The Biological Variation in Serum ACE and CPN/CPB2 Activity in Healthy Individuals as Measured by the Degradation of Dabsylated Bradykinin—Reference Data and the Importance of Pre-Analytical Standardization
Abstract
1. Introduction
1.1. Bradykinin
1.2. Neuropeptide Reporter Assay
2. Materials and Methods
2.1. Sample Cohorts and Permissions
2.2. Method
3. Results and Discussion
3.1. External Standard
3.2. Variability in Sample Quality
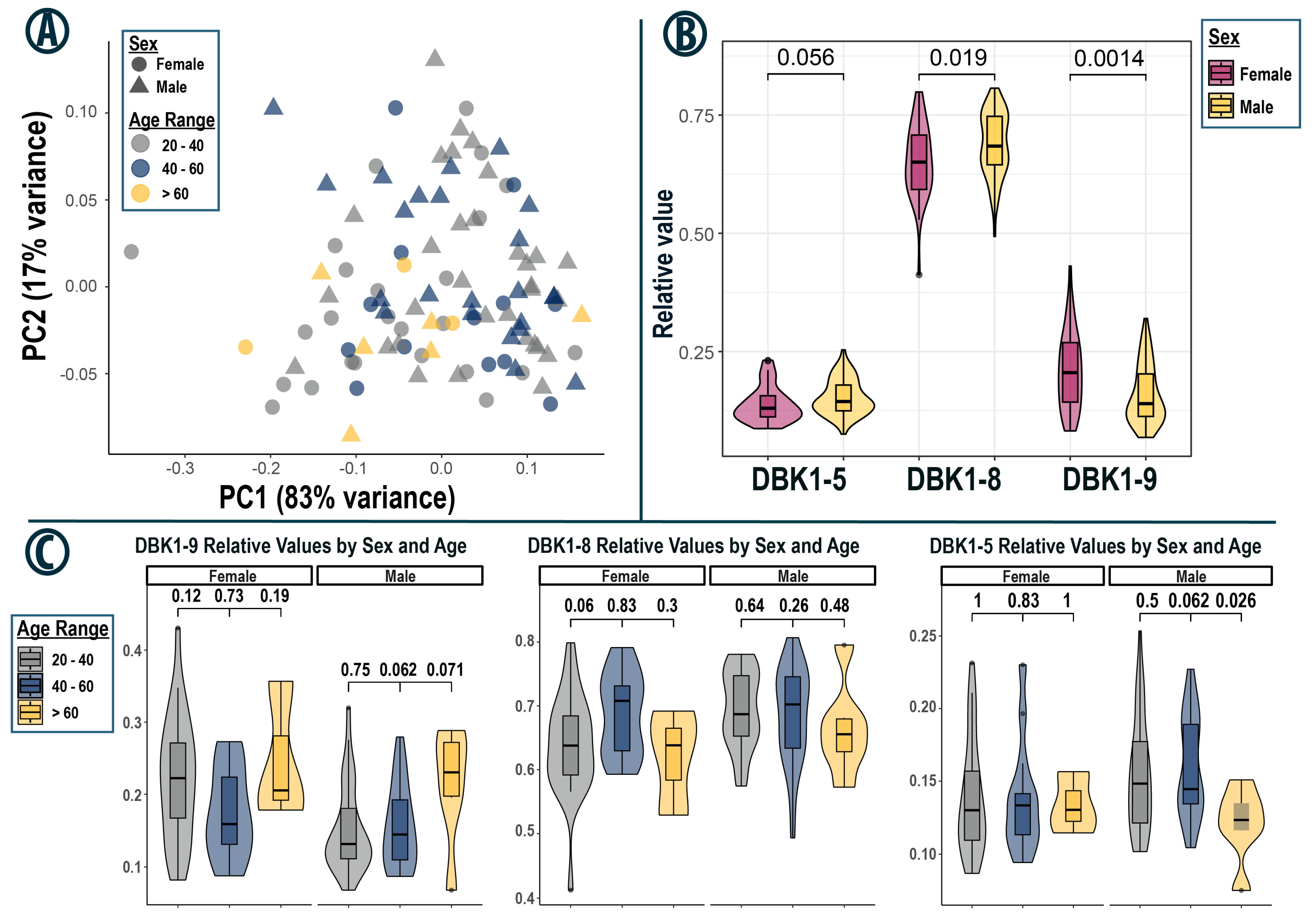
3.3. General Observations
| DBK 1-9 | Std. Dev DBK1-9 | DBK 1-8 | Std. Dev DBK1-8 | DBK 1-5 | Std. Dev DBK1-5 | |
|---|---|---|---|---|---|---|
| Male | ||||||
| Excluding hemolytic samples | 0.193 | 0.086 | 0.649 | 0.089 | 0.158 | 0.046 |
| Taipei 2016 | 0.159 | 0.062 | 0.688 | 0.068 | 0.153 | 0.037 |
| Orlando 2018 | 0.161 | 0.072 | 0.679 | 0.073 | 0.160 | 0.047 |
| Female | ||||||
| Excluding hemolytic samples | 0.234 | 0.089 | 0.618 | 0.092 | 0.148 | 0.045 |
| Taipei 2016 | 0.209 | 0.082 | 0.653 | 0.077 | 0.138 | 0.038 |
| Orlando 2018 | 0.215 | 0.067 | 0.633 | 0.016 | 0.153 | 0.061 |
3.4. Subset Analysis—Taipei
3.5. Subset Analysis—Orlando
3.6. Subset Analysis—Dublin/California
3.7. Statistical Considerations
3.8. ACE-Specific Inhibition
3.9. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ACE2 | Angiotensin-converting enzyme 2 |
| AD | Atopic dermatitis |
| Ang II | Angiotensin II |
| ANOVA | Analysis of variance |
| APP | Aminopeptidase P |
| AT1R, AT2R | Angiotensin receptor 1 and 2 |
| BK | Bradykinin |
| BMI | Body mass index |
| COVID-19 | Coronavirus disease 2019 |
| CPN, CPB2 | Carboxypeptidases N and B2 |
| CRPS | Complex Regional Pain Syndrome |
| DBK or DBK1-9 | Dabsylated bradykinin |
| DBK1-5, DBK1-8 | Enzymatic fragments of DBK |
| HEPES | 2-(4-(2-Hydroxyethyl)-1-piperazinyl)-ethansulfonic acid |
| hPOP | human Personal Omics Profiling, international consortium “Community-Based Personalized Omics Profiling to Assess the Population’s Omics Variation and Dynamics” |
| HUPO | Human Proteome Organisation |
| KKS | Kinin–kallikrein system |
| NIST | National Institute for Standards and Technology |
| NRA | Neuropeptide reporter assay |
| PCA | Principle components analysis |
| RAS | Renin–angiotensin system |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| Std. Dev. | Standard deviation |
