Bidirectional Interaction Between PGE2-Preconditioned Mesenchymal Stem Cells and Myofibroblasts Mediates Anti-Fibrotic Effects: A Proteomic Investigation into Equine Endometrial Fibrosis Reversal
Abstract
1. Introduction
2. Materials and Methods
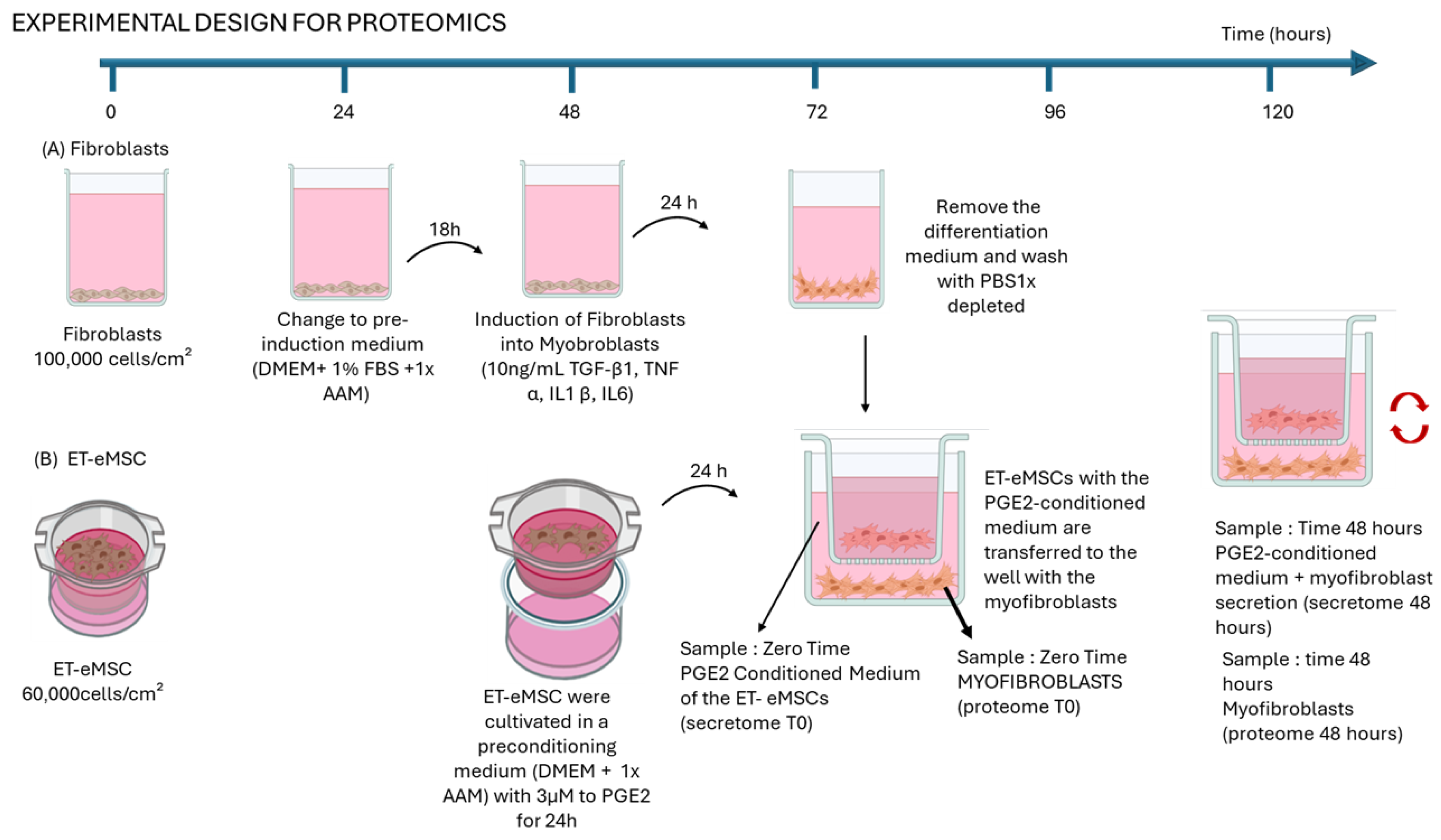
2.1. Experimental Design
2.1.1. Experiment 1: Proteomic Analysis of the Interaction of Endometrial Myofibroblasts with PGE2-Conditioned Equine Mesenchymal Stem Cells Derived from Endometrial Tissue (ET-eMSCs) for the Study of Endometrosis
2.1.2. Induction of Myofibroblasts from Endometrial Fibroblasts
2.1.3. Culture to ET-eMSC
2.1.4. Co-Culture of Myofibroblasts and ET-eMSCs Preconditioned with PGE2
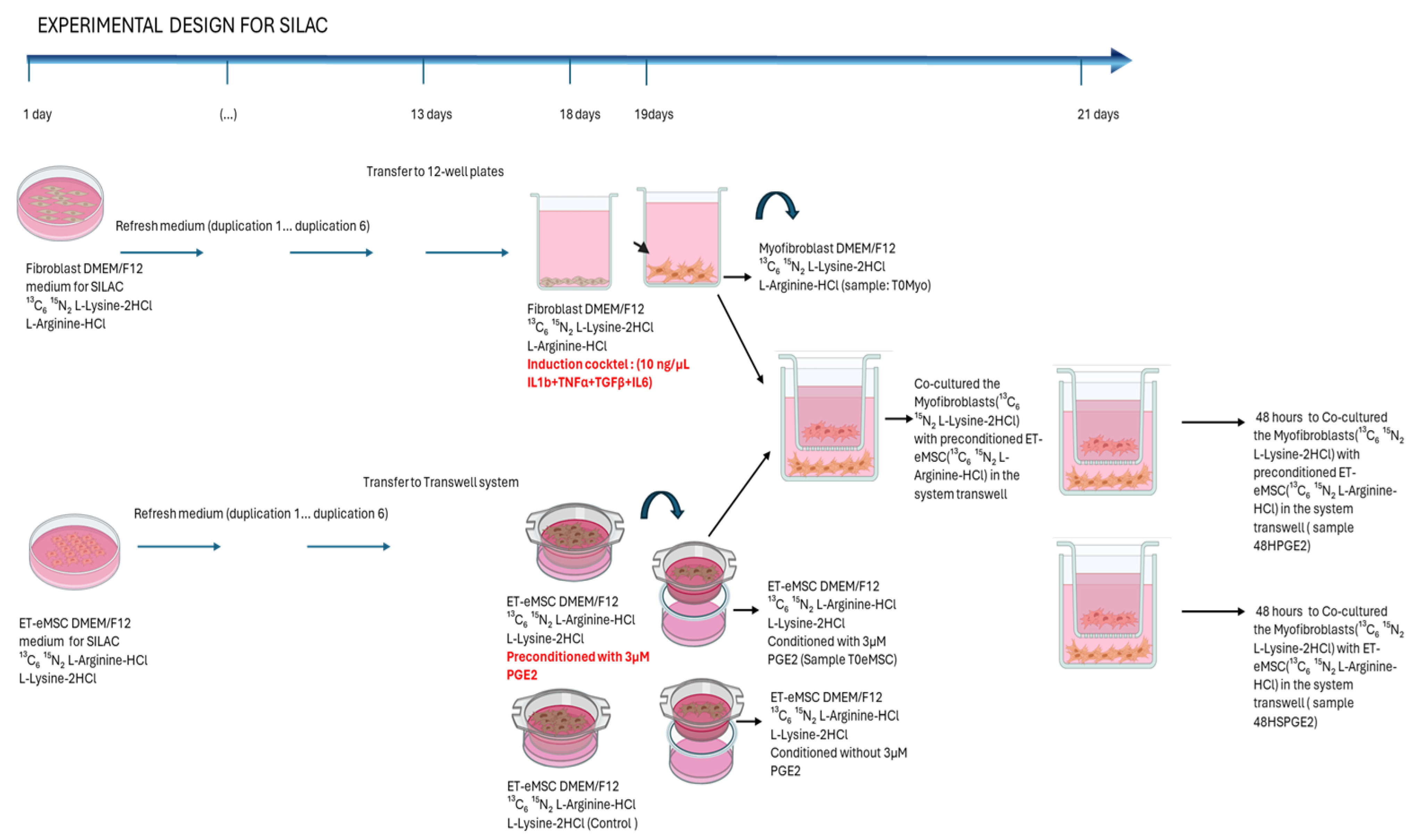
2.2. Experiment 2: Canonical Protein Matching Databases and Relative Quantification of the Interaction Between Endometrial Myofibroblasts and PGE2-Conditioned ET-eMSCs Were Conducted Using the SILAC Protein Quantitation Kit (Trypsin)
2.2.1. Induction of Myofibroblasts from Endometrial Fibroblasts
2.2.2. ET-eMSC Preconditioning with PGE2
2.2.3. Co-Cultures
2.3. Protein Extraction and Preparation for Mass Spectrometry
2.4. Protein Database Matching and Relative Quantification
SILAC-Based Protein Quantification
2.5. Bioinformatics Analysis for the SILAC Experiment
- Secretome at different times: secretome 48 h (MYO-ET-eMSC + PGE2) vs. secretome T0 (ET-eMSC + PGE2).
- Myofibroblast at different times: proteome 48 h vs. proteome T0.
3. Results
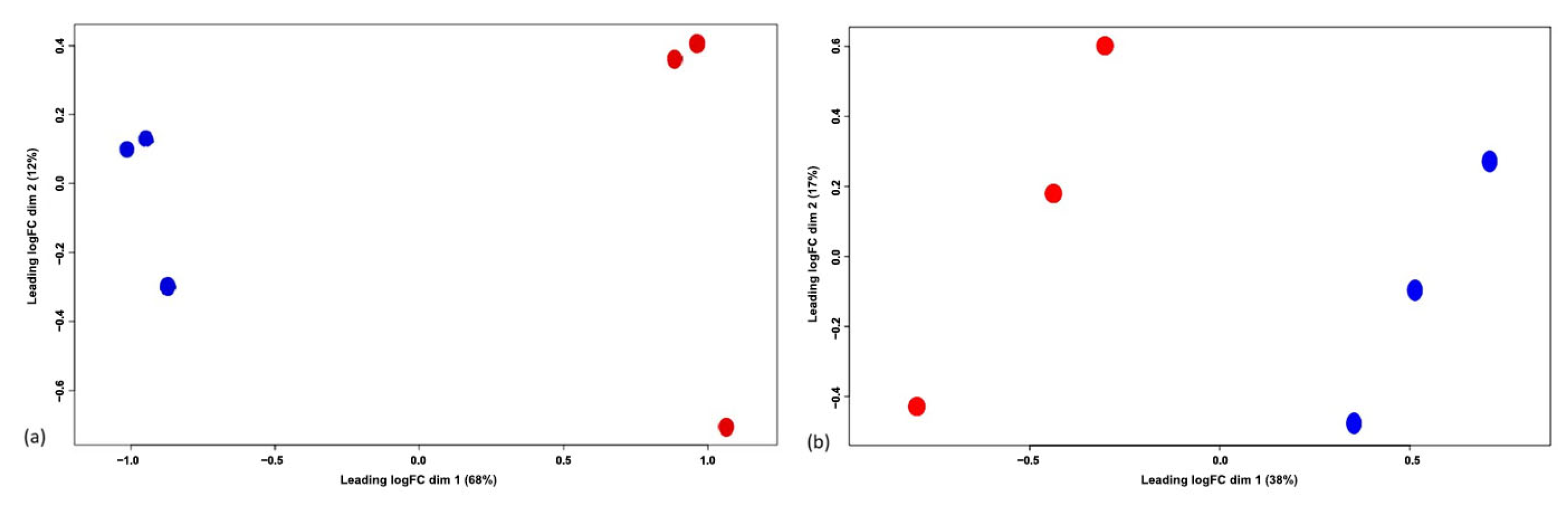
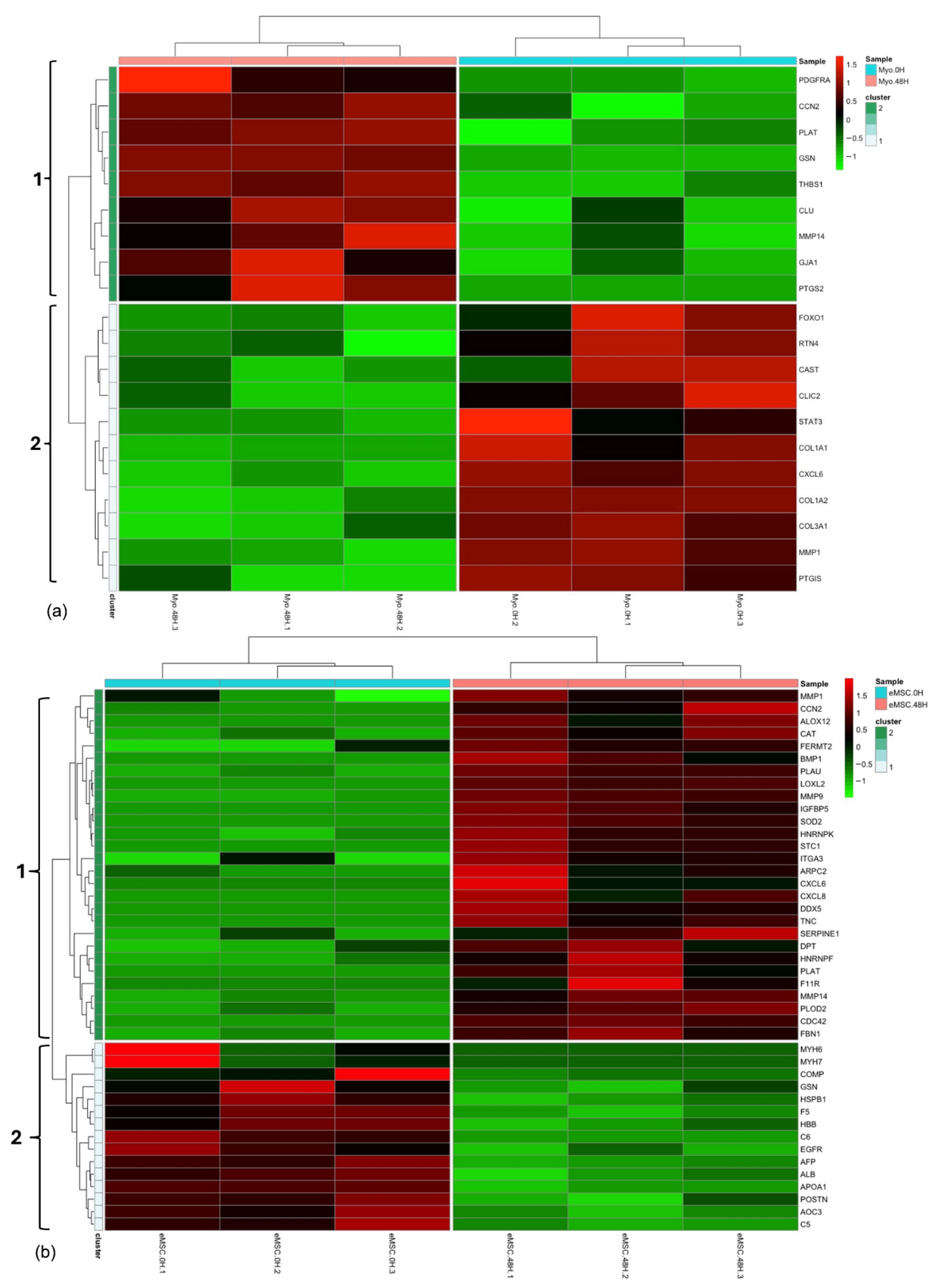
3.1. Experiment 1: Analysis of Differential Protein Abundance During Co-Culture of Myofibroblasts with PGE2-Preconditioned ET-eMSC
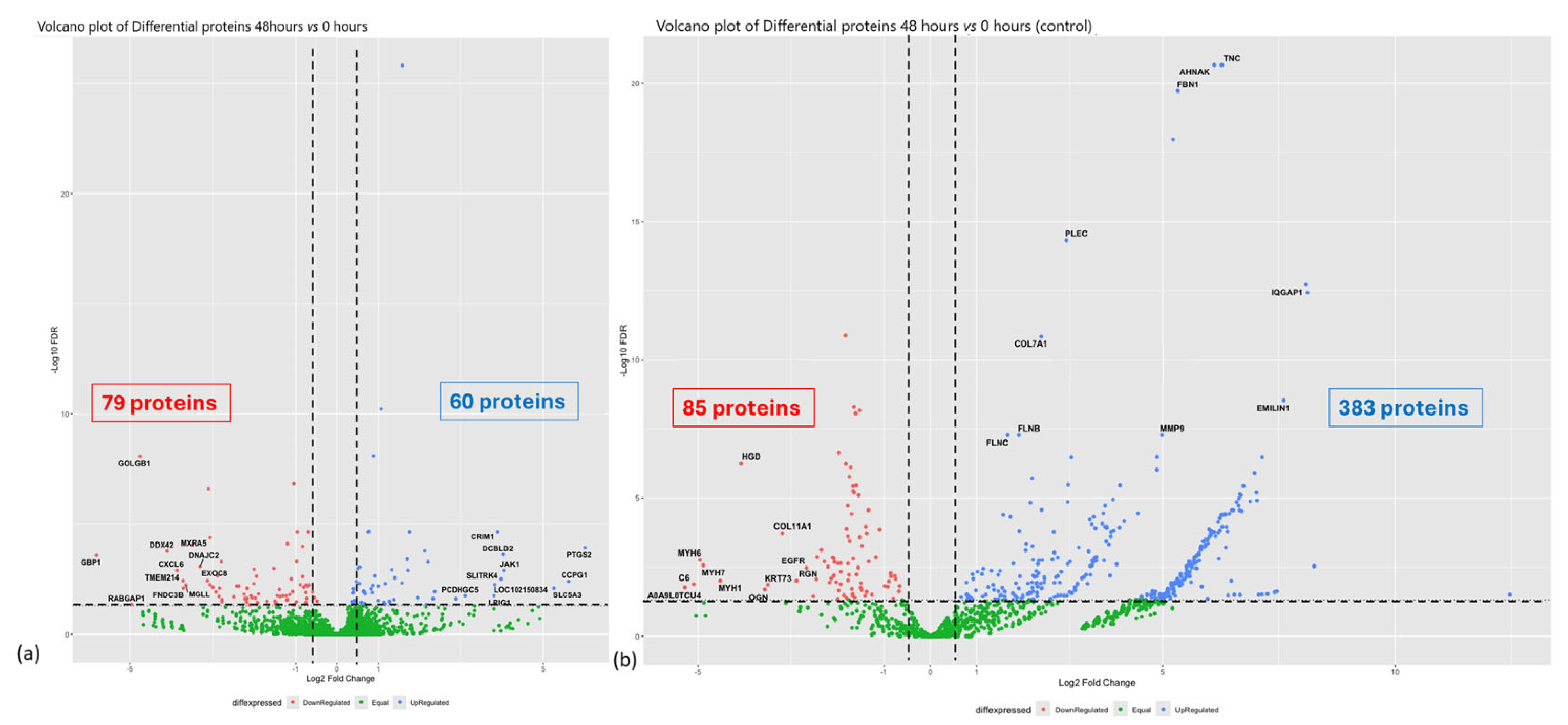
3.1.1. Gene Ontology and Reactome Pathways Enrichment of DAPs in the Secretome of ET-eMSC
3.1.2. Gene Ontology and Reactome Pathways Enrichment of DAPs in the Myofibroblast
3.1.3. Enrichment Analysis of Fibrosis-Related Proteins in Myofibroblasts and Secretome of ET-eMSC
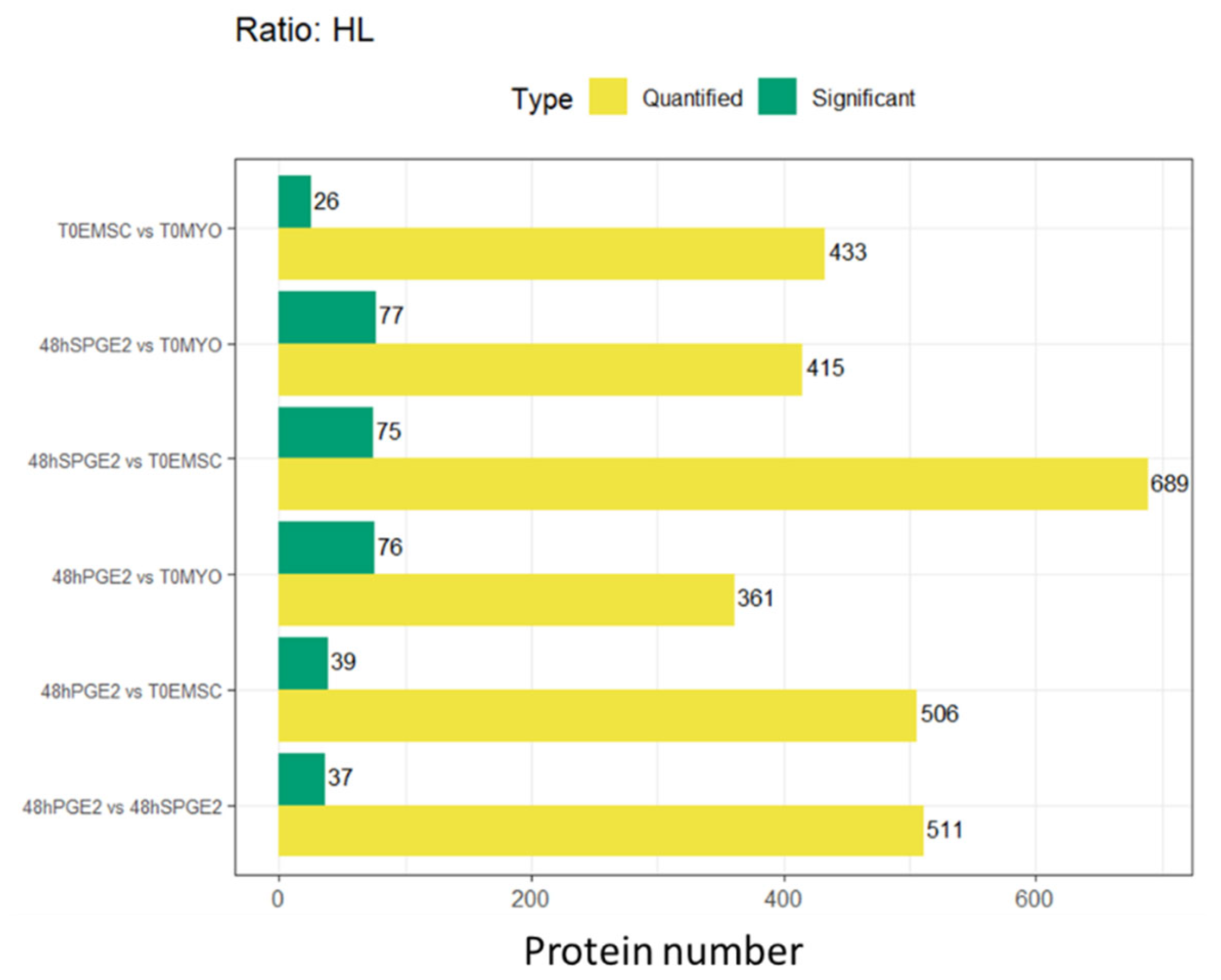
3.2. Experiment 2: SILAC Proteomic Analyses of Myofibroblasts Co-Cultured with Equine Endometrial-Derived MSCs (ET-eMSCs) Preconditioned with PGE2 Reveal Bidirectional Interchange Transfer of Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 48Hpge2 | 48 h with PGE2 (contextual) |
| AAM | Antibiotic-Antimycotic Solution |
| Akt | Proteins Kinase B (PKB) |
| Arg | Arginine |
| ARPC2 | Arp2/3 complex 34 kDa subunit |
| AT-eMSCs | Adipose tissue-derived mesenchymal stem cells |
| AU-rich elements | Elementos ricos en adenina y uracilo |
| AUF1 | AU-rich element RNA-binding factor 1 (hnRNP D0) |
| BP | Biological Process |
| C5 | Complement component 5 |
| C6 | Complement component 6 |
| cAMP | Cyclic adenosine monophosphate |
| CC | Cellular Component |
| CCN1 | Cellular Communication Network Factor 1 |
| CCN2 | Cellular Communication Network Factor 2 |
| CCN2/CTGF | Cellular communication network factor 2/Connective Tissue Growth Factor |
| CLU | Clusterin alpha chain |
| CO2 | Dióxido de carbono |
| COL1A1 | Collagen type I alpha 1 |
| COL2A1 | Collagen type II alpha 1 |
