Proteomic Analysis of the Periodontal Ligament During Orthodontic Movement: A Study in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. OTM Procedure
2.3. Laser Capture Microdissection (LCM) and Protein Extraction
2.4. LC-MS/MS and Bioinformatics Analysis
2.5. Immunohistochemistry (IHC)
2.6. Statistical Analysis
3. Results
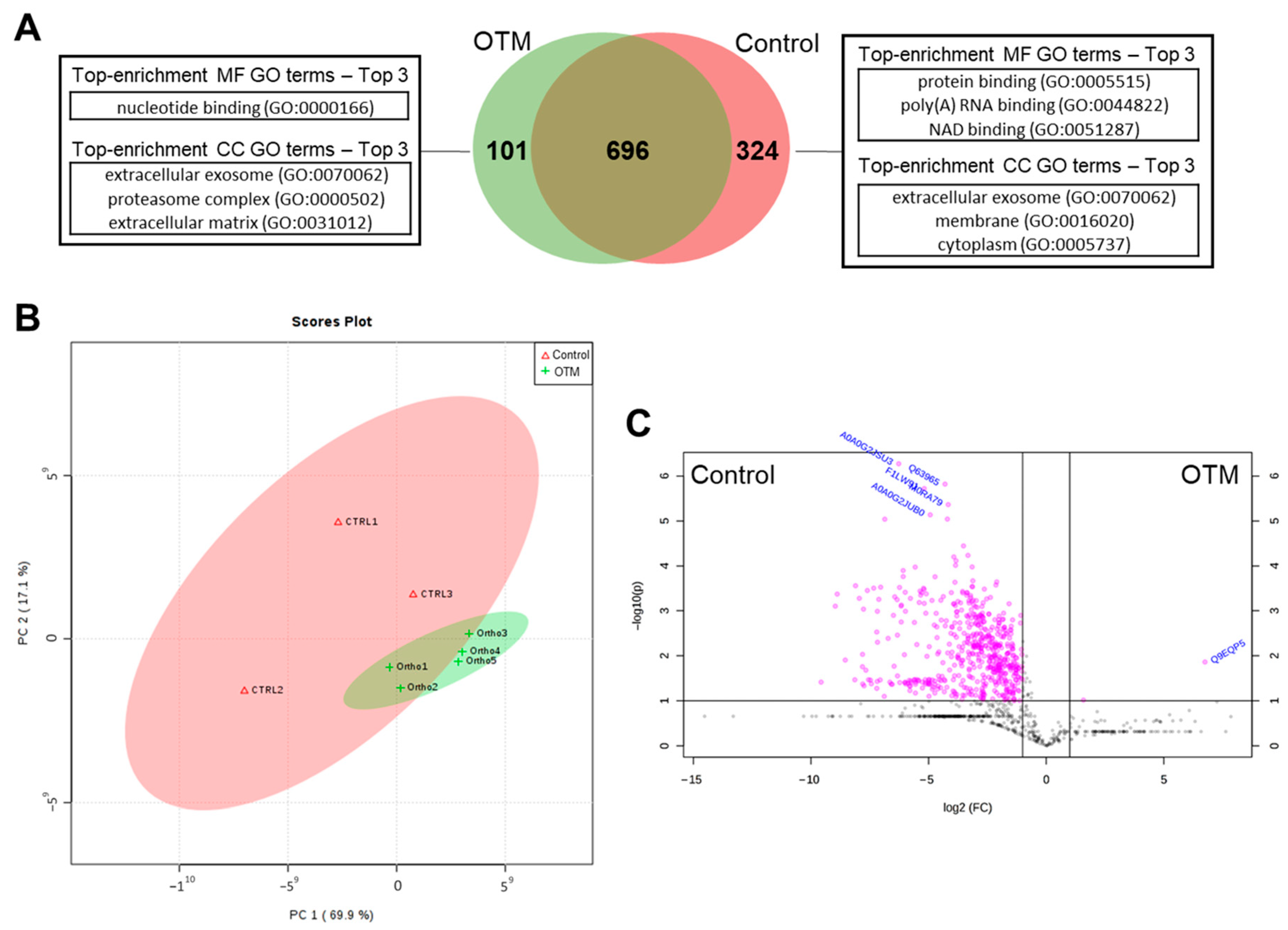
3.1. Proteomic Analysis of PDL in the Control and OTM Groups
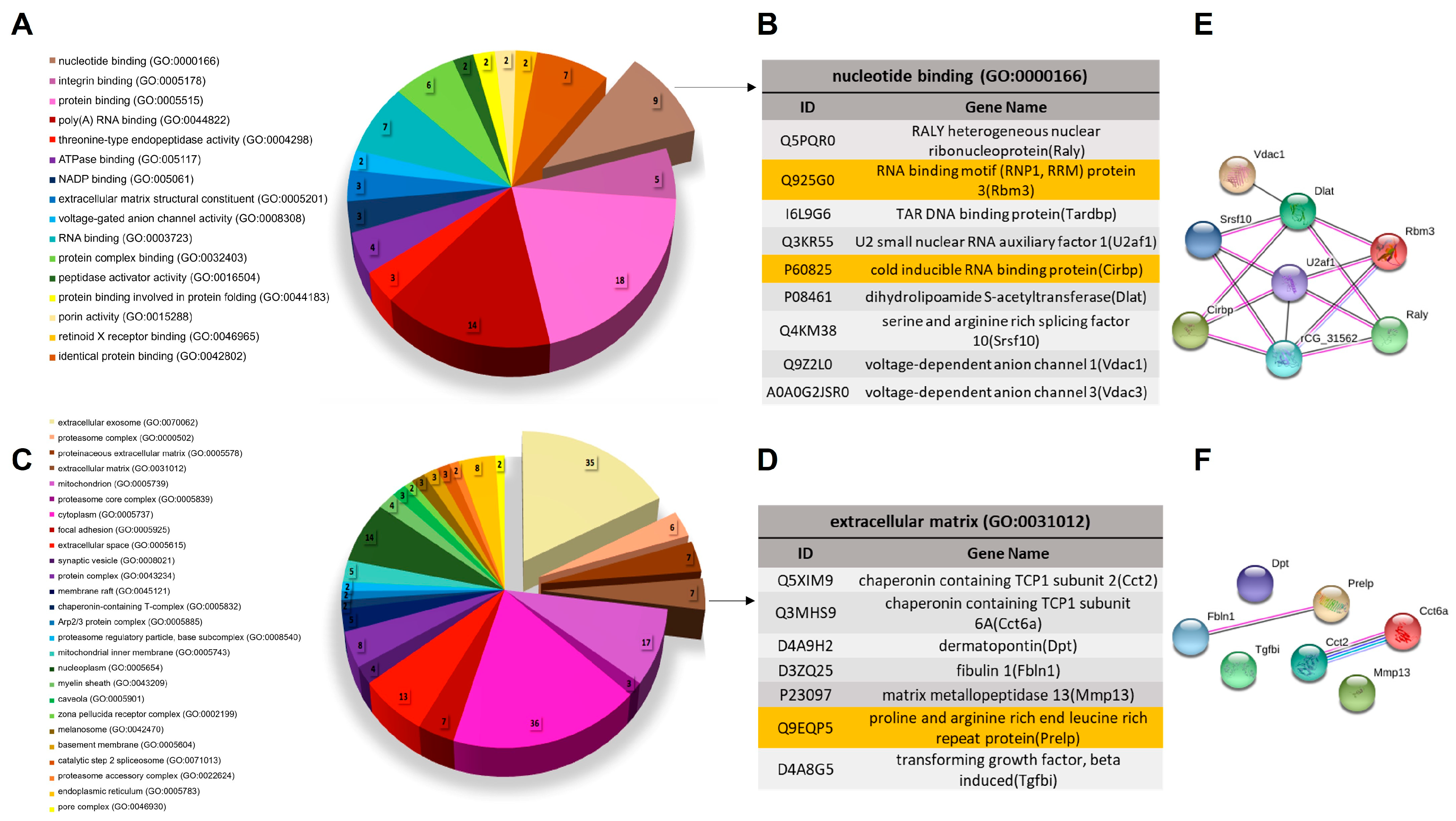
3.2. Biological Characterization of Proteins with Differential Abundance
3.3. Biological Characterization of OTM Exclusive Proteins
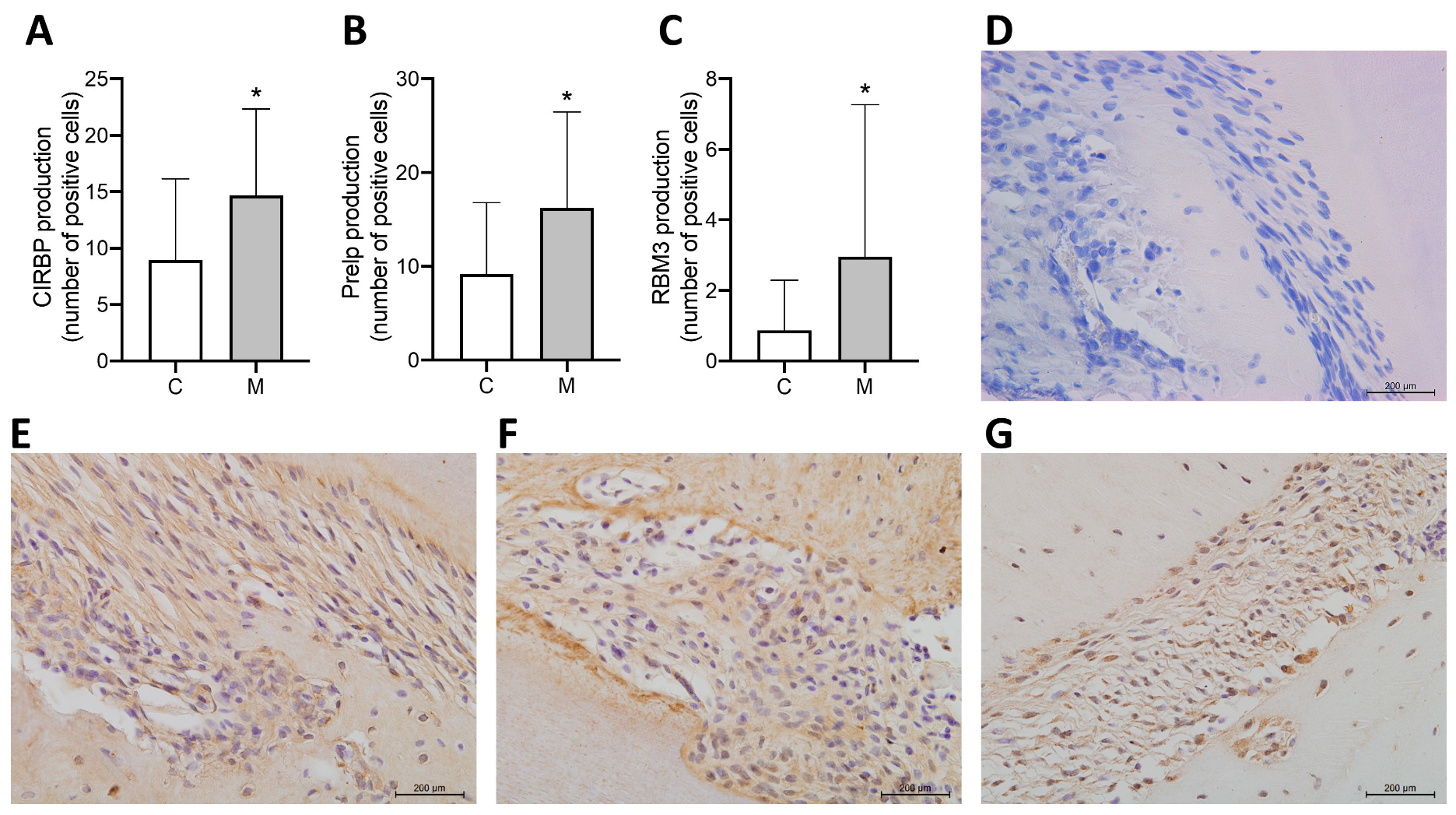
3.4. Subcellular Localization and Abundance Pattern of Selected Proteins Determined by IHC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef]
- Krishnan, V.; Davidovitch, Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J. Dent. Res. 2009, 88, 597–608. [Google Scholar] [CrossRef]
- Alhashimi, N.; Frithiof, L.; Brudvik, P.; Bakhiet, M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Grieve, W.G., 3rd; Johnson, G.K.; Moore, R.N.; Reinhardt, R.A.; DuBois, L.M. Prostaglandin E (PGE) and interleukin-1 beta (IL-1 beta) levels in gingival crevicular fluid during human orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1994, 105, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Park, Y.C.; Yu, H.S.; Choi, S.H.; Yoo, Y.J. Effects of continuous and interrupted orthodontic force on interleukin-1beta and prostaglandin E2 production in gingival crevicular fluid. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.S.; Meister, M. Current concepts in the biology of orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 458–468. [Google Scholar] [CrossRef]