| TLC | Thin-layer chromatography |
References
- Rex, D.A.B.; Deepak, K.; Vaid, N.; Dagamajalu, S.; Kandasamy, R.K.; Flo, T.H.; Keshava Prasad, T.S. A modular map of bradykinin-mediated inflammatory signaling network. J. Cell Commun. Signal. 2022, 16, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Oudit, G.Y.; Verano-Braga, T.; Canta, G.; Steckelings, U.M.; Bader, M. The renin-angiotensin system: Going beyond the classical paradigms. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H958–H970. [Google Scholar] [CrossRef]
- da Graca Naffah-Mazzacoratti, M.; Gouveia, T.L.F.; Simoes, P.S.R.; Perosa, S.R. What have we learned about the kallikrein-kinin and renin-angiotensin systems in neurological disorders? World J. Biol. Chem. 2014, 5, 130–140. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- de Carvalho, P.R.; Sirois, P.; Fernandes, P.D. The role of kallikrein-kinin and renin-angiotensin systems in COVID-19 infection. Peptides 2021, 135, 170428. [Google Scholar] [CrossRef] [PubMed]
- Viel, T.A.; Buck, H.S. Kallikrein-kinin system mediated inflammation in Alzheimer’s disease in vivo. Curr. Alzheimer Res. 2011, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Schieffer, E.; Schieffer, B. The race for ACE: Targeting angiotensin-converting enzymes (ACE) in SARS-CoV-2 infection. J. Renin-Angiotensin-Aldosterone Syst. 2022, 2022, 2549063. [Google Scholar] [CrossRef]
- König, S.; Vollenberg, R.; Tepasse, P. The renin-angiotensin-system in COVID-19: Can Long COVID be predicted? Life 2023, 13, 1462. [Google Scholar] [CrossRef]
- Tabassum, A.; Iqbal, M.S.; Sultan, S.; Alhuthali, R.A.; Alshubaili, D.I.; Sayyam, R.S.; Abyad, L.M.; Qasem, A.H.; Arbaeen, A.F. Dysregulated bradykinin: Mystery in the pathogenesis of COVID-19. Mediat. Inflamm. 2022, 2022, 7423537. [Google Scholar] [CrossRef]
- Coates, D. The angiotensin converting enzyme. Int. J. Biochem. Cell Biol. 2003, 35, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.W.; Mueller-Ortiz, S.L.; Wetsel, R.A. Carboxypeptidase N: A pleiotropic regulator of inflammation. Mol. Immun. 2004, 40, 785–793. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. Thrombin activatable fibrinolysis inhibitor (TAFI): An updated narrative review. Int. J. Mol. Sci. 2021, 22, 3670. [Google Scholar] [CrossRef]
