Adiposome Proteomics Uncover Molecular Signatures of Cardiometabolic Risk in Obese Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Enrollment
2.2. Cardiometabolic Risk Measurements
2.3. Adiposome Isolation and Protein Extraction
2.4. Adiposome Protein Analysis
2.5. Vascular Function Assessment
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Participants
3.2. Adiposome Characteristics
3.3. Obesity-Linked Proteomics Shifts
3.4. Proteomic Shifts in VAT-Derived Adiposomes Mirror Cardiometabolic Risk Profiles in Obesity
3.5. Network-Level Analysis of Obesity-Associated Molecular Dysregulation
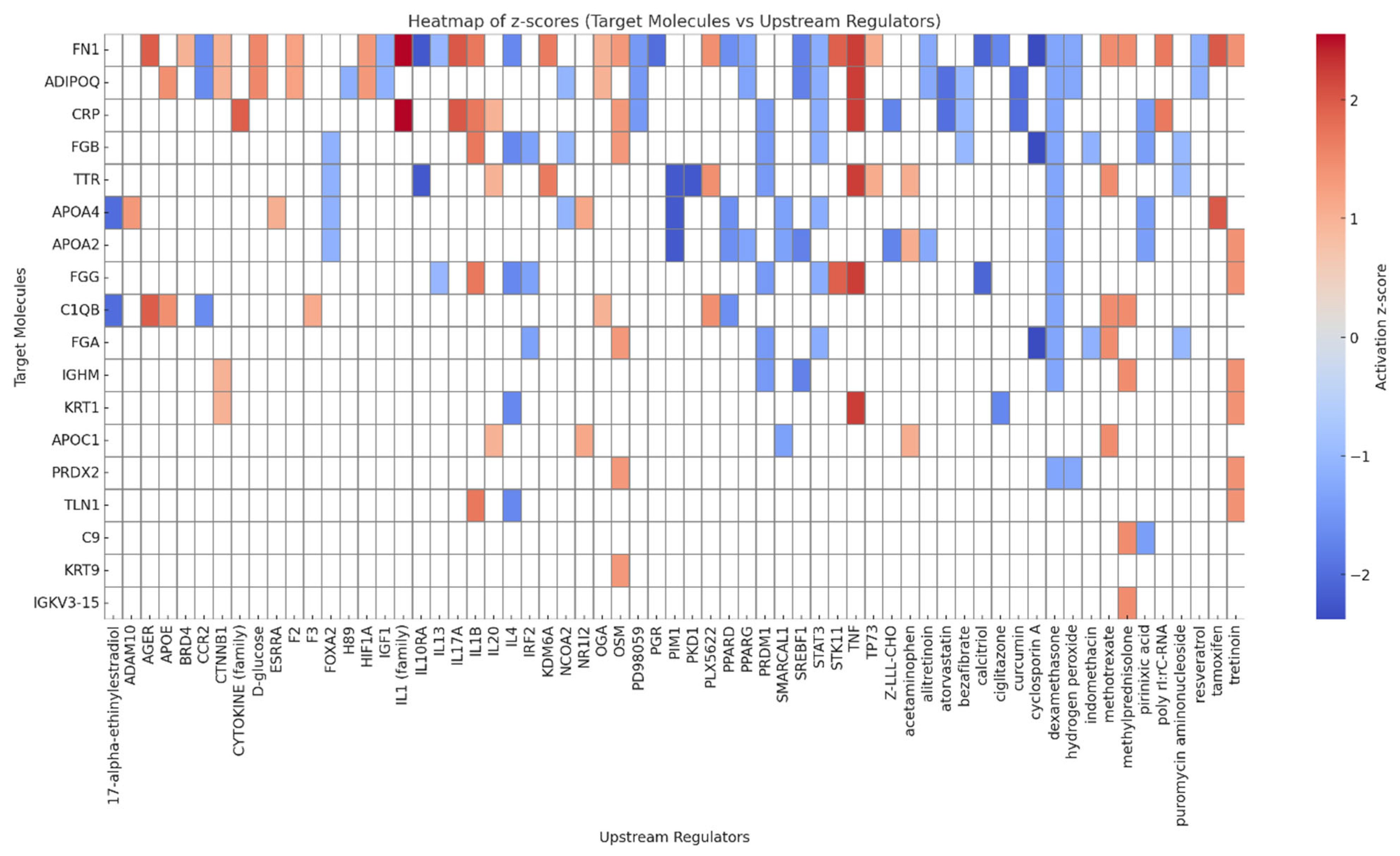
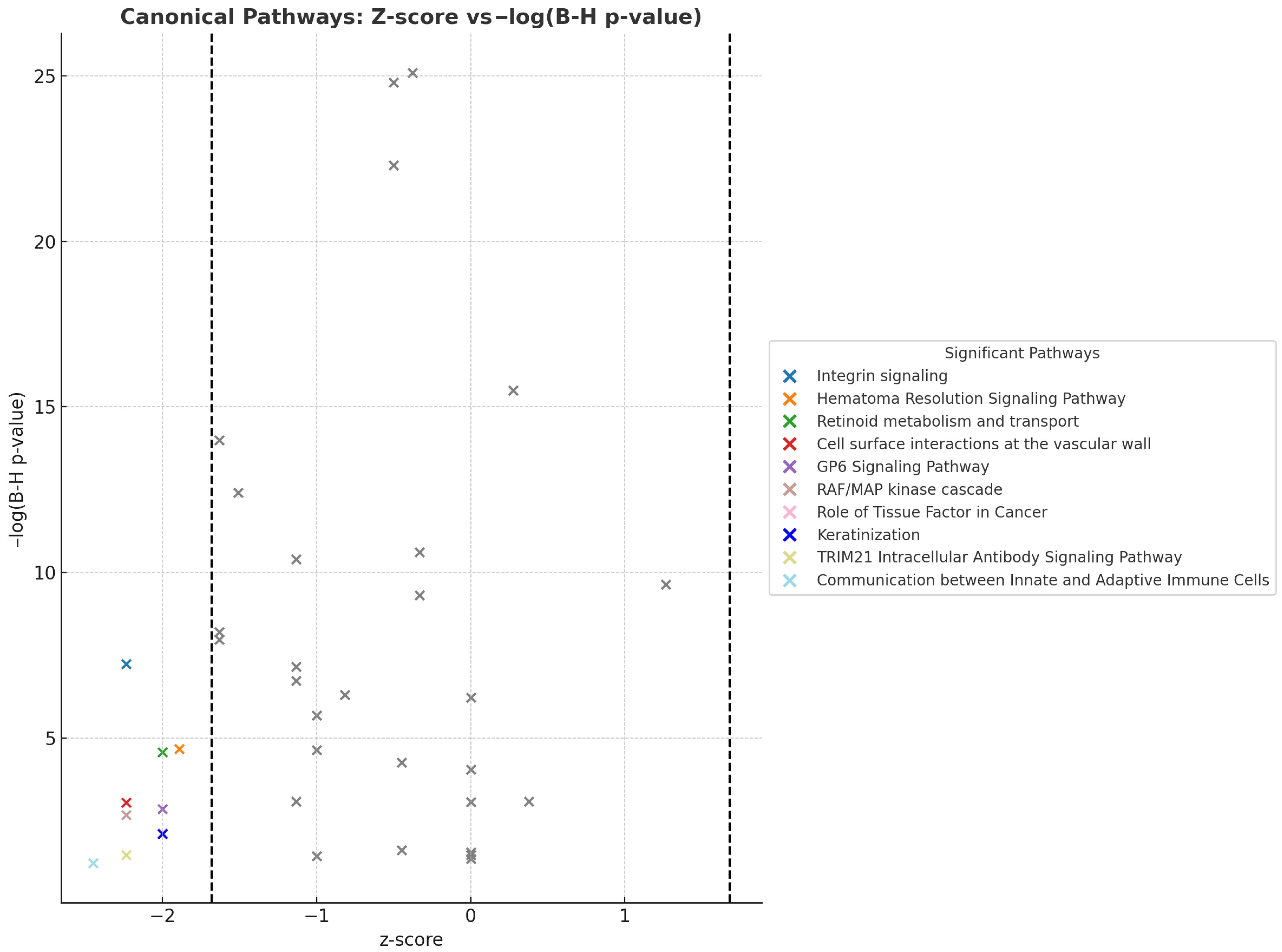
3.6. Upstream Regulators and Canonical Pathways Associated with Adiposome Proteomic Shift
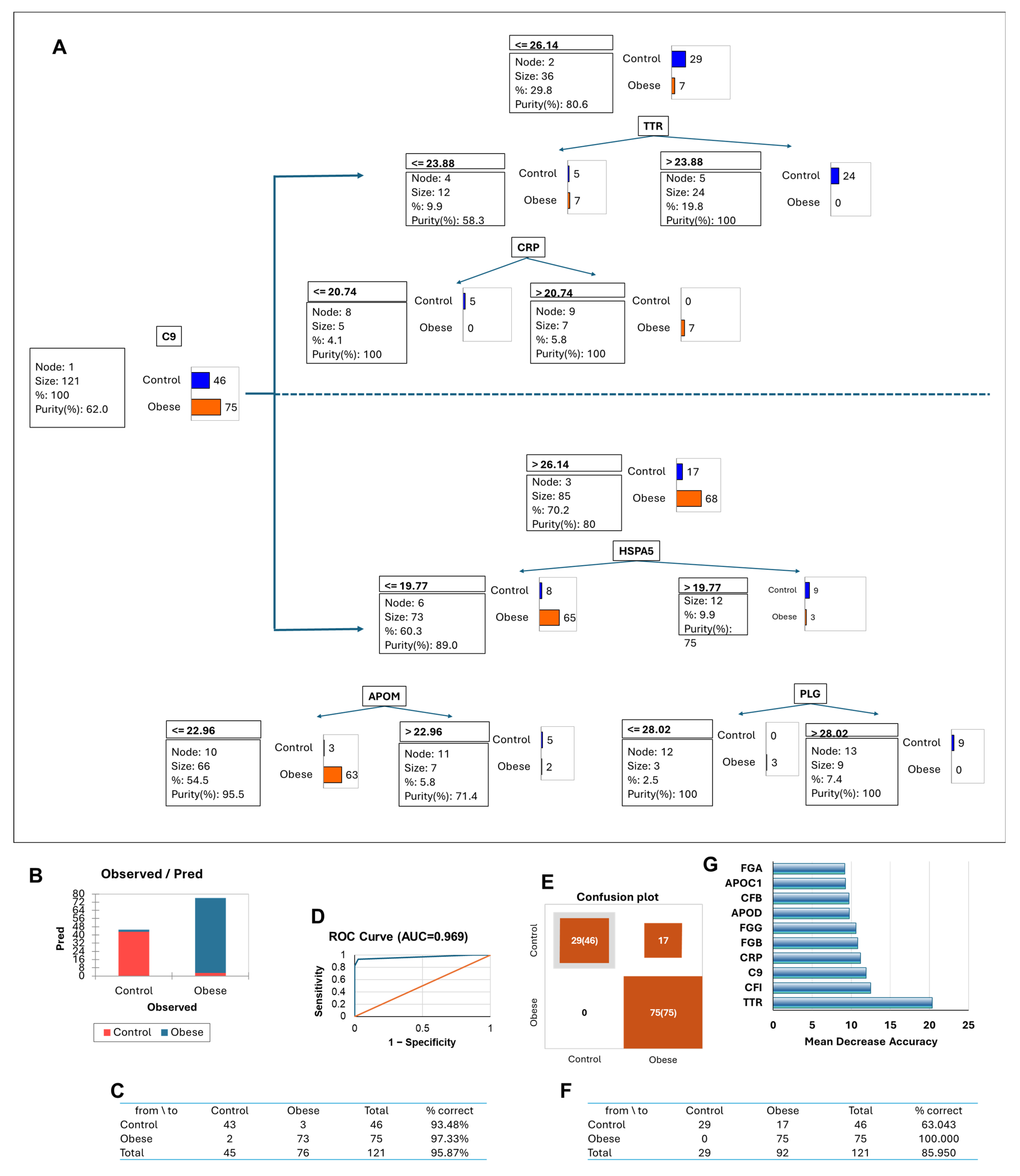
3.7. Adiposome Protein Signatures as Predictors of Cardiometabolic Diseases
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bluher, M. An overview of obesity-related complications: The epidemiological evidence linking body weight and other markers of obesity to adverse health outcomes. Diabetes Obes. Metab. 2025, 27 (Suppl. S2), 3–19. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, T.; Zhao, Q.; Ma, J.; Jiang, J.; Shi, H. Adipose Tissue-Derived Extracellular Vesicles: A Promising Biomarker and Therapeutic Strategy for Metabolic Disorders. Stem Cells Int. 2023, 2023, 9517826. [Google Scholar] [CrossRef]
