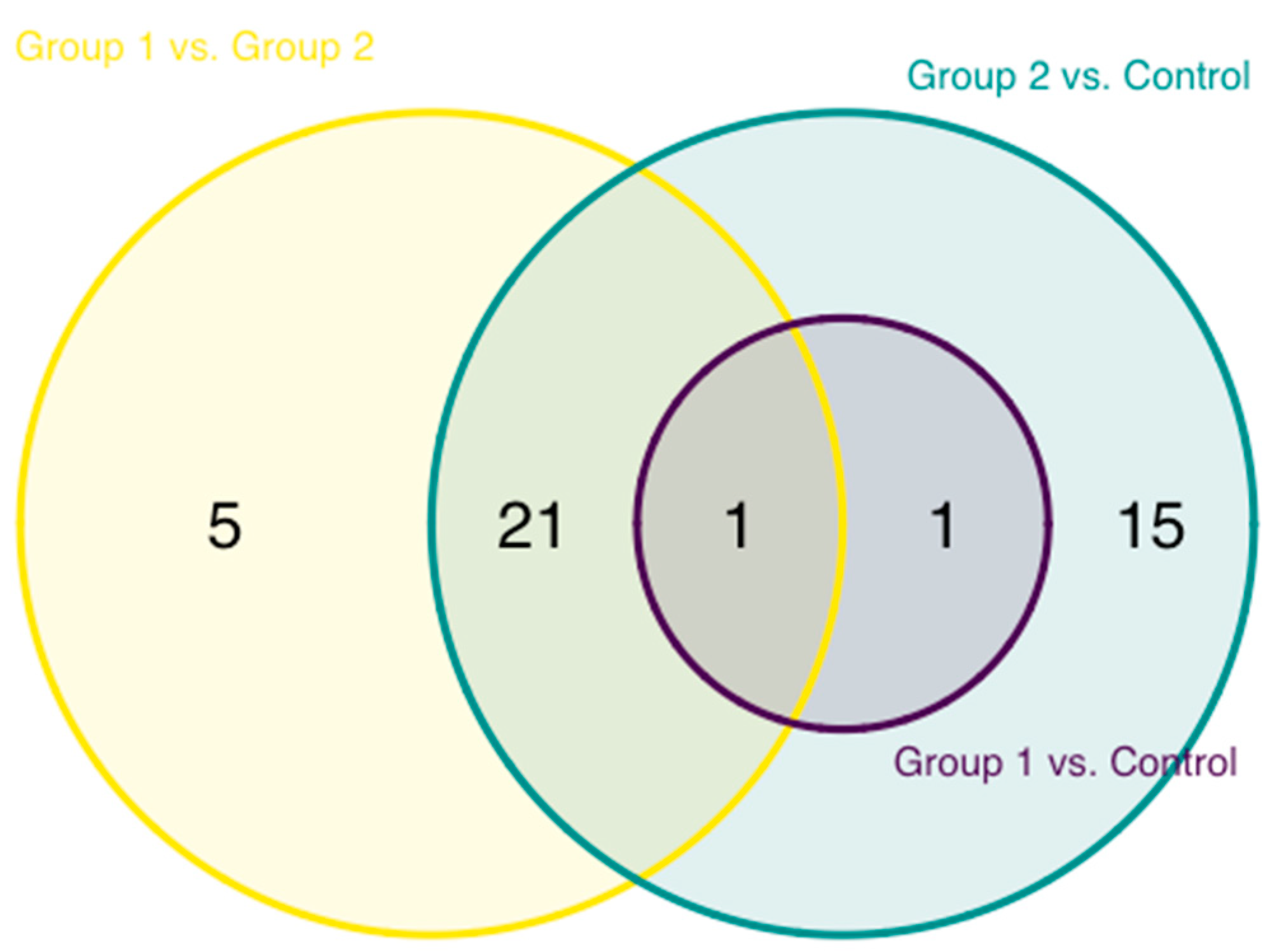
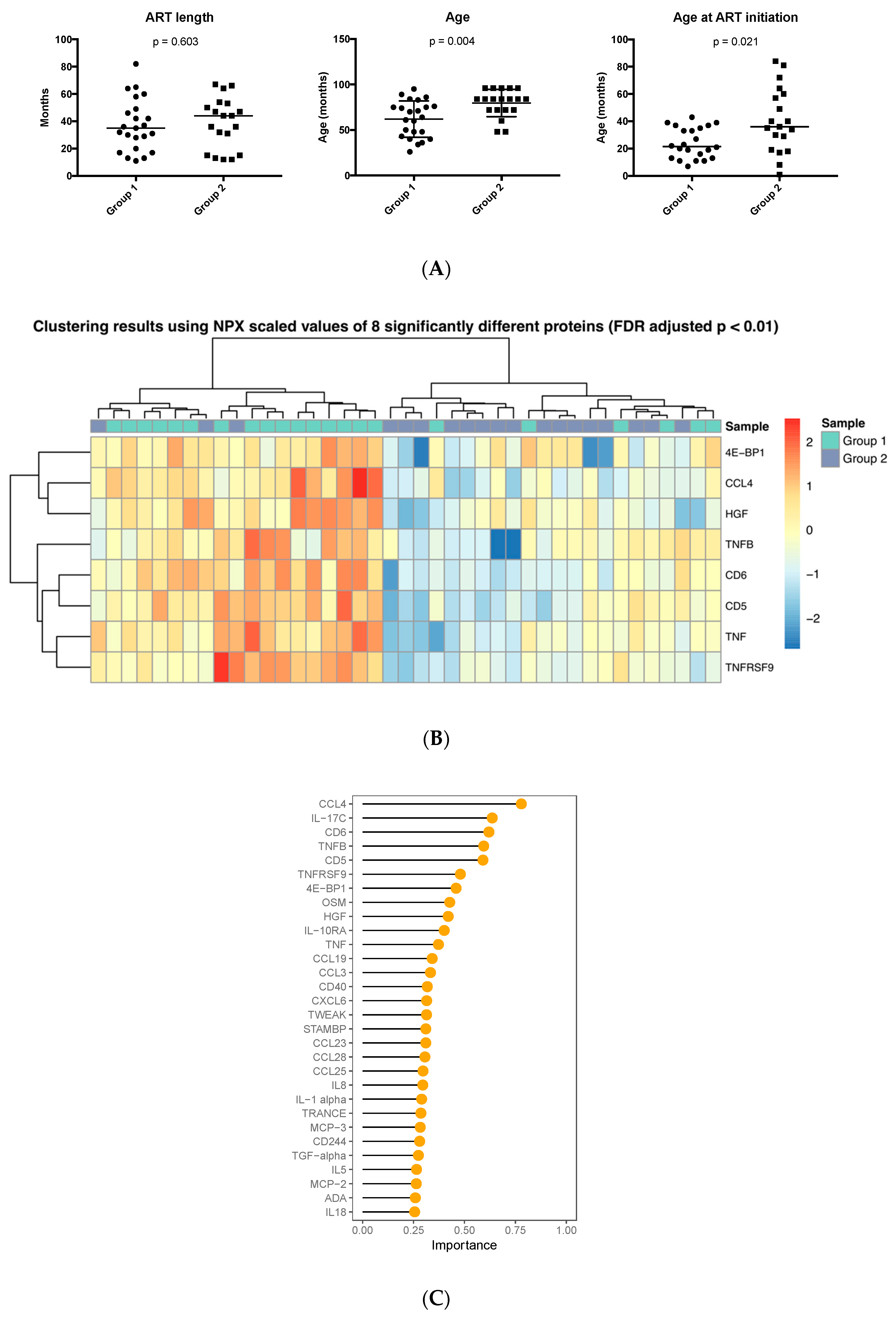
| Characteristics | Controls (n = 43) | HIV-1 Infected (n = 43) | |
|---|---|---|---|
| Median age (range) in months | 60 (43–84) | Group 1 (n = 24) | Group 2 (n = 19) |
| 60.5 (26–95) | 84 (48–96) | ||
| Gender | |||
| Female | 21 | 14 | 7 |
| Male | 22 | 10 | 12 |
| Viral load | |||
| Aviremic | NA | 24 | 19 |
| Viremic | NA | 0 | 0 |
| ART | |||
| ABC + 3TC + LPV/r | NA | 8 | 2 |
| ABC + 3TC + NVP | NA | 2 | 0 |
| AZT + 3TC + NVP | NA | 5 | 15 |
| AZT + 3TC + EFV | NA | 4 | 2 |
| AZT + 3TC + LPV/r | NA | 5 | 0 |
| ART duration (range) in months | NA | 33.5 (11–82) | 44 (12–67) |
| Median age (range) in months at ART initiation | NA | 21.5 (7–43) | 36 (1–84) |
| WHO stage | |||
| Stage I | NA | 23 | 10 |
| Stage II | NA | 1 | 8 |
| Stage III | NA | 0 | 1 |
| Body mass index | 15.4 (12.4–19.8) | 14.3 (8.4–20.2) | 14.9 (12.9–22.7) |
| GO Term ID | GO Term | p | Adj. p | Genes | ||
|---|---|---|---|---|---|---|
| HIV vs. Control | 1 | GO:0007166 | cell surface receptor signaling pathway | 2.90 × 10−9 | 3.23 × 10−5 | 12 |
| 2 | GO:0007165 | signal transduction | 4.83 × 10−8 | 2.66 × 10−4 | 14 | |
| 3 | GO:0051716 | cellular response to stimulus | 7.27×10−8 | 2.67 × 10−4 | 15 | |
| 4 | GO:0023052 | signaling | 1.17 × 10−7 | 2.78 × 10−4 | 14 | |
