A Proteomic View of Salmonella Typhimurium in Response to Phosphate Limitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
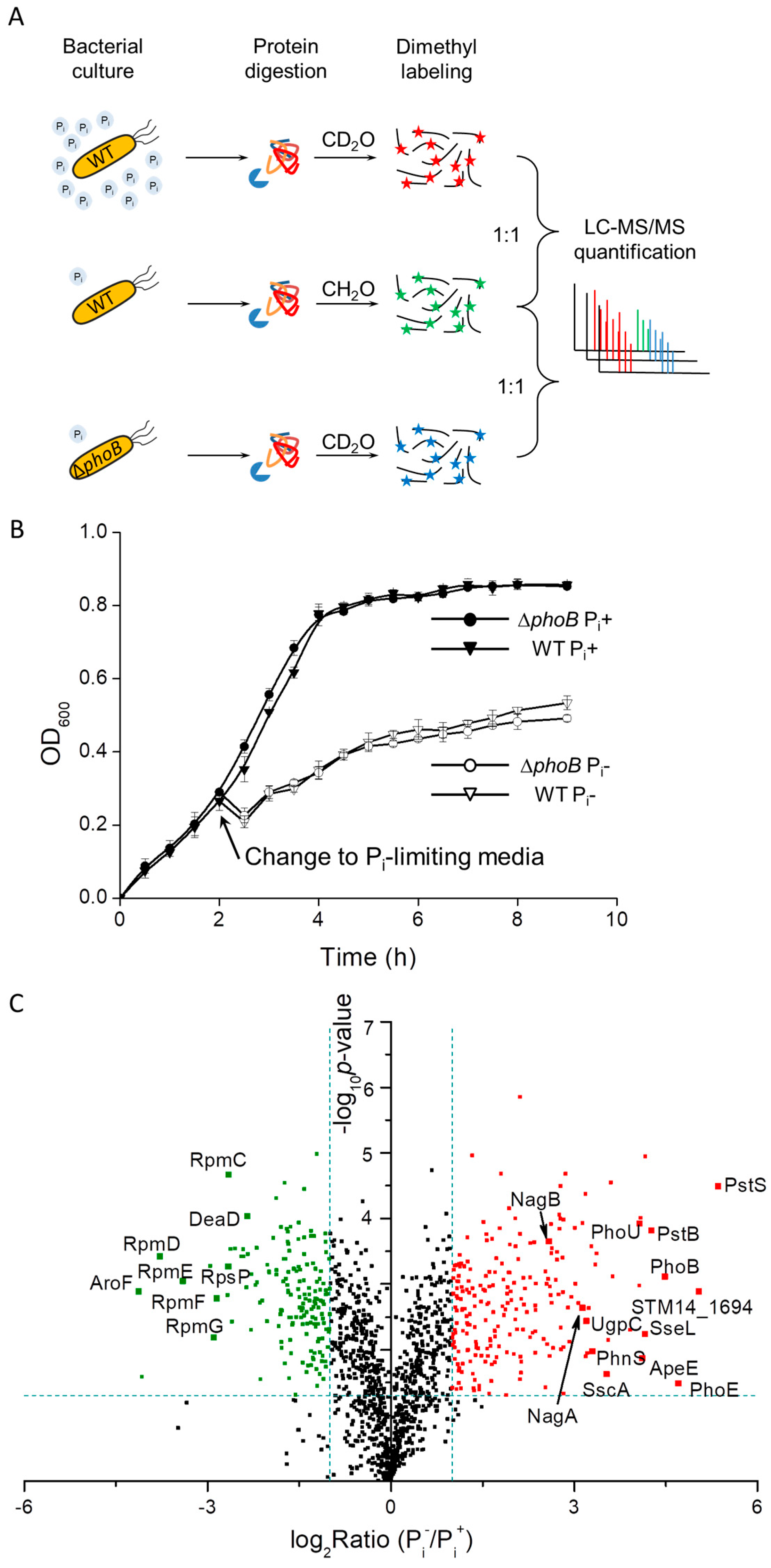
2.2. SDS-PAGE, in-Gel Protein Digestion and Stable Isotope Dimethyl Labeling
2.3. Nanoflow LC-MS/MS Analyses
2.4. Proteomic Data Processing and Bioinformatics Analysis
2.5. Bacterial Growth Competition Assays
2.6. Western Blot Analysis
2.7. β-Galactosidase Activity Assays
3. Results
3.1. Proteomic Analysis of S. Typhimurium in Response to Pi Starvation
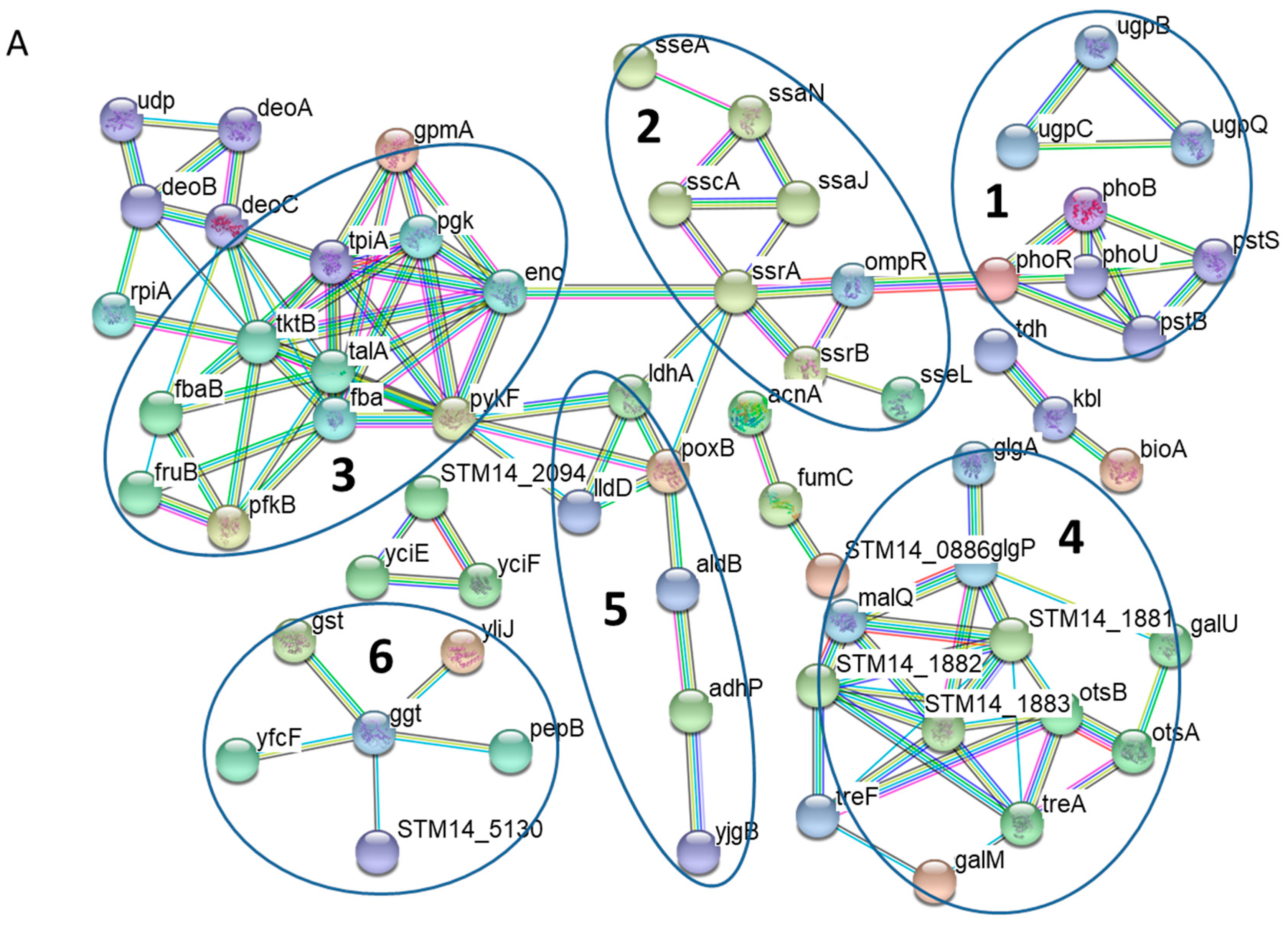
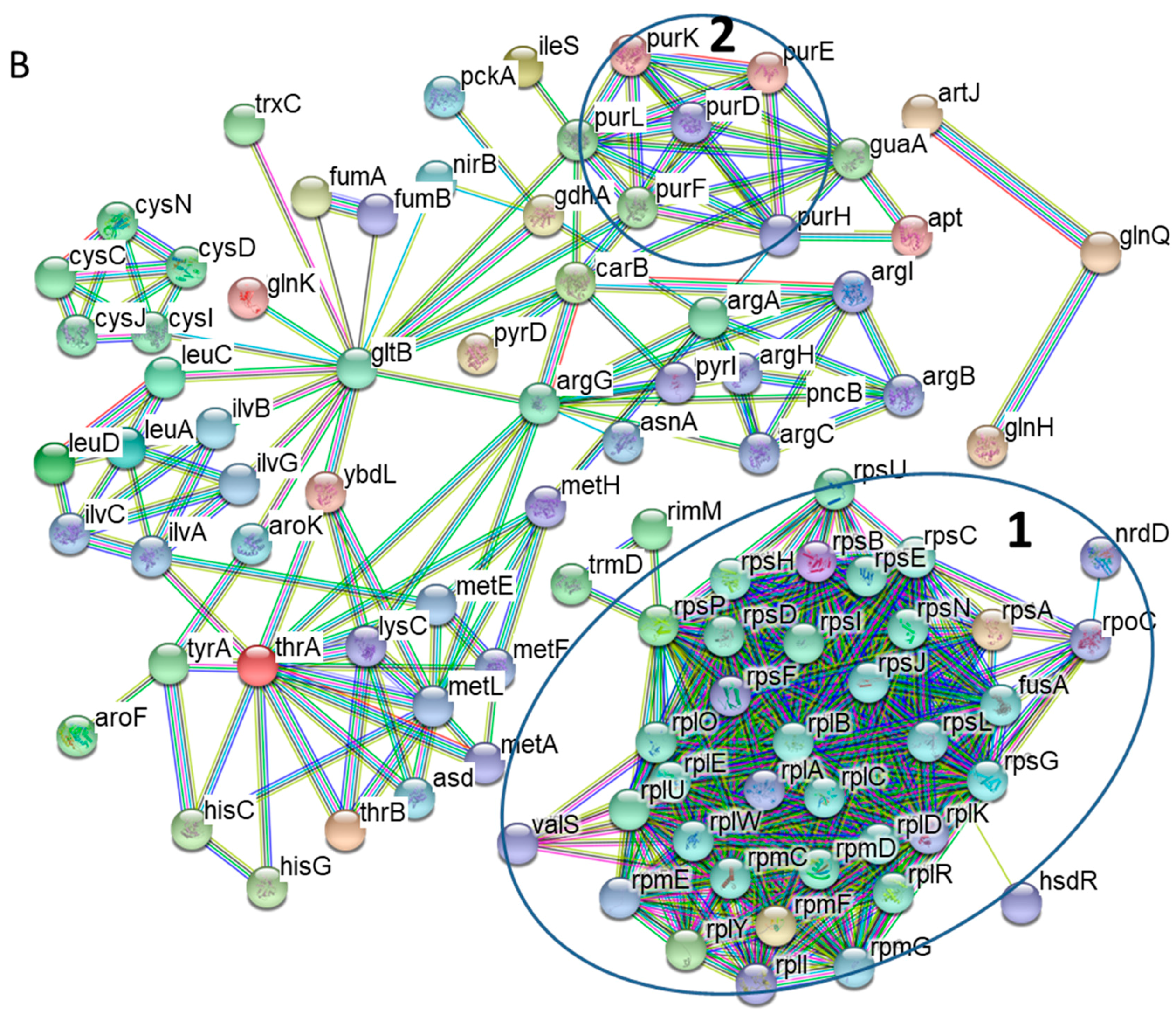
3.2. Protein-Protein Interaction Networks of Altered S. Typhimurium Proteins
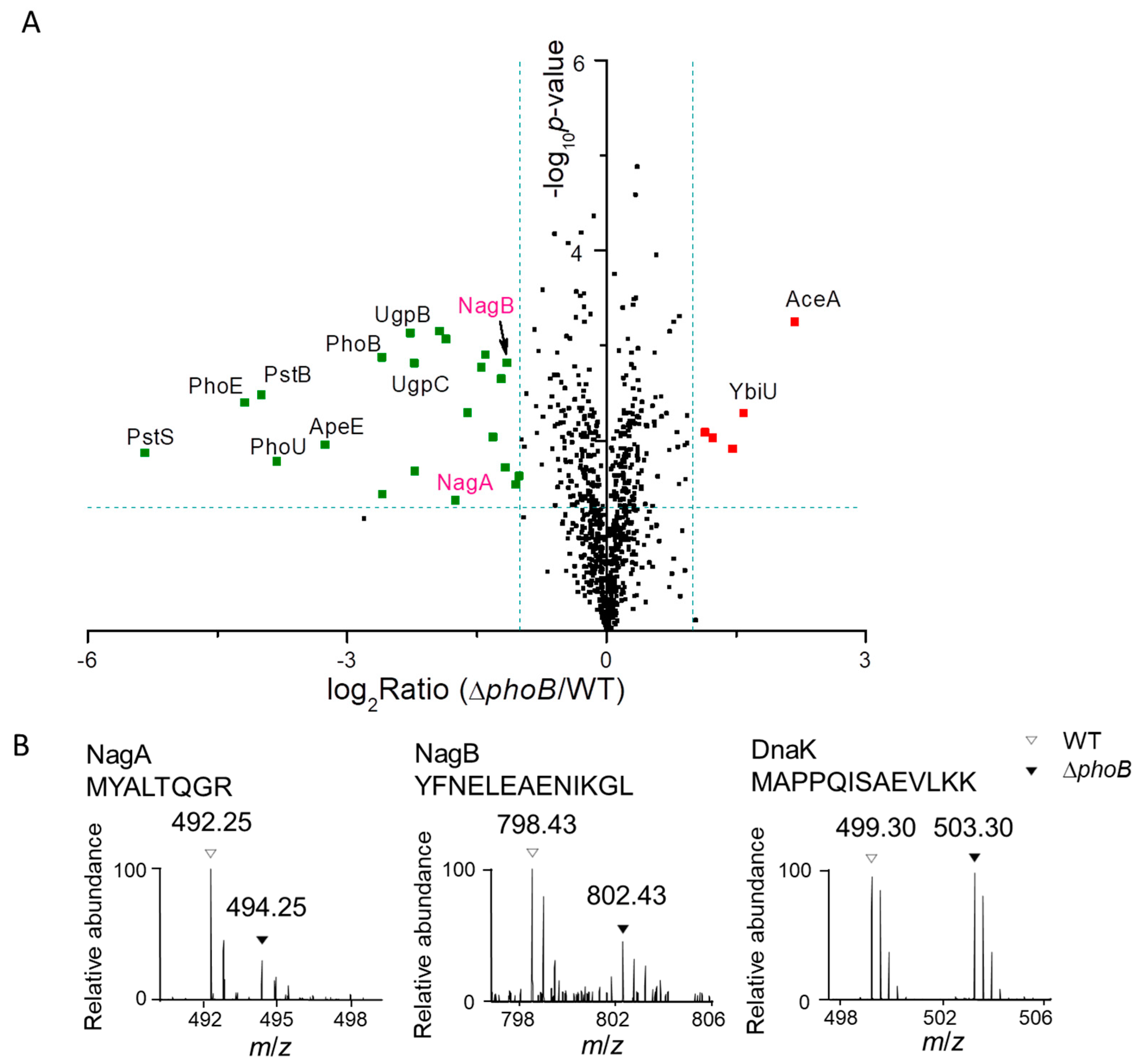
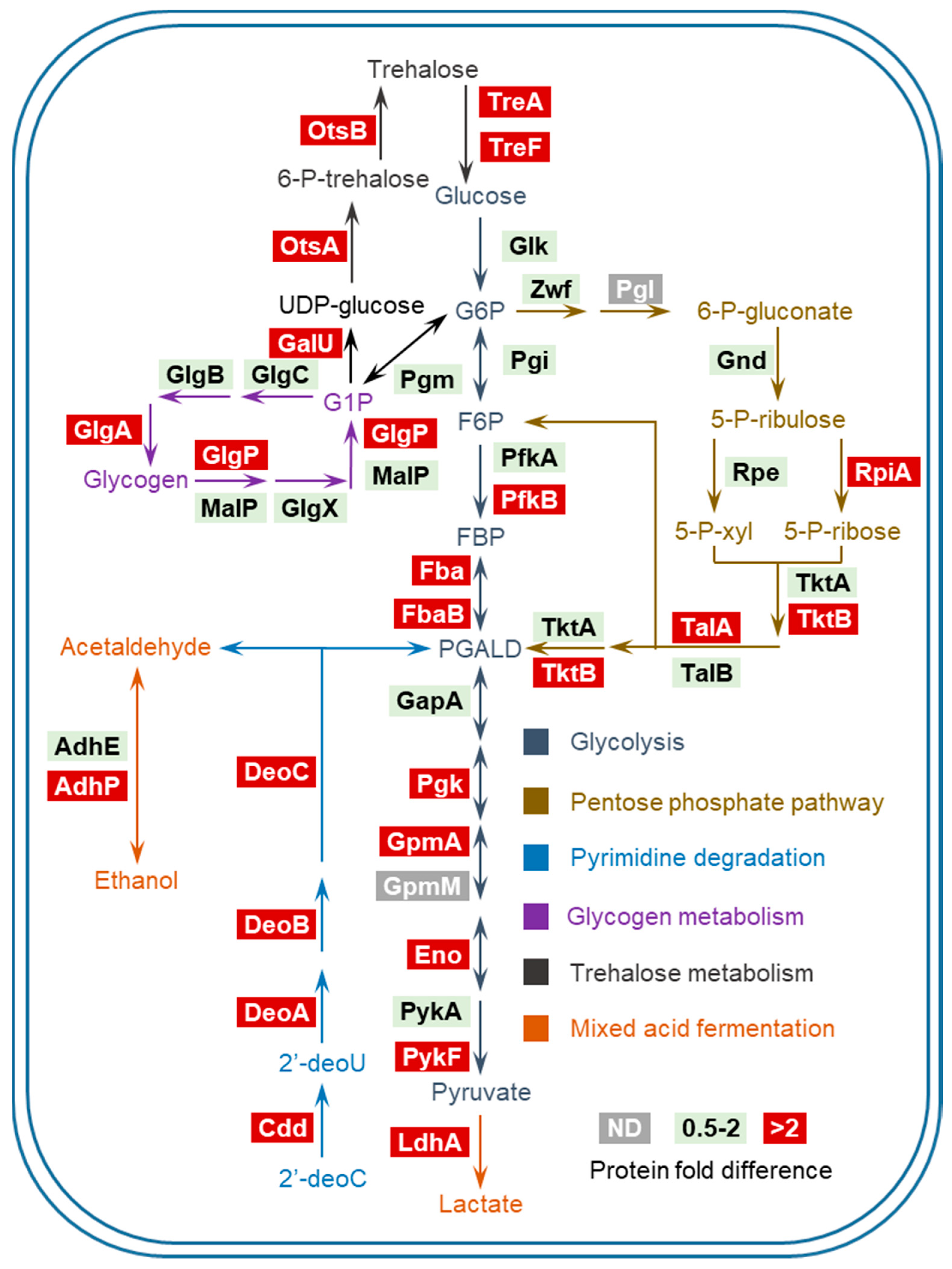
3.3. Assignment of PhoB-regulated and PhoB-independent Pathways under Pi Starvation
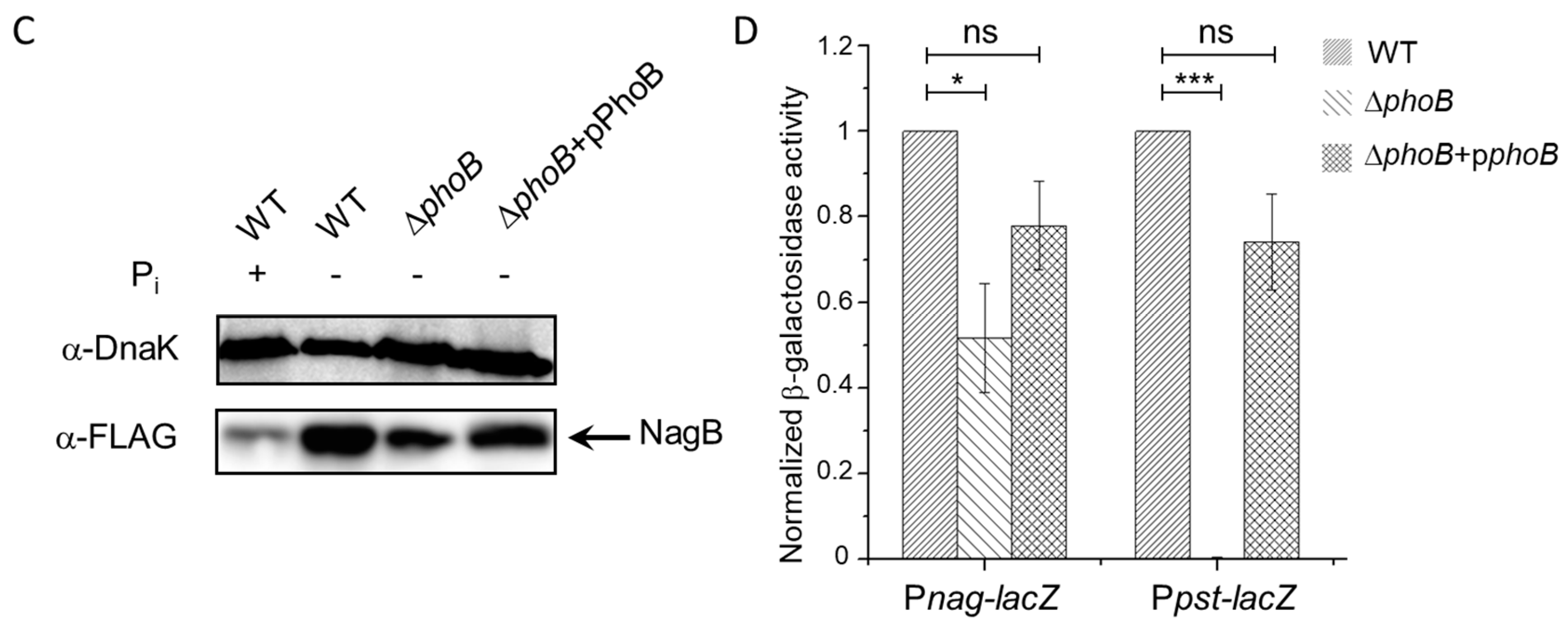
3.4. PhoB-Dependent Induction of S. Typhimurium Nag during Pi Starvation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wanner, B.L. Phosphorus assimilation and control of the phosphate