- Han, Y.; Ye, S.; Liu, B. Roles of extracellular vesicles derived from healthy and obese adipose tissue in inter-organ crosstalk and potential clinical implication. Front. Endocrinol. 2024, 15, 1409000. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ko, J.H.; Kim, S.N. The Extracellular MicroRNAs on Inflammation: A Literature Review of Rodent Studies. Biomedicines 2022, 10, 1601. [Google Scholar] [CrossRef]
- Babuta, M.; Szabo, G. Extracellular vesicles in inflammation: Focus on the microRNA cargo of EVs in modulation of liver diseases. J. Leukoc. Biol. 2022, 111, 75–92. [Google Scholar] [CrossRef]
- Lim, H.J.; Yoon, H.; Kim, H.; Kang, Y.W.; Kim, J.E.; Kim, O.Y.; Lee, E.Y.; Twizere, J.C.; Rak, J.; Kim, D.K. Extracellular Vesicle Proteomes Shed Light on the Evolutionary, Interactive, and Functional Divergence of Their Biogenesis Mechanisms. Front. Cell Dev. Biol. 2021, 9, 734950. [Google Scholar] [CrossRef]
- Martyniak, K.; Masternak, M.M. Changes in adipose tissue cellular composition during obesity and aging as a cause of metabolic dysregulation. Exp. Gerontol. 2017, 94, 59–63. [Google Scholar] [CrossRef]
- Hinte, L.C.; Castellano-Castillo, D.; Ghosh, A.; Melrose, K.; Gasser, E.; Noe, F.; Massier, L.; Dong, H.; Sun, W.; Hoffmann, A.; et al. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature 2024, 636, 457–465. [Google Scholar] [CrossRef]