- Osterhus, V.; König, S. Angiotensin-converting enzyme and blood basic carboxypeptidases CPB2 and CPN activity is an indicator for serum quality—A quick lab test. In Renin-Angiotensin-Aldosterone System—Latest Trends; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Bayer, M.; König, S. A vote for robustness: Monitoring serum enzyme activity by thin-layer chromatography of dabsylated bradykinin products. J. Pharmaceut. Biomed. Anal. 2017, 143, 199–203. [Google Scholar] [CrossRef]
- König, S.; Schlereth, T.; Birklein, F. Molecular signature for complex regional pain syndrome and its analysis. Expert. Rev. Proteom. 2017, 14, 857–867. [Google Scholar] [CrossRef]
- König, S.; Bayer, M.; Dimova, V.; Herrnberger, M.; Escolano-Lozano, F.; Bednarik, J.; Vlckova, E.; Rittner, H.; Schlereth, T.; Birklein, F. The serum protease network–one key to understand Complex Regional Pain Syndrome pathophysiology. Pain 2019, 160, 1402–1409. [Google Scholar] [CrossRef]
- König, S.; Steinebrey, N.; Herrnberger, M.; Escolano-Lozano, F.; Schlereth, T.; Rebhorn, C.; Birklein, F. Reduced serum protease activity in Complex Regional Pain Syndrome: The impact of angiotensin-converting enzyme and carboxypeptidases. J. Pharm. Biomed. Anal. 2021, 205, 114307. [Google Scholar] [CrossRef]
- Tepasse, P.-R.; Vollenberg, R.; Steinebrey, N.; König, S. High angiotensin-converting enzyme and low carboxypeptidase N serum activity correlate with disease severity in COVID-19 patients. J. Pers. Med. 2022, 12, 406. [Google Scholar] [CrossRef]
- Tepasse, P.-R.; Vollenberg, R.; Steinebrey, N.; König, S. The dysregulation of the renin-angiotensin-system in COVID-19 studied by serum proteomics: Angiotensinogen increases with disease severity. Molecules 2022, 27, 2495. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Engl, C.; Bayer, M.; König, S. Neuropeptide reporter assay for serum, capillary blood and blood cards. MethodsX 2020, 7, 100985. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Bayer, M.; König, S. Validation data for the use of bradykinin and substance P protease activity assay with capillary blood and blood cards. Data Brief. 2020, 28, 104873. [Google Scholar] [CrossRef] [PubMed]
- hPOP at Stanford University. Available online: https://med.stanford.edu/hpop.html (accessed on 12 December 2024).