| COL3A1 | Collagen type III alpha 1 |
| COL5A1 | Collagen type V alpha 1 |
| COL7A1 | Collagen type VII alpha 1 |
| COMP | Cartilage oligomeric matrix protein |
| COX-2/PTGS2 | Cyclooxygenase-2/Prostaglandin-endoperoxide synthase 2 |
| CTGF | Connective Tissue Growth Factor |
| CTSK | Cathepsin K |
| CXCL6 | C-X-C motif chemokine 6 |
| CXCL8 | C-X-C motif chemokine 8 |
| DAPs | Differentially Abundant Proteins |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DTT | Dithiothreitol |
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| eMSC.0H | ET-eMSC preconditioned with PGE2 (contextual) |
| eMSC.48H | ET-eMSC after 48 h of co-culture with myofibroblasts (contextual) |
| eMSCs | equine MSCs |
| EP2 | Prostaglandin E2 receptor subtype 2 |
| ER-to-Golgi | Endoplasmic Reticulum to Golgi |
| ET-eMSC | Equine endometrium-derived mesenchymal stem cells |
| ET-eMSCs | Equine mesenchymal stem cells derived from endometrial tissue |
| ET-eMSCs | equine endometrial-derived MSCs |
| F11R | Junctional adhesion molecule A |
| F5 | Factor V |
| FBS | Fetal Bovine Serum |
| FC | Fold-Change |
| FDR | False Discovery Rate |
| FERMT2 | FERM domain containing kindlin 2 |
| FOXO1 | Forkhead box protein O1 |
| FWHM | Full Width at Half Maximum |
| GJA1 | Gap junction protein |
| GO | Gene Ontology |
| GSN | Gelsolin |
| h | hour(s) |
| H/L | Heavy/Light (ratio) |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| hnRNP D0 | Heterogeneous Nuclear Ribonucleoprotein D0 |
| HPLC | High-Performance Liquid Chromatography |
| IGF | Insulin-like Growth Factor |
| IGFBPs | IGF Binding Proteins |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| JAK1 | Janus Kinase 1 |
| K0/R0 | Unlabeled lysine/arginine (SILAC labels) |
| K8/R10 | Lysine-8/Arginine-10 (SILAC labels) |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS/MS | Liquid Chromatography Tandem-Mass Spectrometry |
| logFC | Logarithm of Fold Change |
| LOXL2 | Lysyl oxidase homolog 2 |
| LUM | Lumican |
| Lys | Lysine |
| m/z | Masa por carga (mass-to-charge ratio) |
| MAPK | Mitogen-Activated Protein Kinase |
| MCL | Markov Clustering |
| MF | Molecular Function |
| MMP-1 | Matrix metalloproteinase-1 |
| MMP-14 | Matrix metalloproteinase-14 |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-9 | Matrix metalloproteinase-9 |
| MMP/TIMP | Matrix metalloproteinase/Tissue Inhibitor of metalloproteinases |
| MMPs | Matrix metalloproteinase |
| mRNA | messenger RNA |
| MS | Mass Spectrometry |
| MSCs | Mesenchymal Stem Cells |
| MXR5 | Matrilysin 5 |
| MXR8 | Matrilysin 8 |
| MXRA5 | Matrix Remodeling Associated 5 |
| MXRA8 | Matrix Remodeling Associated 8 |
| MYO-ET-eMSC | Myofibroblasts co-cultured with ET-eMSCs (contextual) |
| Myo.0H | Myofibroblast samples at 0 h (contextual) |
| Myo.48H | Myofibroblast samples at 48 h (contextual) |
| NaCl | Cloruro de sodio |
| PAI-1/SERPINE1 | Plasminogen Activator Inhibitor-1/Serpin Family E Member 1 |
| PBS | Phosphate-Buffered Saline |
| PCA | Principal Component Analysis |
| PCR | Polymerase Chain Reaction |
| PDGF | Platelet-Derived Growth Factor |
| PDGFRα | Platelet-derived growth factor receptor alpha |