- Bond, S.T.; Calkin, A.C.; Drew, B.G. Adipose-Derived Extracellular Vesicles: Systemic Messengers and Metabolic Regulators in Health and Disease. Front. Physiol. 2022, 13, 837001. [Google Scholar] [CrossRef] [PubMed]
- Mirza, I.; Haloul, M.; Hassan, C.; Masrur, M.; Mostafa, A.; Bianco, F.M.; Ali, M.M.; Minshall, R.D.; Mahmoud, A.M. Adiposomes from Obese-Diabetic Individuals Promote Endothelial Dysfunction and Loss of Surface Caveolae. Cells 2023, 12, 2453. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Mirza, I.; Morsy, M.; Mostafa, A.; Hassan, C.; Masrur, M.; Bianco, F.M.; Papasani, S.; Levitan, I.; Mahmoud, A.M. Comparison of Adiposomal Lipids between Obese and Non-Obese Individuals. Metabolites 2024, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 6478. [Google Scholar] [CrossRef]
- Hruska, P.; Kucera, J.; Kuruczova, D.; Buzga, M.; Pekar, M.; Holeczy, P.; Potesil, D.; Zdrahal, Z.; Bienertova-Vasku, J. Unraveling adipose tissue proteomic landscapes in severe obesity: Insights into metabolic complications and potential biomarkers. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E562–E580. [Google Scholar] [CrossRef]
- Sandoval-Borquez, A.; Carrion, P.; Hernandez, M.P.; Perez, J.A.; Tapia-Castillo, A.; Vecchiola, A.; Fardella, C.E.; Carvajal, C.A. Adipose Tissue Dysfunction and the Role of Adipocyte-Derived Extracellular Vesicles in Obesity and Metabolic Syndrome. J. Endocr. Soc. 2024, 8, bvae126. [Google Scholar] [CrossRef]
