Proteomics of Human Retinal Pigment Epithelium (RPE) Cells
Abstract
1. Introduction
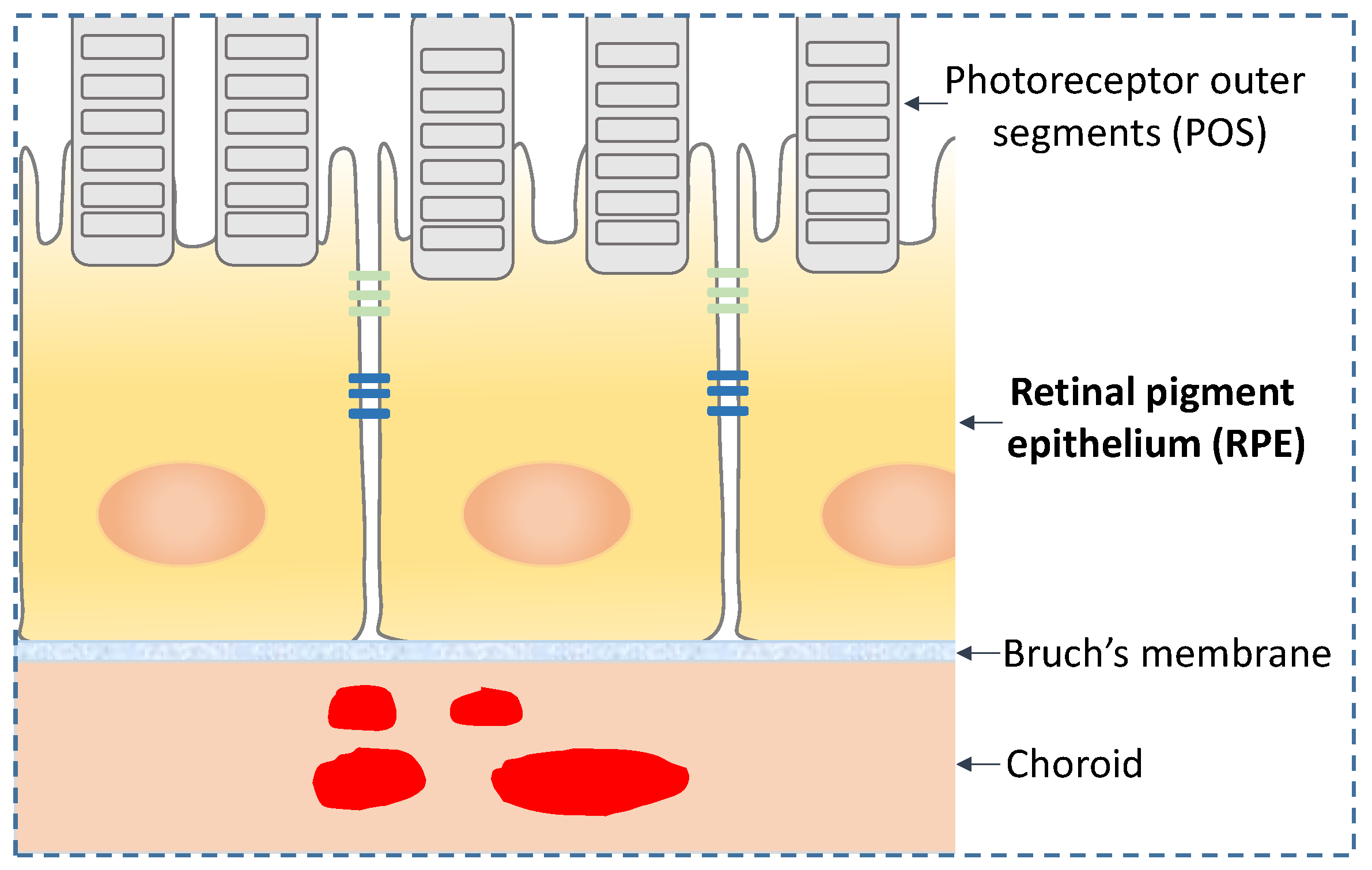
2. RPE Morphology and Function
3. RPE and AMD
4. In Vitro Models of RPE
5. Proteomics of RPE
5.1. Proteome Mapping
5.2. Proteome Profiling: Intracellular
5.3. Proteome Profiling: Extracellular
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Semba, R.D.; Enghild, J.J.; Venkatraman, V.; Dyrlund, T.F.; Van Eyk, J.E. The Human Eye Proteome Project: Perspectives on an emerging proteome. Proteomics 2013, 13, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S. The proteomes of the human eye, a highly compartmentalized organ. Proteomics 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.T.; Zhang, P.; Dufresne, C.; Ferrucci, L.; Semba, R.D. The Human Eye Proteome Project: Updates on an Emerging Proteome. Proteomics 2018, 18, 1700394. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Sparrrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J.; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [PubMed]
- Cardarelli, W.J.; Smith, R.A. Managed care implications of age-related ocular conditions. Am. J. Manag. Care 2013, 19 (Suppl. 5), S85–S91. [Google Scholar] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Dietzel, M.; Pauleikhoff, D.; Holz, F.; Bird, A. Early AMD. In Age-Related Macular Degeneration; Springer: New York, NY, USA, 2013; pp. 101–109. [Google Scholar]
- Klein, M.L.; Francis, P.J.; Ferris, F.L., 3rd; Hamon, S.C.; Clemons, T.E. Risk assessment model for development of advanced age-related macular degeneration. Arch. Ophthalmol. 2011, 129, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Mitchell, P.; Leeder, S.R. Smoking and age-related maculopathy. The Blue Mountains Eye Study. Arch. Ophthalmol. 1996, 114, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, G.R.; Curcio, C.A.; Swaroop, A. Age-Related Macular Degeneration: Genetics and Biology Coming Together. Annu. Rev. Genom. Hum. Genet. 2014, 15, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta 2017, 1862, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Johnson, M.; Rudolf, M.; Huang, J.D. The oil spill in ageing Bruch membrane. Br. J. Ophthalmol. 2011, 95, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.; Mullins, R. Molecular composition of drusen as related to substructural phenotype. Mol. Vis. 1999, 5, 28. [Google Scholar] [PubMed]
- Spaide, R.F.; Ho-Spaide, W.C.; Browne, R.W.; Armstrong, D. Characterization of peroxidized lipids in Bruch’s membrane. Retina 1999, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gu, X.; Crabb, J.S.; Yue, X.; Shadrach, K.; Hollyfield, J.G.; Crabb, J.W. Quantitative proteomic comparison of the macular Bruch’s membrane/choroid complex from age-related macular degeneration and normal eyes. Mol. Cell. Proteom. 