| PGE2 | Prostaglandin E2 |
| PI3K/AKT | Phosphoinositide 3-kinase/Protein Kinase B |
| PKA | ProteinKinase A |
| PLAT | Plasminogen activator, urokinase-type |
| PLAU | Urokinase-type plasminogen activator |
| PPI | Protein-ProteinInteraction |
| PPM | Parts per million |
| PTGIS | Prostacyclin synthase |
| PTGS2 | Prostaglandin G/H synthase 2 |
| PTM | Post-Translational Modifications |
| Rho/ROCK | Rho-associated protein kinase |
| SGCA | Sarcoglycan alpha |
| SILAC | Stable isotope labeling by amino acids in cell culture |
| SPD | Samples per Day |
| sPGE2 | Sin PGE2 (referring to conditioned medium without PGE2) (contextual) |
| STAT3 | Signal transducer and activator of transcription 3 |
| T0EMSC | ET-eMSCs at time zero (contextual) |
| T0MYO | Myofibroblasts at time zero (contextual) |
| TGF-β1 | Transforming Growth Factor-beta 1 |
| THBS1 | Thrombospondin-1 |
| THBS2 | Thrombospondin 2 |
| TIMP-1 | Tissue Inhibitor of Metalloproteinase-1 |
| TIMP-2 | Tissue Inhibitor of Metalloproteinase-2 |
| TMM | Trimmed Mean of M-values |
| TNF-α | Tumor Necrosis Factor-alpha |
| TriC/CCT | TCP-1 Ring Complex/Chaperonin Containing TCP-1 |
| uPA | Urokinase-type plasminogen activator |
| YAP/TAZ | Yes-associated protein/Transcriptional coactivator with PDZ-binding motif |
| α-SMA | α-smooth muscle actin |
| µL | microliters |
| μM | micromolar |
References
- Trundell, D.A. Mare Can Teach Us When Dealing. In Endometriosis: Recent Advances, New Perspectives and Treatments; Intechopen: London, UK, 2022. [Google Scholar]
- Buczkowska, J.; Kozdrowski, R.; Nowak, M.; Ras, A.; Mrowiec, J. Endometrosis-significance for horse reproduction, pathogenesis, diagnosos, and proposed therapeutic methods. Pol. J. Vet. Sci. 2014, 17, 547–554. [Google Scholar] [CrossRef]
- Hanada, M.; Maeda, Y.; Oikawa, M.A. Histopathological characteristics of endometrosis in thoroughbred mares in Japan: Results from 50 necropsy cases. J. Equine Sci. 2014, 25, 45–52. [Google Scholar] [CrossRef]
- Ferreira-Dias, G.; Rebordão, M.; Galvão, A.; Roberto-da-Costa, R.; Amaral, A.; Fernandes, C.; Pinto-Bravo, P.; Morazzo, S.; Alexandre-Pires, G.; Lukasik, K. What goes wrong from a mare healthy endometrium to endometrosis? In Advances in Animal Health, Medicine and Production: A Research Portrait of the Centre for Interdisciplinary Research in Animal Health (CIISA), University of Lisbon, Portugal; Springer: Cham, Switzerland, 2020; pp. 528–540. [Google Scholar]
- Rebordao, M.R.; Galvao, A.; Szostek, A.; Amaral, A.; Mateus, L.; Skarzynski, D.J.; Ferreira-Dias, G. Physiopathologic mechanisms involved in mare endometrosis. Reprod. Domest. Anim. 2014, 49 (Suppl. S4), 82–87. [Google Scholar] [CrossRef]
- Hoffmann, C.; Ellenberger, C.; Mattos, R.C.; Aupperle, H.; Dhein, S.; Stief, B.; Schoon, H.A. The equine endometrosis: New insights into the pathogenesis. Anim. Reprod. Sci. 2009, 111, 261–278. [Google Scholar] [CrossRef]
- Gerarduzzi, C.; Di Battista, J.A. Myofibroblast repair mechanisms post-inflammatory response: A fibrotic perspective. Inflamm. Res. 2017, 66, 451–465. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Morris, L.H.; McCue, P.; Aurich, C. Equine endometritis: A review of challenges and new approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef] [PubMed]