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard Operating Procedures for serum and plasma collection: Early detection research network consensus statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. 2010. Available online: http://www.who.int/publications/i/item/9789241599221 (accessed on 18 December 2024).
- Sharp, M.K.; Mohammad, S.F. Scaling of hemolysis in needles and catheters. Ann. Biomed. Eng. 1998, 26, 788–797. [Google Scholar] [CrossRef]
- Saenger, J.A.; Atamaniuk, J.; Gaggl, M.; Asenbaum, J.; Huber, F.A.; Grieb, A.; Födinger, M. Increased hemolysis rate in plasma tubes after implementation of a fully automated sample delivery and acceptance system. J. Lab. Med. 2023, 47, 63–68. [Google Scholar] [CrossRef]
- Krasowski, M.D. Educational case: Hemolysis and lipemia interference with laboratory testing. Acad. Pathol. 2019, 6, 2374289519888754. [Google Scholar] [CrossRef]
- Wan Azman, W.N.; Omar, J.; Koon, T.S.; Tuan Ismail, T.S. Hemolyzed specimens: Major challenge for identifying and rejecting specimens in clinical laboratories. Oman Med. J. 2019, 34, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, M.; Hur, A.; Atay, A.; Cuhadar, S. Effects of hemolysis interferences on routine biochemistry parameters. Biochem. Medica 2011, 21, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Roman, Y.; Bomsel-Demontoy, M.-C.; Levrier, J.; Chaste-Duvernoy, D.; Jalme, M.S. Effect of hemolysis on plasma protein levels and plasma electrophoresis in birds. J. Wildl. Dis. 2009, 45, 73–80. [Google Scholar] [CrossRef]
- El-Khoury, J.M.; Bunch, D.R.; Wang, S. Is the effect of hemolysis on plasma ammonia measurement overrated? Arch. Pathol. Lab. Med. 2012, 136, 471–472. [Google Scholar] [CrossRef]
- Marques-Garcia, F.; Jung, D.H.H.; Pérez, S.E. Impact of individualized hemolysis management based on biological variation cut-offs in a clinical laboratory. Ann. Lab. Med. 2022, 42, 169–177. [Google Scholar] [CrossRef]
- CDC Reference Tool to Determine Hemolysis Status. Available online: https://www.cdc.gov/vector-borne-diseases/php/laboratories/reference-tool-for-hemolysis-status.html (accessed on 22 May 2024).