- Berkovitz, B.K.; Moxham, B.J. The development of the periodontal ligament with special reference to collagen fibre ontogeny. J. Biol. Buccale 1990, 18, 227–236. [Google Scholar]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Songhee Song, K.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Proff, P.; Romer, P. The molecular mechanism behind bone remodelling: A review. Clin. Oral Investig. 2009, 13, 355–362. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef]
- Yong, J.; Groeger, S.; Meyle, J.; Ruf, S. MAPK and beta-Catenin signaling: Implication and interplay in orthodontic tooth movement. Front. Biosci. (Landmark Ed.) 2022, 27, 54. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Weissinger, F.; Rausch, M.A.; Andrukhov, O.; Jonke, E. MMPs and TIMPs Expression Levels in the Periodontal Ligament During Orthodontic Tooth Movement: A Systematic Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2021, 22, 6967. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Park, W.; Choi, S.H.; Hong, N.; Huh, J.; Jung, S. Effect of anti-sclerostin antibody on orthodontic tooth movement in ovariectomized rats. Prog. Orthod. 2024, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Belda-Ferre, P.; Williamson, J.; Simon-Soro, A.; Artacho, A.; Jensen, O.N.; Mira, A. The human oral metaproteome reveals potential biomarkers for caries disease. Proteomics 2015, 15, 3497–3507. [Google Scholar] [CrossRef]
- Choi, Y.J.; Heo, S.H.; Lee, J.M.; Cho, J.Y. Identification of azurocidin as a potential periodontitis biomarker by a proteomic analysis of gingival crevicular fluid. Proteome Sci. 2011, 9, 42. [Google Scholar] [CrossRef]
- Giovani, P.A.; Salmon, C.R.; Martins, L.; Leme, A.F.P.; Puppin-Rontani, R.M.; Mofatto, L.S.; Nociti, F.H., Jr.; Kantovitz, K.R. Membrane proteome characterization of periodontal ligament cell sets from deciduous and permanent teeth. J. Periodontol. 2019, 90, 775–787. [Google Scholar] [CrossRef]
- Salmon, C.R.; Giorgetti, A.P.; Paes Leme, A.F.; Domingues, R.R.; Sallum, E.A.; Alves, M.C.; Kolli, T.N.; Foster, B.L.; Nociti, F.H., Jr. Global proteome profiling of dental cementum under experimentally-induced apposition. J. Proteom. 2016, 141, 12–23. [Google Scholar] [CrossRef]