- Mambelli, L.I.; Mattos, R.C.; Winter, G.H.; Madeiro, D.S.; Morais, B.P.; Malschitzky, E.; Miglino, M.A.; Kerkis, A.; Kerkis, I. Changes in expression pattern of selected endometrial proteins following mesenchymal stem cells infusion in mares with endometrosis. PLoS ONE 2014, 9, e97889. [Google Scholar] [CrossRef] [PubMed]
- Mambelli, L.I.; Winter, G.H.; Kerkis, A.; Malschitzky, E.; Mattos, R.C.; Kerkis, I. A novel strategy of mesenchymal stem cells delivery in the uterus of mares with endometrosis. Theriogenology 2013, 79, 744–750. [Google Scholar] [CrossRef]
- Rezakhani, L.; Kelishadrokhi, A.F.; Soleimanizadeh, A.; Rahmati, S. Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients Infected with COVID-19: Real opportunities and range of promises. Chem. Phys. Lipids 2021, 234, 105009. [Google Scholar] [CrossRef]
- Perrini, C.; Strillacci, M.G.; Bagnato, A.; Esposti, P.; Marini, M.G.; Corradetti, B.; Bizzaro, D.; Idda, A.; Ledda, S.; Capra, E.; et al. Microvesicles secreted from equine amniotic-derived cells and their potential role in reducing inflammation in endometrial cells in an in-vitro model. Stem Cell Res. Ther. 2016, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; Saravia, F.; Cisterna, G.; Rojas, F.; Silva, P.P.; Rodriguez-Alvarez, L.; Rojas, D.; Cabezas, J.; Mancanares, A.C.F.; Castro, F.O. Assessment of the anti-inflammatory and engraftment potential of horse endometrial and adipose mesenchymal stem cells in an in vivo model of post breeding induced endometritis. Theriogenology 2020, 155, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, J.; Rojas, D.; Wong, Y.; Telleria, F.; Manriquez, J.; Mancanares, A.C.F.; Rodriguez-Alvarez, L.L.; Castro, F.O. In vitro preconditioning of equine adipose mesenchymal stem cells with prostaglandin E(2), substance P and their combination changes the cellular protein secretomics and improves their immunomodulatory competence without compromising stemness. Vet. Immunol. Immunopathol. 2020, 228, 110100. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.S.; Mancanares, A.C.; Navarrete, F.; Poblete, P.; Mendez-Perez, L.; Cabezas, J.; Riadi, G.; Rodriguez-Alvarez, L.; Castro, F.O. Extracellular vesicles secreted by equine adipose mesenchymal stem cells preconditioned with transforming growth factor beta-1 are enriched in anti-fibrotic miRNAs and inhibit the expression of fibrotic genes in an in vitro system of endometrial stromal cells fibrosis. Vet. Q. 2024, 44, 1–11. [Google Scholar] [CrossRef]
- Wong, Y.S.; Mancanares, A.C.; Navarrete, F.; Poblete, P.; Mendez-Perez, L.; Rodriguez-Alvarez, L.; Castro, F.O. Short preconditioning with TGFbeta of equine adipose tissue-derived mesenchymal stem cells predisposes towards an anti-fibrotic secretory phenotype: A possible tool for treatment of endometrosis in mares. Theriogenology 2024, 225, 119–129. [Google Scholar] [CrossRef]