2010, 9, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Dentchev, T.; Hahn, P.; Dunaief, J.L. Strong labeling for iron and the iron-handling proteins ferritin and ferroportin in the photoreceptor layer in age-related macular degeneration. Arch. Ophthalmol. 2005, 123, 1745–1746. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Tian, J.; Yang, Y.; Cutler, R.G.; Wu, T.; Telljohann, R.S.; Mattson, M.P.; Handa, J.T. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. J. Neurochem. 2008, 105, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.-P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Bernstein, P.S.; Bok, D.; Turner, J.; Nachtigal, M.; Hunt, R.C. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest. Ophthalmol. Vis. Sci. 1995, 36, 955–964. [Google Scholar] [PubMed]
- Dunn, K.; Aotaki-Keen, A.; Putkey, F.; Hjelmeland, L. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis. 2017, 23, 60. [Google Scholar] [PubMed]
- Luo, Y.; Zhuo, Y.; Fukuhara, M.; Rizzolo, L.J. Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3644–3655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kokkinaki, M.; Sahibzada, N.; Golestaneh, N. Human Induced Pluripotent Stem-Derived Retinal Pigment Epithelium (RPE) Cells Exhibit Ion Transport, Membrane Potential, Polarized Vascular Endothelial Growth Factor Secretion, and Gene Expression Pattern Similar to Native RPE. Stem Cells 2011, 29, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Bok, D. The use of cultured human fetal retinal pigment epithelium in studies of the classical retinoid visual cycle and retinoid-based disease processes. Exp. Eye Res. 2014, 126, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.D.; Goodlett, D.R.; Masselon, C.D. Multiplexed and data-independent tandem mass spectrometry for global proteome profiling. Mass Spectrom. Rev. 2014, 33, 452–470. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Yan, L.; Shadrach, K.; Sun, J.; Hasan, A.; Miyagi, M.; Crabb, J.S.; Hollyfield, J.G.; Marmorstein, A.D.; Crabb, J.W. Protein database, human retinal pigment epithelium. Mol. Cell. Proteom. 2003, 2, 37–49. [Google Scholar] [CrossRef]
- Schutt, F.; Ueberle, B.; Schnolzer, M.; Holz, F.G.; Kopitz, J. Proteome analysis of lipofuscin in human retinal pigment epithelial cells. FEBS Lett. 2002, 528, 217–221. [Google Scholar] [CrossRef]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3663–3668. [Google Scholar] [CrossRef] [PubMed]
- Warburton, S.; Southwick, K.; Hardman, R.M.; Secrest, A.M.; Grow, R.K.; Xin, H.; Woolley, A.T.; Burton, G.F.; Thulin, C.D. Examining the proteins of functional retinal lipofuscin using proteomic analysis as a guide for understanding its origin. Mol. Vis. 2005, 11, 1122–1134. [Google Scholar] [PubMed]
- Warburton, S.; Davis, W.E.; Southwick, K.; Xin, H.; Woolley, A.T.; Burton, G.F.; Thulin, C.D. Proteomic and phototoxic characterization of melanolipofuscin: Correlation to disease and model for its origin. Mol. Vis. 2007, 13, 318–329. [Google Scholar] [PubMed]
