De Novo Sequencing of Top-Down Tandem Mass Spectra: A Next Step towards Retrieving a Complete Protein Sequence
Abstract
:1. Introduction
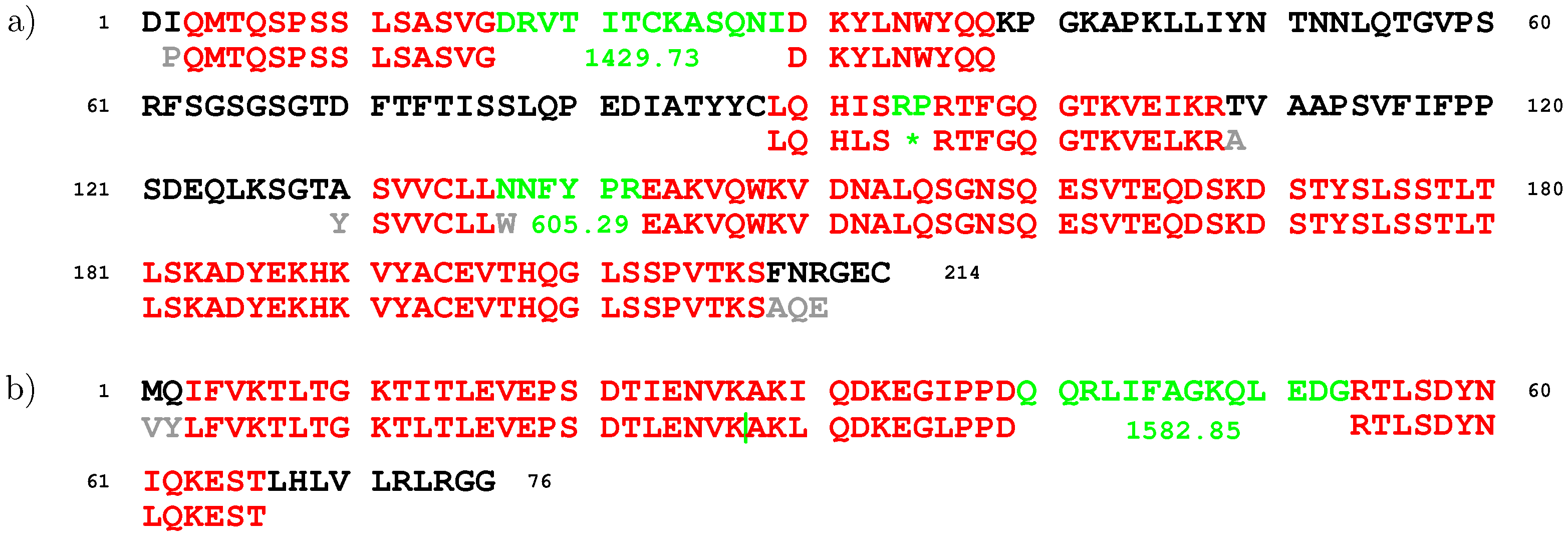
2. Results
- tag length: ;
- maximum gap size: Da;
- minimum multiplicity of a binned distance: ;
- minimum number of amino acids supporting a reliable binned distance (see Section 4.6): ;
- tolerance for comparing mass offsets: ppm.
3. Discussion
4. Materials and Methods
4.1. Data Sets
4.2. Deconvolution
4.3. Tags
4.4. Sequence Fragments
4.5. Tag Convolution
4.6. Gap Estimation
Supplementary Materials
Acknowledgments
Conflicts of Interest
Abbreviations
| MS/MS | tandem mass spectrometry |
| CAD | collisionally activated dissociation |
| ECD | electron capture dissociation |
| CAH2 | carbonic anhydrase 2 |
| CDR | complementarity determining region |
| RPLC | reversed-phase liquid chromatography |
| MS | mass spectrometry |
| ETD | electron-transfer dissociation |
| CID | collision-induced dissociation |
| HCD | higher-energy C-trap dissociation |
References
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Pevzner, P. PepNovo: De Novo Peptide Sequencing via Probabilistic Network Modeling. Anal. Chem. 2005, 77, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Sun, R.X.; Yang, B.; Song, C.Q.; Wang, L.H.; Liu, C.; Fu, Y.; Yuan, Z.F.; Wang, H.P.; He, S.M.; et al. pNovo: De novo Peptide Sequencing and Identification Using HCD Spectra. J. Proteom. Res. 2010, 9, 2713–2724. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Johnson, R.S. Sequence database searches via de novo peptide sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1067–1075. [Google Scholar] [CrossRef]
- Dancik, V.; Addona, T.A.; Clauser, K.R.; Vath, J.E.; Pevzner, P.A. De Novo Peptide Sequencing via Tandem Mass Spectrometry. J. Comput. Biol. 1999, 6, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Park, B.; McDonald, W.; Carey, P.; Banfield, J.; VerBerkmoes, N.; Hettich, R.; Samatova, N. A high-throughput de novo sequencing approach for shotgun proteomics using high-resolution tandem mass spectrometry. BMC Bioinform. 2010, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Ma, B. Novor: Real-time peptide de novo sequencing software. J. Am. Soc. Mass Spectrom. 2015, 26, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Rahman, M.Z.; He, L.; Shan, B.; Li, M. Complete De Novo Assembly of Monoclonal Antibody Sequences. Sci. Rep. 2016, 6, 31730. [Google Scholar] [CrossRef] [PubMed]
- Robotham, S.A.; Horton, A.P.; Cannon, J.R.; Cotham, V.C.; Marcotte, E.M.; Brodbelt, J.S. UVnovo: A de Novo Sequencing Algorithm Using Single Series of Fragment Ions via Chromophore Tagging and 351 nm Ultraviolet Photodissociation Mass Spectrometry. Anal. Chem. 2016, 88, 3990–3997. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, N.; Tang, H.; Bafna, V.; Pevzner, P. Shotgun Protein Sequencing by Tandem Mass Spectra Assembly. Anal. Chem. 2004, 76, 7221–7233. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, N.; Clauser, K.R.; Pevzner, P.A. Shotgun Protein Sequencing: Assembly of Peptide Tandem Mass Spectra from Mixtures of Modified Proteins. Mol. Cell. Proteom. 2007, 6, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, N.; Pham, V.; Pevzner, P.; Arnott, D.; Lill, J.R. Automated de novo protein sequencing of monoclonal antibodies. Nat. Biotechnol. 