| 5 | GO:0048522 | positive regulation of cellular process | 1.26 × 10−7 | 2.78 × 10−4 | 14 | |
| 6 | GO:0007154 | cell communication | 1.53 × 10−7 | 2.81 × 10−4 | 14 | |
| 7 | GO:1902533 | positive regulation of intracellular signal transduction | 1.93 × 10−7 | 3.04 × 10−4 | 8 | |
| 8 | GO:0048518 | positive regulation of biological process | 7.67 × 10−7 | 1.06 × 10−3 | 14 | |
| 9 | GO:0034612 | response to tumor necrosis factor | 1.02 × 10−6 | 1.25 × 10−3 | 5 | |
| 10 | GO:1902531 | regulation of intracellular signal transduction | 1.37 × 10−6 | 1.51 × 10−3 | 9 | |
| Group 1 vs. Group 2 | 1 | GO:0002874 | regulation of chronic inflammatory response to antigenic stimulus | 4.30 × 10−7 | 3.61 × 10−3 | 2 |
| 2 | GO:0002678 | positive regulation of chronic inflammatory response | 8.59 × 10−7 | 3.61 × 10−3 | 2 | |
| 3 | GO:0071356 | cellular response to tumor necrosis factor | 1.33 × 10−6 | 3.61 × 10−3 | 4 | |
| 4 | GO:0002925 | positive regulation of humoral immune response mediated by circulating immunoglobulin | 1.43 × 10−6 | 3.61 × 10−3 | 2 | |
| 5 | GO:0034612 | response to tumor necrosis factor | 1.96 × 10−6 | 3.61 × 10−3 | 4 | |
| 6 | GO:0019221 | cytokine−mediated signaling pathway | 2.15 × 10−6 | 3.61 × 10−3 | 5 | |
| 7 | GO:0071310 | cellular response to organic substance | 2.39 × 10−6 | 3.61 × 10−3 | 7 | |