regulon. In Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed.; Neidhardt, F.C., Curtiss, R., III, Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; ASM Press: Washington, DC, USA, 1996; pp. 1357–1381. [Google Scholar]
- VanBogelen, R.A.; Olson, E.R.; Wanner, B.L.; Neidhardt, F.C. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 1996, 178, 4344–4366. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Wanner, B.L. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Darwin, K.H.; Miller, V.L. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 1999, 12, 405–428. [Google Scholar] [PubMed]
- Galán, J.E.; Lara-Tejero, M.; Marlovits, T.C.; Wagner, S. Bacterial type III secretion systems: Specialized nanomachines for protein delivery into target cells. Annu. Rev. Microbiol. 2014, 68, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.; Thurston, T.L.M.; Holden, D.W. Salmonella SPI-2 Type III Secretion System Effectors: Molecular Mechanisms And Physiological Consequences. Cell Host Microbe 2017, 22, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Q.; Hu, M.; Yu, K.; Fu, J.; Zhou, F.; Liu, X. Proteomic analyses of intracellular Salmonella enterica Serovar Typhimurium reveal extensive bacterial adaptations to infected host epithelial cells. Infect. Immun. 2015, 83, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, K.; Zhou, F.; Ding, T.; Yang, Y.; Hu, M.; Liu, X. Quantitative proteomics charts the landscape of Salmonella carbon metabolism within host epithelial cells. J. Proteome Res. 2017, 16, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Qi, L.; Ding, T.; Wang, Z.; Fu, J.; Hu, M.; Li, M.; Song, J.; Liu, X.; et al. Temporal regulation of a Salmonella Typhimurium virulence factor by the transcriptional regulator YdcR. Mol. Cell. Proteom. 2017, 16, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.Y. Transcriptome analysis of phosphate starvation response in Escherichia coli. J. Microbiol. Biotechnol. 2007, 17, 244–252. [Google Scholar] [PubMed]
- Chekabab, S.M.; Jubelin, G.; Dozois, C.M.; Harel, J. PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation. PLoS ONE 2014, 9, e94285. [Google Scholar] [CrossRef] [PubMed]