- Mendez-Perez, L.; Ibanez, B.O.; Rodriguez, S.; Sen Wong, Y.; Caamano, D.; Navarrete, F.I.; Cabezas, J.; Mancanares, A.C.; Escudero, C.; Rodriguez-Alvarez, L.; et al. Extracellular Vesicles Derived From Endometrial Stem Cells Preconditioned With PGE2-Reverse Myofibroblast Phenotype in Mare Endometrial Cells: A Novel Anti-Fibrotic Approach. Mol. Reprod Dev. 2025, 92, e70053. [Google Scholar] [CrossRef]
- Cabezas, J.; Rojas, D.; Navarrete, F.; Ortiz, R.; Rivera, G.; Saravia, F.; Rodriguez-Alvarez, L.; Castro, F.O. Equine mesenchymal stem cells derived from endometrial or adipose tissue share significant biological properties, but have distinctive pattern of surface markers and migration. Theriogenology 2018, 106, 93–102. [Google Scholar] [CrossRef]
- Kenney, R.; Doig, P. Equine endometrial biopsy. Curr. Ther. Theriogenology 1986, 2, 723–729. [Google Scholar]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vazquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martin-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.S.; Mancanares, A.C.; Navarrete, F.I.; Poblete, P.M.; Mendez-Perez, L.; Ferreira-Dias, G.M.L.; Rodriguez-Alvarez, L.; Castro, F.O. Mare stromal endometrial cells differentially modulate inflammation depending on oestrus cycle status: An in vitro study. Front. Vet. Sci. 2023, 10, 1271240. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.T.; Leprevost, F.V.; Avtonomov, D.M.; Mellacheruvu, D.; Nesvizhskii, A.I. MSFragger: Ultrafast and comprehensive peptide identification in mass spectrometry-based proteomics. Nat Methods 2017, 14, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, D.; Liu, J.; Xu, H.; Liu, Y.; Li, Y.; Diao, L.; Wang, X.; Wang, D.; Tian, L.; et al. FibroAtlas: A Database for the Exploration of Fibrotic Diseases and Their Genes. Cardiol. Res. Pract 2019, 2019, 4237285. [Google Scholar] [CrossRef]
- Alpoim-Moreira, J.; Fernandes, C.; Pimenta, J.; Bliebernicht, M.; Rebordao, M.R.; Castelo-Branco, P.; Szostek-Mioduchowska, A.; Skarzynski, D.J.; Ferreira-Dias, G. Metallopeptidades 2 and 9 genes epigenetically modulate equine endometrial fibrosis. Front. Vet. Sci. 2022, 9, 970003. [Google Scholar] [CrossRef]
- Alpoim-Moreira, J.; Fernandes, C.; Rebordao, M.R.; Costa, A.L.; Bliebernicht, M.; Nunes, T.; Szostek-Mioduchowska, A.; Skarzynski, D.J.; Ferreira-Dias, G. Collagen Type III as a Possible Blood Biomarker of Fibrosis in Equine Endometrium. Animals 2022, 12, 1854. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y. TGF-beta in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019, 9, 98. [Google Scholar] [CrossRef]
- Aresu, L.; Benali, S.; Giannuzzi, D.; Mantovani, R.; Castagnaro, M.; Falomo, M.E. The role of inflammation and matrix metalloproteinases in equine endometriosis. J. Vet. Sci. 2012, 13, 171–177. [Google Scholar] [CrossRef]
- Centeno, L.A.M.; Bastos, H.B.A.; Bueno, V.L.C.; Trentin, J.M.; Fiorenza, M.; Panziera, W.; Winter, G.H.Z.; Kretzmann, N.A.; Fiala-Rechsteiner, S.; Mattos, R.C.; et al. Collagen and collagenases in mare’s endometrium with endometrosis. Theriogenology 2024, 230, 28–36. [Google Scholar] [CrossRef]