| 8 | GO:0007166 | cell surface receptor signaling pathway | 2.62 × 10−6 | 3.61 × 10−3 | 7 | |
| 9 | GO:0002676 | regulation of chronic inflammatory response | 5.15 × 10−6 | 5.31 × 10−3 | 2 | |
| 10 | GO:0060693 | regulation of branching involved in salivary gland morphogenesis | 5.15 × 10-6 | 5.31 × 10-3 | 2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemma, M.; Petkov, S.; Bekele, Y.; Petros, B.; Howe, R.; Chiodi, F. Profiling of Inflammatory Proteins in Plasma of HIV-1-Infected Children Receiving Antiretroviral Therapy. Proteomes 2020, 8, 24. https://doi.org/10.3390/proteomes8030024
Lemma M, Petkov S, Bekele Y, Petros B, Howe R, Chiodi F. Profiling of Inflammatory Proteins in Plasma of HIV-1-Infected Children Receiving Antiretroviral Therapy. Proteomes. 2020; 8(3):24. https://doi.org/10.3390/proteomes8030024
Chicago/Turabian StyleLemma, Mahlet, Stefan Petkov, Yonas Bekele, Beyene Petros, Rawleigh Howe, and Francesca Chiodi. 2020. "Profiling of Inflammatory Proteins in Plasma of HIV-1-Infected Children Receiving Antiretroviral Therapy" Proteomes 8, no. 3: 24. https://doi.org/10.3390/proteomes8030024
APA StyleLemma, M., Petkov, S., Bekele, Y., Petros, B., Howe, R., & Chiodi, F. (2020). Profiling of Inflammatory Proteins in Plasma of HIV-1-Infected Children Receiving Antiretroviral Therapy. Proteomes, 8(3), 24. https://doi.org/10.3390/proteomes8030024