- Ni, J.; Zhu, W.; Wang, Y.; Wei, X.; Li, J.; Peng, L.; Zhang, K.; Bai, B. A Reference chart for clinical biochemical tests of hemolyzed serum samples. J. Clin. Lab. Anal. 2021, 35, e23561. [Google Scholar] [CrossRef]
- Lippi, G.; Cadamuro, J. Visual assessment of sample quality: Quo usque tandem? Clin. Chem. Lab. Med. 2018, 56, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Gislefoss, R.E.; Berge, U.; Lauritzen, M.; Langseth, H.; Wojewodzic, M.W. A Simple and cost-effective method for measuring hemolysis in biobank serum specimens. Biopreserv. Biobank. 2021, 19, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, A.; Nishioka, M.; Morishige, A.; Yoneshiro, M.; Shinkawa, K.; Fujinaga, A.; Kobayashi, T.; Suehiro, Y.; Yamasaki, T. Determination of the optimal wavelength of the hemolysis index measurement. J. Clin. Med. 2023, 12, 5864. [Google Scholar] [CrossRef]
- Larrán, B.; López-Alonso, M.; Miranda, M.; Pereira, V.; Rigueira, L.; Suárez, M.L.; Herrero-Latorre, C. Measuring haemolysis in cattle serum by direct UV-VIS and RGB digital image-based methods. Sci. Rep. 2022, 12, 13523. [Google Scholar] [CrossRef]
- Hernaningsih, Y.; Akualing, J.S. The effects of hemolysis on plasma prothrombin time and activated partial thromboplastin time tests using photo-optical method. Medicine 2017, 96, e7976. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, D.; Sun, D.; Zhang, S.; Zhang, P.; Xiong, Y.; Zhao, M.; Qi, T.; Situ, B.; Zheng, L. A deep learning-based system for assessment of serum quality using sample images. Clin. Chim. Acta 2022, 531, 254–260. [Google Scholar] [CrossRef]
- König, S.; Schork, K.; Eisenacher, M. Observations from the proteomics bench. Proteomes 2024, 12, 6. [Google Scholar] [CrossRef]
- Gegner, H.M.; Naake, T.; Dugourd, A.; Müller, T.; Czernilofsky, F.; Kliewer, G.; Jäger, E.; Helm, B.; Kunze-Rohrbach, N.; Klingmüller, U.; et al. Pre-analytical processing of plasma and serum samples for combined proteome and metabolome analysis. Front. Mol. Biosci. 2022, 9, 961448. [Google Scholar] [CrossRef]
- Coorssen, J.; Padula, M.P. Proteomics–The state of the field: The definition and analysis of proteomes should be based in reality, not convenience. Proteomes 2024, 12, 14. [Google Scholar] [CrossRef]