- Ng, K.P.; Gugiu, B.; Renganathan, K.; Davies, M.W.; Gu, X.; Crabb, J.S.; Kim, S.R.; Rozanowska, M.B.; Bonilha, V.L.; Rayborn, M.E.; et al. Retinal pigment epithelium lipofuscin proteomics. Mol. Cell. Proteom. 2008, 7, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Alge, C.S.; Suppmann, S.; Priglinger, S.G.; Neubauer, A.S.; May, C.A.; Hauck, S.; Welge-Lussen, U.; Ueffing, M.; Kampik, A. Comparative proteome analysis of native differentiated and cultured dedifferentiated human RPE cells. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3629–3641. [Google Scholar] [CrossRef] [PubMed]
- Alge, C.S.; Hauck, S.M.; Priglinger, S.G.; Kampik, A.; Ueffing, M. Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival. J. Proteome Res. 2006, 5, 862–878. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, H.; Jylha, A.; Nattinen, J.; Rieck, J.; Ilmarinen, T.; Vereb, Z.; Aapola, U.; Beuerman, R.; Petrovski, G.; Uusitalo, H.; et al. Comparative proteomic analysis of human embryonic stem cell-derived and primary human retinal pigment epithelium. Sci. Rep. 2017, 7, 6016. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, L.; Sato, K.; Reinisalo, M.; Kidron, H.; Tachikawa, M.; Watanabe, M.; Uchida, Y.; Urtti, A.; Terasaki, T. LC-MS/MS Based Quantitation of ABC and SLC Transporter Proteins in Plasma Membranes of Cultured Primary Human Retinal Pigment Epithelium Cells and Immortalized ARPE19 Cell Line. Mol. Pharm. 2017, 14, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Tachikawa, M.; Obuchi, W.; Hoshi, Y.; Tomioka, Y.; Ohtsuki, S.; Terasaki, T. A study protocol for quantitative targeted absolute proteomics (QTAP) by LC-MS/MS: Application for inter-strain differences in protein expression levels of transporters, receptors, claudin-5, and marker proteins at the blood–brain barrier in ddY, FVB, and C57BL/6J mice. Fluids Barriers CNS 2013, 10, 21. [Google Scholar] [PubMed]
- Liao, W.L.; Turko, I.V. Accumulation of large protein fragments in prematurely senescent ARPE-19 cells. Invest. Ophthalmol. Vis. Sci. 2009, 50, 4992–4997. [Google Scholar] [CrossRef] [PubMed]
- Arnouk, H.; Lee, H.; Zhang, R.; Chung, H.; Hunt, R.C.; Jahng, W.J. Early biosignature of oxidative stress in the retinal pigment epithelium. J. Proteom. 2011, 74, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sripathi, S.R.; He, W.; Prigge, C.L.; Sylvester, O.; Um, J.Y.; Powell, F.L.; Neksumi, M.; Bernstein, P.S.; Choo, D.W.; Bartoli, M.; et al. Interactome Mapping Guided by Tissue-Specific Phosphorylation in Age-Related Macular Degeneration. Int. J. Sci. Eng. Res. 2017, 8, 680–699. [Google Scholar] [CrossRef] [PubMed]