- Salmon, C.R.; Giorgetti, A.P.O.; Paes Leme, A.F.; Domingues, R.R.; Kolli, T.N.; Foster, B.L.; Nociti, F.H., Jr. Microproteome of dentoalveolar tissues. Bone 2017, 101, 219–229. [Google Scholar] [CrossRef]
- Han, N.Y.; Hong, J.Y.; Park, J.M.; Shin, C.; Lee, S.; Lee, H.; Yun, J.H. Label-free quantitative proteomic analysis of human periodontal ligament stem cells by high-resolution mass spectrometry. J. Periodontal Res. 2019, 54, 53–62. [Google Scholar] [CrossRef]
- McKnight, H.; Kelsey, W.P.; Hooper, D.A.; Hart, T.C.; Mariotti, A. Proteomic analyses of human gingival and periodontal ligament fibroblasts. J. Periodontol. 2014, 85, 810–818. [Google Scholar] [CrossRef]
- Reichenberg, E.; Redlich, M.; Cancemi, P.; Zaks, B.; Pitaru, S.; Fontana, S.; Pucci-Minafra, I.; Palmon, A. Proteomic analysis of protein components in periodontal ligament fibroblasts. J. Periodontol. 2005, 76, 1645–1653. [Google Scholar] [CrossRef]
- Nogueira, A.V.; de Molon, R.S.; Nokhbehsaim, M.; Deschner, J.; Cirelli, J.A. Contribution of biomechanical forces to inflammation-induced bone resorption. J. Clin. Periodontol. 2017, 44, 31–41. [Google Scholar] [CrossRef]
- Foster, B.; Ao, M.; Salmon, C.; Chavez, M.; Kolli, T.; Tran, A.; Chu, E.; Kantovitz, K.; Yadav, M.; Narisawa, S. Osteopontin regulates dentin and alveolar bone development and mineralization. Bone 2018, 107, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.L. Methods for studying tooth root cementum by light microscopy. Int. J. Oral Sci. 2012, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kou, X.; Jiang, N.; Liu, Y.; Tay, F.R.; Zhou, Y. Effect of intraoral mechanical stress application on the expression of a force-responsive prognostic marker associated with system disease progression. J. Dent. 2017, 57, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ellias, M.F.; Zainal Ariffin, S.H.; Karsani, S.A.; Abdul Rahman, M.; Senafi, S.; Megat Abdul Wahab, R. Proteomic analysis of saliva identifies potential biomarkers for orthodontic tooth movement. Sci. World J. 2012, 2012, 647240. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Zheng, H.; Zhou, Y.; Chen, F.; Lin, J. Magnetic bead-based salivary peptidome profiling analysis during orthodontic treatment durations. Biochem. Biophys. Res. Commun. 2012, 421, 844–849. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Deutsch, O.; Zaks, B.; Krief, G.; Chaushu, S.; Palmon, A. Identification of salivary protein biomarkers for orthodontically induced inflammatory root resorption. Proteom. Clin. Appl. 2017, 11, 1600119. [Google Scholar] [CrossRef]
- Wu, J.Q.; Jiang, J.H.; Xu, L.; Liang, C.; Wang, X.J.; Bai, Y. Magnetic Bead-based Salivary Peptidome Profiling for Accelerated Osteogenic Orthodontic Treatments. Chin. J. Dent. Res. 2018, 21, 41–49. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Li, R.; Cao, T.; Zheng, H.; Wang, X.; Zhou, Y.; Du, N.; Chen, F.; Lin, J. Magnetic bead-based salivary peptidome profiling for periodontal-orthodontic treatment. Proteome Sci. 2012, 10, 63. [Google Scholar] [CrossRef]
- Bengtsson, E.; Morgelin, M.; Sasaki, T.; Timpl, R.; Heinegard, D.; Aspberg, A. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J. Biol. Chem. 2002, 277, 15061–15068. [Google Scholar] [CrossRef]
- Bengtsson, E.; Lindblom, K.; Tillgren, V.; Aspberg, A. The leucine-rich repeat protein PRELP binds fibroblast cell-surface proteoglycans and enhances focal adhesion formation. Biochem. J. 2016, 473, 1153–1164. [Google Scholar] [CrossRef]
- Tsuru, M.; Soejima, T.; Shiba, N.; Kimura, K.; Sato, K.; Toyama, Y.; Nagata, K. Proline/arginine-rich end leucine-rich repeat protein converts stem cells to ligament tissue and Zn(II) influences its nuclear expression. Stem Cells Dev. 2013, 22, 2057–2070. [Google Scholar] [CrossRef]