- Brockbals, L.; Ueland, M.; Fu, S.; Padula, M.P. Development and thourough evaluation of a multi-omics sample preparation workflow for comprehensive LC-MS/MS-based metabolomics, lipidomics and proteomics datasets. Talanta 2025, 286, 127442. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.N.; Ko, G.T.C.; Leung, D.H.Y.; Cheing, R.C.K.; Cheing, M.Y.F.; So, W.-Y.; Swaminathan, R.; Nicholls, M.G.; Critchley, J.A.J.H.; Cockram, C.S. Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int. 2000, 57, 590–600. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite concentration changes in humans after a bout of exercise: A systematic review of exercise metabolomics studies. Sports Med.-Open 2020, 6, 11. [Google Scholar] [CrossRef]
- König, S.; Jockenhöfer, C.; Billich, C.; Beer, M.; Machann, J.; Schmidt-Trucksäss, A.; Schütz, U. Long distance running-can bioprofiling predict success in endurance athletes? Med. Hypotheses 2020, 146, 110474. [Google Scholar] [CrossRef]
- Monteonofrio, L.; Florio, M.C.; AIGhatrif, M.; Lakatta, E.G.; Capogrossi, M.C. Aging- and gender-related modulation of RAAS: Potential implications in COVID-19 disease. Vasc. Biol. 2021, 3, R1–R14. [Google Scholar] [CrossRef]
- Nwia, S.M.; Leite, A.P.O.; Li, X.C.; Zhuo, J.L. Sex differences in the renin-angiotensin-aldosterone system and its roles in hypertension, cardiovascular, and kidney diseases. Front. Cardiovasc. Med. 2023, 10, 1198090. [Google Scholar] [CrossRef]
- Komukai, K.; Mochizuki, S.; Yoshimura, M. Gender and the renin-angiotensin-aldosterone system. Fundam. Clin. Pharmacol. 2010, 24, 687–698. [Google Scholar] [CrossRef]
- Conti, S.; Cassis, P.; Benigni, A. Aging and the renin-angiotensin system. Hypertension 2012, 60, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R. The science of soy: What do we really know? Environ. Health Perspect. 2006, 114, A352–A358. [Google Scholar] [CrossRef] [PubMed]
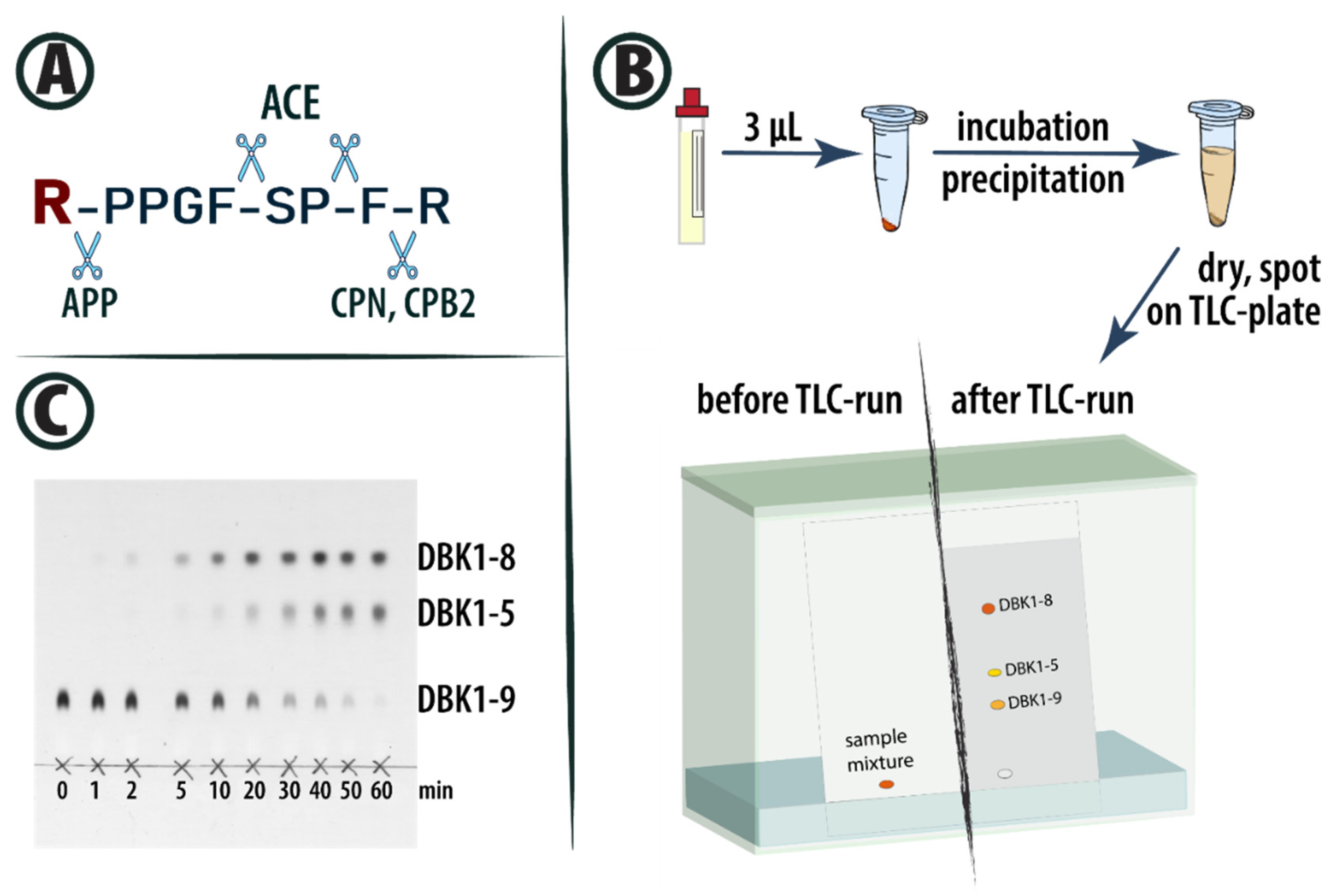


| Boston 2016 | Taipei 2016 | Dublin 2017 | Orlando 2018 | California 2018 | ||
|---|---|---|---|---|---|---|
| Code # | 1 | 2 | 3 | 4 | 5 | |
| Subjects | 24 | 105 | 110 | 83 | 18 | |
| Gender | Female | 10 | 41 | 44 | 22 | 7 |
| Male | 14 | 62 | 66 | 61 | 11 | |
| Ethnicity | Asian | 6 | 50 | 17 | 22 | 1 |
| Caucasian | 18 | 51 | 84 | 56 | 17 | |
| BL/HO | - | 4 | 9 | 5 | ||