- Marzan, L.W.; Shimizu, K. Metabolic regulation of Escherichia coli and its phoB and phoR genes knockout mutants under phosphate and nitrogen limitations as well as at acidic condition. Microb. Cell. Fact. 2011, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, T.; Loffler, M.; Hurler, T.; Takors, R. Phosphate limited fed-batch processes: Impact on carbon usage and energy metabolism in Escherichia coli. J. Biotechnol. 2014, 190, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Sugiyama, S.; Oyamada, T.; Yokoyama, K.; Makino, K. Novel members of the phosphate regulon in Escherichia coli O157:H7 identified using a whole-genome shotgun approach. Gene 2012, 502, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.G.; Canals, A.; Coll, M. PhoB transcriptional activator binds hierarchically to pho box promoters. Biol. Chem. 2012, 393, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Chekabab, S.M.; Harel, J.; Dozois, C.M. Interplay between genetic regulation of phosphate homeostasis and bacterial virulence. Virulence 2014, 5, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Torriani, A. From cell membrane to nucleotides: The phosphate regulon in Escherichia coli. Bioessays 1990, 12, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.Y.; Torriani, A. PstB protein of the phosphate-specific transport system of Escherichia coli is an ATPase. J. Bacteriol. 1996, 178, 3974–3977. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Uzzau, S.; Figueroa-Bossi, N.; Rubino, S.; Bossi, L. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. USA 2001, 98, 15264–15269. [Google Scholar] [CrossRef] [PubMed]
- Elledge, S.J.; Davis, R.W. Position and density effects on repression by stationary and mobile DNA-binding proteins. Genes Dev. 1989, 3, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Neidhardt, F.C.; Bloch, P.L.; Smith, D.F. Culture medium for enterobacteria. J. Bacteriol. 1974, 119, 736–747. [Google Scholar] [PubMed]
- Hu, M.; Liu, Y.; Yu, K.; Liu, X. Decreasing the amount of trypsin in in-gel digestion leads to diminished chemical noise and improved protein identifications. J. Proteom. 2014, 109, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Huang, S.Y.; Chow, N.H.; Chen, S.H. Stable-isotope dimethyl labeling for quantitative proteomics. Anal. Chem. 2003, 75, 6843–6852. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Plumbridge, J.A. Sequence of the nagBACD operon in Escherichia coli K12 and pattern of transcription within the nag regulon. Mol. Microbiol. 1989, 3, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Plumbridge, J. How to achieve constitutive expression of a gene within an inducible operon: The example of the nagC gene of Escherichia coli. J. Bacteriol. 1996, 178, 2629–2636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plumbridge, J.A. Repression and induction of the nag regulon of Escherichia coli K-12: The roles of nagC and nagA in maintenance of the uninduced state. Mol. Microbiol. 1991, 5, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, L.W.; Dunaway-Mariano, D.; Allen, K.N. Structure and activity analyses of Escherichia coli K-12 NagD provide insight into the evolution of biochemical function in the haloalkanoic acid dehalogenase superfamily. Biochemistry 2006, 45, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Deiwick, J.; Nikolaus, T.; Erdogan, S.; Hensel, M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol. Microbiol. 1999, 31, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Yu, K.; Qi, L.; Liu, Y.; Cheng, S.; Wu, M.; Wang, Z.; Fu, J.; Liu, X. A Proteomic View of Salmonella Typhimurium in Response to Phosphate Limitation. Proteomes 2018, 6, 19. https://doi.org/10.3390/proteomes6020019
Jiang J, Yu K, Qi L, Liu Y, Cheng S, Wu M, Wang Z, Fu J, Liu X. A Proteomic View of Salmonella Typhimurium in Response to Phosphate Limitation. Proteomes. 2018; 6(2):19. https://doi.org/10.3390/proteomes6020019
Chicago/Turabian StyleJiang, Jiezhang, Kaiwen Yu, Linlu Qi, Yanhua Liu, Sen Cheng, Mei Wu, Zhen Wang, Jiaqi Fu, and Xiaoyun Liu. 2018. "A Proteomic View of Salmonella Typhimurium in Response to Phosphate Limitation" Proteomes 6, no. 2: 19. https://doi.org/10.3390/proteomes6020019
APA StyleJiang, J., Yu, K., Qi, L., Liu, Y., Cheng, S., Wu, M., Wang, Z., Fu, J., & Liu, X. (2018). A Proteomic View of Salmonella Typhimurium in Response to Phosphate Limitation. Proteomes, 6(2), 19. https://doi.org/10.3390/proteomes6020019