- Oddsdóttir, C. Development of Endometrial Fibrosis in the Mare: Factors Involved in Tissue Remodelling and Collagen Deposition. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 2008. [Google Scholar]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Wipff, P.J.; Rifkin, D.B.; Meister, J.J.; Hinz, B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007, 179, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Garrison, G.; Huang, S.K.; Okunishi, K.; Scott, J.P.; Kumar Penke, L.R.; Scruggs, A.M.; Peters-Golden, M. Reversal of myofibroblast differentiation by prostaglandin E(2). Am. J. Respir. Cell Mol. Biol. 2013, 48, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, D.; Swindell, W.R.; Xia, W.; Weng, S.; Fu, J.; Worthen, C.A.; Okubo, T.; Johnston, A.; Gudjonsson, J.E.; et al. Age-Associated Increase in Skin Fibroblast-Derived Prostaglandin E2 Contributes to Reduced Collagen Levels in Elderly Human Skin. J. Investig. Dermatol. 2015, 135, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shu, B.; Chen, L.; Tang, J.; Zhang, L.; Xie, J.; Liu, X.; Xu, Y.; Qi, S. Prostaglandin E2 inhibits collagen synthesis in dermal fibroblasts and prevents hypertrophic scar formation in vivo. Exp. Dermatol. 2016, 25, 604–610. [Google Scholar] [CrossRef]
- Cilli, F.; Khan, M.; Fu, F.; Wang, J.H. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin. J. Sport Med. 2004, 14, 232–236. [Google Scholar] [CrossRef]
- Becerra, A.; Rojas, M.; Vallejos, A.; Villegas, V.; Perez, L.; Cabello-Verrugio, C.; Simon, F. Endothelial fibrosis induced by suppressed STAT3 expression mediated by signaling involving the TGF-beta1/ALK5/Smad pathway. Lab. Investig. 2017, 97, 1033–1046. [Google Scholar] [CrossRef]
- Zhao, J.; Qi, Y.F.; Yu, Y.R. STAT3: A key regulator in liver fibrosis. Ann. Hepatol. 2021, 21, 100224. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, S.J.; Hahn, Y.I.; Yoon, H.J.; Han, B.; Kim, K.; Lee, S.; Kim, K.P.; Suh, Y.G.; Na, H.K.; et al. 15-Keto prostaglandin E(2) suppresses STAT3 signaling and inhibits breast cancer cell growth and progression. Redox Biol. 2019, 23, 101175. [Google Scholar] [CrossRef]
- Bozyk, P.D.; Moore, B.B. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 445–452. [Google Scholar] [CrossRef]
- Stamov, D.R.; Muller, A.; Wegrowski, Y.; Brezillon, S.; Franz, C.M. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J. Struct. Biol. 2013, 183, 394–403. [Google Scholar] [CrossRef]
- Rixon, C.; Andreassen, K.; Shen, X.; Erusappan, P.M.; Almaas, V.M.; Palmero, S.; Dahl, C.P.; Ueland, T.; Sjaastad, I.; Louch, W.E.; et al. Lumican accumulates with fibrillar collagen in fibrosis in hypertrophic cardiomyopathy. ESC Heart Fail. 2023, 10, 858–871. [Google Scholar] [CrossRef]
- Sweetwyne, M.T.; Murphy-Ullrich, J.E. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012, 31, 178–186. [Google Scholar] [CrossRef]