- Pillai, V.S.; Kundargi, R.R.; Edathadathil, F.; Nair, S.; Thilak, J.; Mathew, R.A.; Xavier, T.; Shenoy, P.; Menon, K.N. Identification of prolargin expression in articular cartilage and its significance in rheumatoid arthritis pathology. Int. J. Biol. Macromol. 2018, 110, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Versteegden, E.E.; Nagarajan, R.; Beggs, M.L.; Bearden, E.D.; Simpson, P.M.; Peterson, C.A. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1263–R1273. [Google Scholar] [CrossRef] [PubMed]
- Al-Astal, H.I.; Massad, M.; AlMatar, M.; Ekal, H. Cellular Functions of RNA-Binding Motif Protein 3 (RBM3): Clues in Hypothermia, Cancer Biology and Apoptosis. Protein Pept. Lett. 2016, 23, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, A.C.; Sharma, A.; Yang, W.L.; Nicastro, J.; Coppa, G.F.; Wang, P. Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner. Cell. Mol. Immunol. 2018, 15, 38–47. [Google Scholar] [CrossRef]
- Qiang, X.; Yang, W.L.; Wu, R.; Zhou, M.; Jacob, A.; Dong, W.; Kuncewitch, M.; Ji, Y.; Yang, H.; Wang, H.; et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat. Med. 2013, 19, 1489–1495. [Google Scholar] [CrossRef]
- Yang, W.L.; Sharma, A.; Wang, Z.; Li, Z.; Fan, J.; Wang, P. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci. Rep. 2016, 6, 26571. [Google Scholar] [CrossRef]
- Ode, Y.; Aziz, M.; Wang, P. CIRP increases ICAM-1(+) phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J. Leukoc. Biol. 2018, 103, 693–707. [Google Scholar] [CrossRef]
- Thant, L.; Kaku, M.; Kakihara, Y.; Mizukoshi, M.; Kitami, M.; Arai, M.; Kitami, K.; Kobayashi, D.; Yoshida, Y.; Maeda, T. Extracellular matrix-oriented proteomic analysis of periodontal ligament under mechanical stress. Front. Physiol. 2022, 13, 899699. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef]


| Protein Symbol | Description | Fold-Change |
|---|---|---|
| Upregulated in OTM | ||