- Sripathi, S.R.; He, W.; Sylvester, O.; Neksumi, M.; Um, J.Y.; Dluya, T.; Bernstein, P.S.; Jahng, W.J. Altered Cytoskeleton as a Mitochondrial Decay Signature in the Retinal Pigment Epithelium. Protein J. 2016, 35, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Sripathi, S.R.; Sylvester, O.; He, W.; Moser, T.; Um, J.Y.; Lamoke, F.; Ramakrishna, W.; Bernstein, P.S.; Bartoli, M.; Jahng, W.J. Prohibitin as the Molecular Binding Switch in the Retinal Pigment Epithelium. Protein J. 2016, 35, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.V.; Mahaffy, H.; Dasari, S.; Oliver, M.; Chen, M.; Boulton, M.E.; Xu, H.; Curry, W.J.; Stitt, A.W. Proteomic profiling of human retinal pigment epithelium exposed to an advanced glycation-modified substrate. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 349–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velilla, S.; García-Medina, J.J.; García-Layana, A.; Dolz-Marco, R.; Pons-Vázquez, S.; Pinazo-Durán, M.D.; Gómez-Ulla, F.; Arévalo, J.F.; Díaz-Llopis, M.; Gallego-Pinazo, R. Smoking and age-related macular degeneration: Review and update. J. Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef] [PubMed]
- Woodell, A.; Rohrer, B. A mechanistic review of cigarette smoke and age-related macular degeneration. In Retinal Degenerative Diseases; Springer: New York, NY, USA, 2014; pp. 301–307. [Google Scholar]
- Merl-Pham, J.; Gruhn, F.; Hauck, S.M. Proteomic Profiling of Cigarette Smoke Induced Changes in Retinal Pigment Epithelium Cells. Adv. Exp. Med. Biol. 2016, 854, 785–791. [Google Scholar] [PubMed]
- Nordgaard, C.L.; Berg, K.M.; Kapphahn, R.J.; Reilly, C.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Proteomics of the retinal pigment epithelium reveals altered protein expression at progressive stages of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2006, 47, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Nordgaard, C.L.; Karunadharma, P.P.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2008, 49, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Smeitink, J.A.; Elpeleg, O.; Antonicka, H.; Diepstra, H.; Saada, A.; Smits, P.; Sasarman, F.; Vriend, G.; Jacob-Hirsch, J.; Shaag, A. Distinct clinical phenotypes associated with a mutation in the mitochondrial translation elongation factor EFTs. Am. J. Hum. Genet. 2006, 79, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Murad, N.; Kokkinaki, M.; Gunawardena, N.; Gunawan, M.S.; Hathout, Y.; Janczura, K.J.; Theos, A.C.; Golestaneh, N. miR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in human retinal pigment epithelium and is downregulated in age-related macular degeneration. FEBS J. 2014, 281, 5251–5264. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Chan, L.; Tsai, Y.T.; Wu, W.H.; Nguyen, H.V.; Hsu, C.W.; Li, X.; Brown, L.M.; Egli, D.; et al. Validation of genome-wide association study (GWAS)-identified disease risk alleles with patient-specific stem cell lines. Hum. Mol. Genet. 2014, 23, 3445–3455. [Google Scholar] [CrossRef] [PubMed]