- Kubota, S.; Takigawa, M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin. Sci. 2015, 128, 181–196. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T. Connective Tissue Growth Factor in Idiopathic Pulmonary Fibrosis: Breaking the Bridge. Int. J. Mol. Sci. 2022, 23, 6064. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y. Periostin in Skin Tissue Skin-Related Diseases. Allergol. Int. 2014, 63, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Liu, X.; Wang, B.; Wei, S. The role of periostin in cardiac fibrosis. Heart Fail. Rev. 2024, 29, 191–206. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Aoki, H. Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front. Physiol. 2014, 5, 283. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wang, W.; Morales-Nebreda, L.; Feng, G.; Wu, M.; Zhou, X.; Lafyatis, R.; Lee, J.; Hinchcliff, M.; Feghali-Bostwick, C.; et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 2016, 7, 11703. [Google Scholar] [CrossRef]
- Rathaur, P.; Rodriguez, J.; Kuchtey, J.; Insignares, S.; Jones, W.B.; Kuchtey, R.W.; Bassnett, S. The Biomechanics of Fibrillin Microfibrils: Lessons from the Ciliary Zonule. Cells 2024, 13, 2097. [Google Scholar] [CrossRef]
- Kato, A.; Okamoto, O.; Wu, W.; Matsuo, N.; Kumai, J.; Yamada, Y.; Katagiri, F.; Nomizu, M.; Fujiwara, S. Identification of fibronectin binding sites in dermatopontin and their biological function. J. Dermatol. Sci. 2014, 76, 51–59. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.L.; Yang, H.X.; Liu, Q.P.; Rahman, K.; Zhang, H. CXCL6: A potential therapeutic target for inflammation and cancer. Clin. Exp. Med. 2023, 23, 4413–4427. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.J.; Kim, D.; Lu, M.; Farina, M.; Bowman, R.L.; Yang, J.L.; Park, Y.; Karzai, A.; Xiao, W.; Zaroogian, Z.; et al. CXCL8/CXCR2 signaling mediates bone marrow fibrosis and is a therapeutic target in myelofibrosis. Blood 2023, 141, 2508–2519. [Google Scholar] [CrossRef]
- Lawler, J. The functions of thrombospondin-1 and-2. Curr. Opin. Cell Biol. 2000, 12, 634–640. [Google Scholar] [CrossRef]
- Poveda, J.; Sanz, A.B.; Fernandez-Fernandez, B.; Carrasco, S.; Ruiz-Ortega, M.; Cannata-Ortiz, P.; Ortiz, A.; Sanchez-Niño, M.D. MXRA 5 is a TGF-β1-regulated human protein with anti-inflammatory and anti-fibrotic properties. J. Cell. Mol. Med. 2017, 21, 154–164. [Google Scholar] [CrossRef]
- Peng, S.Q.; Zhu, X.R.; Zhao, M.Z.; Zhang, Y.F.; Wang, A.R.; Chen, M.B.; Ye, Z.Y. Identification of matrix-remodeling associated 5 as a possible molecular oncotarget of pancreatic cancer. Cell Death Dis. 2023, 14, 157. [Google Scholar] [CrossRef]
- Ichihara, R.; Shiraki, Y.; Mizutani, Y.; Iida, T.; Miyai, Y.; Esaki, N.; Kato, A.; Mii, S.; Ando, R.; Hayashi, M.; et al. Matrix remodeling-associated protein 8 is a marker of a subset of cancer-associated fibroblasts in pancreatic cancer. Pathol. Int. 2022, 72, 161–175. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Song, L.; Yuan, F.; Yan, Y. Matrix Remodeling-Associated Protein 8 as a Novel Indicator Contributing to Glioma Immune Response by Regulating Ferroptosis. Front. Immunol. 2022, 13, 834595. [Google Scholar] [CrossRef]
- Kebschull, M.; Demmer, R.; Behle, J.H.; Pollreisz, A.; Heidemann, J.; Belusko, P.B.; Celenti, R.; Pavlidis, P.; Papapanou, P.N. Granulocyte chemotactic protein 2 (gcp-2/cxcl6) complements interleukin-8 in periodontal disease. J. Periodontal Res. 2009, 44, 465–471. [Google Scholar] [CrossRef]