2008, 26, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; Yuen, D.; Ma, B. Automated Protein (Re)Sequencing with MS/MS and a Homologous Database Yields Almost Full Coverage and Accuracy. Bioinformatics 2009, 25, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Castellana, N.E.; Pham, V.; Arnott, D.; Lill, J.R.; Bafna, V. Template Proteogenomics: Sequencing Whole Proteins Using an Imperfect Database. Mol. Cell. Proteom. 2010, 9, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Savitski, M.; Nielsen, M.L.; Zubarev, R.A. New data base-independent, sequence tag-based scoring of peptide MS/MS data validates Mowse scores, recovers below threshold data, singles out modified peptides, and assesses the quality of MS/MS techniques. Mol. Cell. Proteom. 2005, 4, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Bern, M. Spectrum Fusion: Using Multiple Mass Spectra for De Novo Peptide Sequencing. In Research in Computational Molecular Biology; Vingron, M., Wong, L., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 4955, pp. 140–153. [Google Scholar]
- Bertsch, A.; Leinenbach, A.; Pervukhin, A.; Lubeck, M.; Hartmer, R.; Baessmann, C.; Elnakady, Y.A.; Müller, R.; Böcker, S.; Huber, C.G.; et al. De novo peptide sequencing by tandem MS using complementary CID and electron transfer dissociation. Electrophoresis 2009, 30, 3736–3747. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ma, B. ADEPTS: Advanced Peptide De Novo Sequencing with a Pair of Tandem Mass Spectra. J. Bioinform. Comput. Biol. 2010, 8, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Chen, H.; He, K.; Wu, L.; Yang, B.; Sun, R.X.; Liu, J.; Zeng, W.F.; Song, C.Q.; He, S.M.; et al. pNovo+: De Novo Peptide Sequencing Using Complementary HCD and ETD Tandem Mass Spectra. J. Proteom. Res. 2013, 12, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Guthals, A.; Clauser, K.R.; Frank, A.M.; Bandeira, N. Sequencing-Grade De novo Analysis of MS/MS Triplets (CID/HCD/ETD) From Overlapping Peptides. J. Proteom. Res. 2013, 12, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Perkins, D.N.; Pappin, D.J.C.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- LeDuc, R.D.; Taylor, G.K.; Kim, Y.B.; Januszyk, T.E.; Bynum, L.H.; Sola, J.V.; Garavelli, J.S.; Kelleher, N.L. ProSight PTM: An integrated environment for protein identification and characterization by top-down mass spectrometry. Nucleic Acids Res. 2004, 32, W340–W345. [Google Scholar] [CrossRef] [PubMed]
- Zamdborg, L.; LeDuc, R.D.; Glowacz, K.J.; Kim, Y.B.; Viswanathan, V.; Spaulding, I.T.; Early, B.P.; Bluhm, E.J.; Babai, S.; Kelleher, N.L. ProSight PTM 2.0: Improved protein identification and characterization for top down mass spectrometry. Nucleic Acids Res. 2007, 35, W701–W706. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sirotkin, Y.; Shen, Y.; Anderson, G.; Tsai, Y.S.; Ting, Y.S.; Goodlett, D.R.; Smith, R.D.; Bafna, V.; Pevzner, P.A. Protein Identification Using Top-Down Spectra. Mol. Cell. Proteom. 2012, 11, M111.008524. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, P.V.; Second, T.P.; Zabrouskov, V.; Makarov, A.A.; Zhang, Z. Mass Measurement and Top-Down HPLC/MS Analysis of Intact Monoclonal Antibodies on a Hybrid Linear Quadrupole Ion Trap-Orbitrap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2009, 20, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Kellie, J.F.; Tran, J.C.; Lee, J.E.; Ahlf, D.R.; Thomas, H.M.; Ntai, I.; Catherman, A.D.; Durbin, K.R.; Zamdborg, L.; Vellaichamy, A.; et al. The emerging process of Top Down mass spectrometry for protein analysis: biomarkers, protein-therapeutics, and achieving high throughput. Mol. BioSyst. 2010, 6, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Fornelli, L.; Damoc, E.; Thomas, P.M.; Kelleher, N.L.; Aizikov, K.; Denisov, E.; Makarov, A.; Tsybin, Y.O. Analysis of Intact Monoclonal Antibody IgG1 by Electron Transfer Dissociation Orbitrap FTMS. Mol. Cell. Proteom. 2012, 11, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.M.; Zubarev, R.A.; McLafferty, F.W. Automated de novo sequencing of proteins by tandem high-resolution mass spectrometry. Proc. Natl. Acad. Sci. USA 2000, 97, 10313–10317. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dekker, L.; Wu, S.; Vanduijn, M.M.; Luider, T.M.; Tolić, N.; Dvorkin, M.; Alexandrova, S.; Vyatkina, K.; Paša-Tolić, L.; et al. De Novo Protein Sequencing by Combining Top-Down and Bottom-Up Tandem Mass Spectra. J. Proteom. Res. 2014, 13, 3241–3248. [Google Scholar] [CrossRef] [PubMed]
- Vyatkina, K.; Wu, S.; Dekker, L.J.; VanDuijn, M.M.; Liu, X.; Tolić, N.; Dvorkin, M.; Alexandrova, S.; Luider, T.M.; Paša-Tolić, L.; et al. De novo sequencing of peptides from top-down tandem mass spectra. J. Proteom. Res. 2015, 14, 4450–4462. [Google Scholar] [CrossRef] [PubMed]
- Vyatkina, K.; Wu, S.; Dekker, L.J.; VanDuijn, M.M.; Liu, X.; Tolić, N.; Dvorkin, M.; Alexandrova, S.; Luider, T.M.; Paša-Tolić, L.; et al. Top-down analysis of protein samples by de novo sequencing techniques. Bioinformatics 2016, 32, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Vyatkina, K.; Dekker, L.J.M.; Wu, S.; VanDuijn, M.M.; Liu, X.; Tolić, N.; Dvorkin, M.; Alexandrova, S.; Luider, T.M.; Paša-Tolić, L. Tag convolution as a means for validating de novo peptide sequences. 2017. submitted. [Google Scholar]
- Liu, X.; Inbar, Y.; Dorrestein, P.C.; Wynne, C.; Edwards, N.; Souda, P.; Whitelegge, J.P.; Bafna, V.; Pevzner, P.A. Deconvolution and Database Search of Complex Tandem Mass Spectra of Intact Proteins: A Combinatorial Approach. Mol. Cell. Proteom. 2010, 9, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyatkina, K. De Novo Sequencing of Top-Down Tandem Mass Spectra: A Next Step towards Retrieving a Complete Protein Sequence. Proteomes 2017, 5, 6. https://doi.org/10.3390/proteomes5010006
Vyatkina K. De Novo Sequencing of Top-Down Tandem Mass Spectra: A Next Step towards Retrieving a Complete Protein Sequence. Proteomes. 2017; 5(1):6. https://doi.org/10.3390/proteomes5010006
Chicago/Turabian StyleVyatkina, Kira. 2017. "De Novo Sequencing of Top-Down Tandem Mass Spectra: A Next Step towards Retrieving a Complete Protein Sequence" Proteomes 5, no. 1: 6. https://doi.org/10.3390/proteomes5010006
APA StyleVyatkina, K. (2017). De Novo Sequencing of Top-Down Tandem Mass Spectra: A Next Step towards Retrieving a Complete Protein Sequence. Proteomes, 5(1), 6. https://doi.org/10.3390/proteomes5010006