| Age range | 20–40 | 12 | 59 | 57 | 34 | 1 |
| 40–60 | 8 | 37 | 42 | 37 | 14 | |
| >60 | 4 | 9 | 11 | 12 | 3 | |
| BMI | <25 | 15 | 34 | 62 | 37 | 12 |
| 25–30 | 8 | 15 | 31 | 35 | 5 | |
| 30+ | 1 | 5 | 17 | 10 | 1 | |
| Samples | Hemolytic | - | 1 | 7 | 1 | - |
| Lipemic | 1 | - | 3 | 3 | - |
| DBK 1-9 | Std. Dev DBK1-9 | DBK 1-8 | Std. Dev DBK1-8 | DBK 1-5 | Std. Dev DBK1-5 | |
|---|---|---|---|---|---|---|
| All samples | 0.231 | 0.112 | 0.613 | 0.117 | 0.156 | 0.047 |
| Excluding hemolytic samples | 0.227 | 0.109 | 0.619 | 0.110 | 0.154 | 0.045 |
| Hemolytic samples | 0.387 | 0.120 | 0.388 | 0.145 | 0.225 | 0.063 |
| Boston 2016 | 0.445 | 0.088 | 0.395 | 0.077 | 0.160 | 0.036 |
| Dublin 2017 | 0.254 | 0.086 | 0.587 | 0.090 | 0.159 | 0.049 |
| California 2018 | 0.261 | 0.106 | 0.592 | 0.095 | 0.147 | 0.039 |
| Taipei 2016 | 0.180 | 0.074 | 0.674 | 0.073 | 0.146 | 0.038 |
| Orlando 2018 | 0.175 | 0.074 | 0.667 | 0.076 | 0.158 | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayer, M.; Snyder, M.; König, S. The Biological Variation in Serum ACE and CPN/CPB2 Activity in Healthy Individuals as Measured by the Degradation of Dabsylated Bradykinin—Reference Data and the Importance of Pre-Analytical Standardization. Proteomes 2025, 13, 40. https://doi.org/10.3390/proteomes13030040
Bayer M, Snyder M, König S. The Biological Variation in Serum ACE and CPN/CPB2 Activity in Healthy Individuals as Measured by the Degradation of Dabsylated Bradykinin—Reference Data and the Importance of Pre-Analytical Standardization. Proteomes. 2025; 13(3):40. https://doi.org/10.3390/proteomes13030040
Chicago/Turabian StyleBayer, Malte, Michael Snyder, and Simone König. 2025. "The Biological Variation in Serum ACE and CPN/CPB2 Activity in Healthy Individuals as Measured by the Degradation of Dabsylated Bradykinin—Reference Data and the Importance of Pre-Analytical Standardization" Proteomes 13, no. 3: 40. https://doi.org/10.3390/proteomes13030040
APA StyleBayer, M., Snyder, M., & König, S. (2025). The Biological Variation in Serum ACE and CPN/CPB2 Activity in Healthy Individuals as Measured by the Degradation of Dabsylated Bradykinin—Reference Data and the Importance of Pre-Analytical Standardization. Proteomes, 13(3), 40. https://doi.org/10.3390/proteomes13030040