- Gharaee-Kermani, M.; Kasina, S.; Moore, B.B.; Thomas, D.; Mehra, R.; Macoska, J.A. CXC-type chemokines promote myofibroblast phenoconversion and prostatic fibrosis. PLoS ONE 2012, 7, e49278. [Google Scholar] [CrossRef]
- Tan, A.B.; Kress, S.; Castro, L.; Sheppard, A.; Raghunath, M. Cellular re- and de-programming by microenvironmental memory: Why short TGF-beta1 pulses can have long effects. Fibrogenesis Tissue Repair. 2013, 6, 12. [Google Scholar] [CrossRef]
| Secretome Experiment 1 (Abundance) | Secretome Experiment 2 (in Donor Samples) | Origin Cell | ||||
|---|---|---|---|---|---|---|
| Gene Name | Secretome 48 h vs. 0 h | 48H PGE2 | 48H SPGE2 | T0MSC | T0MYO | |
| MMP1 | high abundance | + | + | + | + | both |
| MMP2 | Equal | + | + | + | + | both |
| MMP9 | high abundance | + | + | + | + | both |
| MMP14 | high abundance | + | + | - | + | both |
| TIMP1 | Equal | + | + | + | + | both |
| TIMP2 | Equal | + | + | + | + | both |
| MXRA5 | high abundance | + | + | - | + | both |
| MXRA8 | high abundance | + | + | - | - | ET-eMSC |
| CXCL6 | high abundance | + | + | - | + | MYO |
| CXCL8 | high abundance | + | + | - | - | both |
| C-C motif chemo kine | Equal | + | + | - | + | MYO |
| HMGB1 | Equal | + | + | - | - | both |
| THBS 1 | Equal | + | + | + | + | both |
| THBS 2 | Equal | + | + | + | + | both |
| PLAU | high abundance | + | + | + | + | both |
| SERPINE 1 | high abundance | + | + | + | + | both |
| CCN2/CTGF | high abundance | - | + | - | + | both |
| TGF β1 | Equal | - | + | - | - | both |
| INHBA | high abundance | + | + | - | + | both |
| PCOLCE | high abundance | + | + | + | + | both |
| PCOLCE2 | Equal | + | + | + | + | both |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Pérez, L.; Wong, Y.S.; Ibáñez, B.O.; Martinez-Hormaza, I.; Rodríguez-Álvarez, L.; Castro, F.O. Bidirectional Interaction Between PGE2-Preconditioned Mesenchymal Stem Cells and Myofibroblasts Mediates Anti-Fibrotic Effects: A Proteomic Investigation into Equine Endometrial Fibrosis Reversal. Proteomes 2025, 13, 41. https://doi.org/10.3390/proteomes13030041
Méndez-Pérez L, Wong YS, Ibáñez BO, Martinez-Hormaza I, Rodríguez-Álvarez L, Castro FO. Bidirectional Interaction Between PGE2-Preconditioned Mesenchymal Stem Cells and Myofibroblasts Mediates Anti-Fibrotic Effects: A Proteomic Investigation into Equine Endometrial Fibrosis Reversal. Proteomes. 2025; 13(3):41. https://doi.org/10.3390/proteomes13030041
Chicago/Turabian StyleMéndez-Pérez, Lidice, Yat Sen Wong, Belén O. Ibáñez, Ioanna Martinez-Hormaza, Lleretny Rodríguez-Álvarez, and Fidel Ovidio Castro. 2025. "Bidirectional Interaction Between PGE2-Preconditioned Mesenchymal Stem Cells and Myofibroblasts Mediates Anti-Fibrotic Effects: A Proteomic Investigation into Equine Endometrial Fibrosis Reversal" Proteomes 13, no. 3: 41. https://doi.org/10.3390/proteomes13030041
APA StyleMéndez-Pérez, L., Wong, Y. S., Ibáñez, B. O., Martinez-Hormaza, I., Rodríguez-Álvarez, L., & Castro, F. O. (2025). Bidirectional Interaction Between PGE2-Preconditioned Mesenchymal Stem Cells and Myofibroblasts Mediates Anti-Fibrotic Effects: A Proteomic Investigation into Equine Endometrial Fibrosis Reversal. Proteomes, 13(3), 41. https://doi.org/10.3390/proteomes13030041