- An, E.; Lu, X.; Flippin, J.; Devaney, J.M.; Halligan, B.; Hoffman, E.P.; Strunnikova, N.; Csaky, K.; Hathout, Y. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J. Proteome Res. 2006, 5, 2599–2610. [Google Scholar] [CrossRef] [PubMed]
- Zareparsi, S.; Branham, K.E.; Li, M.; Shah, S.; Klein, R.J.; Ott, J.; Hoh, J.; Abecasis, G.R.; Swaroop, A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am. J. Hum. Genet. 2005, 77, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Zhang, L.; Zhang, M.; Du, H.; Zhao, L.; Lee, C.; Grob, S.; Lim, S.L.; Hughes, G.; Lee, J.; et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2012, 109, 13757–13762. [Google Scholar] [CrossRef] [PubMed]
- An, E.; Sen, S.; Park, S.K.; Gordish-Dressman, H.; Hathout, Y. Identification of novel substrates for the serine protease HTRA1 in the human RPE secretome. Invest. Ophthalmol. Vis. Sci. 2010, 51, 3379–3386. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, O.; Hawkridge, A.M.; Collier, T.S.; Cousins, S.W.; Bhattacharya, S.K.; Muddiman, D.C.; Marin-Castano, M.E. Proteomics characterization of cell membrane blebs in human retinal pigment epithelium cells. Mol. Cell. Proteom. 2009, 8, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, A.P. The toxicology of hydroquinone—Relevance to occupational and environmental exposure. Crit. Rev. Toxicol. 1999, 29, 283–330. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Chiechi, A.; Couch, R.; Liotta, L.A.; Espina, V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013, 319, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Gutierrez-Vazquez, C.; Sanchez-Madrid, F.; Mittelbrunn, M. Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Liotta, L.A.; Petricoin, E., III. Monitoring proteins and protein networks using reverse phase protein arrays. Dis. Markers 2010, 28, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.C.; Choi, S.; Yoon, S.; Kim, H.C.; Baek, J.H.; Park, H.S.; et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J. Proteome Res. 2014, 13, 581–595. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beranova-Giorgianni, S.; Giorgianni, F. Proteomics of Human Retinal Pigment Epithelium (RPE) Cells. Proteomes 2018, 6, 22. https://doi.org/10.3390/proteomes6020022
Beranova-Giorgianni S, Giorgianni F. Proteomics of Human Retinal Pigment Epithelium (RPE) Cells. Proteomes. 2018; 6(2):22. https://doi.org/10.3390/proteomes6020022
Chicago/Turabian StyleBeranova-Giorgianni, Sarka, and Francesco Giorgianni. 2018. "Proteomics of Human Retinal Pigment Epithelium (RPE) Cells" Proteomes 6, no. 2: 22. https://doi.org/10.3390/proteomes6020022
APA StyleBeranova-Giorgianni, S., & Giorgianni, F. (2018). Proteomics of Human Retinal Pigment Epithelium (RPE) Cells. Proteomes, 6(2), 22. https://doi.org/10.3390/proteomes6020022