| Prelp | Prolargin | 7,867,141.88 |
| Downregulated in OTM | ||
| D3zn79 | Similar to 60S ribosomal protein L35 | −36,595,282.2 |
| S10a4 | Protein S100-A4 | −34,574,876.8 |
| D3zku5 | Similar to ribosomal protein L31 | −20,157,790.6 |
| Q9wuh9 | Fibrillin-2 | −18,039,348.1 |
| Aqp1 | Aquaporin-1 | −15,943,980 |
| Crip1 | Cysteine-rich protein 1 | −14,228,021.1 |
| D3zjd3 | Similar to ribosomal protein L28 | −11,804,227.7 |
| Hmgb2 | High mobility group protein B2 | −10,598,886 |
| A0a0h2uhg7 | 40S ribosomal protein S20 | −10,152,529 |
| Imb1 | Importin subunit beta-1 | −9,620,749.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcantonio, C.C.; Lopes, M.E.S.; Soares, L.F.F.; Salmon, C.R.; Nociti Junior, F.H.; Deschner, J.; Nogueira, A.V.B.; Cirelli, J.A. Proteomic Analysis of the Periodontal Ligament During Orthodontic Movement: A Study in Rats. Proteomes 2025, 13, 42. https://doi.org/10.3390/proteomes13030042
Marcantonio CC, Lopes MES, Soares LFF, Salmon CR, Nociti Junior FH, Deschner J, Nogueira AVB, Cirelli JA. Proteomic Analysis of the Periodontal Ligament During Orthodontic Movement: A Study in Rats. Proteomes. 2025; 13(3):42. https://doi.org/10.3390/proteomes13030042
Chicago/Turabian StyleMarcantonio, Camila Chierici, Maria Eduarda Scordamaia Lopes, Lélio Fernando Ferreira Soares, Cristiane Ribeiro Salmon, Francisco Humberto Nociti Junior, James Deschner, Andressa Vilas Boas Nogueira, and Joni Augusto Cirelli. 2025. "Proteomic Analysis of the Periodontal Ligament During Orthodontic Movement: A Study in Rats" Proteomes 13, no. 3: 42. https://doi.org/10.3390/proteomes13030042
APA StyleMarcantonio, C. C., Lopes, M. E. S., Soares, L. F. F., Salmon, C. R., Nociti Junior, F. H., Deschner, J., Nogueira, A. V. B., & Cirelli, J. A. (2025). Proteomic Analysis of the Periodontal Ligament During Orthodontic Movement: A Study in Rats. Proteomes, 13(3), 42. https://doi.org/10.3390/proteomes13030042