- Hruska, P.; Kucera, J.; Pekar, M.; Holeczy, P.; Mazur, M.; Buzga, M.; Kuruczova, D.; Lenart, P.; Fialova Kucerova, J.; Potesil, D.; et al. Proteomic Signatures of Human Visceral and Subcutaneous Adipocytes. J. Clin. Endocrinol. Metab. 2022, 107, 755–775. [Google Scholar] [CrossRef]
- Haloul, M.; Vinjamuri, S.J.; Naquiallah, D.; Mirza, M.I.; Qureshi, M.; Hassan, C.; Masrur, M.; Bianco, F.M.; Frederick, P.; Cristoforo, G.P.; et al. Hyperhomocysteinemia and Low Folate and Vitamin B12 Are Associated with Vascular Dysfunction and Impaired Nitric Oxide Sensitivity in Morbidly Obese Patients. Nutrients 2020, 12, 2014. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Szczurek, M.R.; Blackburn, B.K.; Mey, J.T.; Chen, Z.; Robinson, A.T.; Bian, J.T.; Unterman, T.G.; Minshall, R.D.; Brown, M.D.; et al. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol. Rep. 2016, 4, e12895. [Google Scholar] [CrossRef]
- Niada, S.; Giannasi, C.; Magagnotti, C.; Andolfo, A.; Brini, A.T. Proteomic analysis of extracellular vesicles and conditioned medium from human adipose-derived stem/stromal cells and dermal fibroblasts. J. Proteom. 2021, 232, 104069. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120. [Google Scholar] [CrossRef]
- Wang, Z.G.; He, Z.Y.; Liang, S.; Yang, Q.; Cheng, P.; Chen, A.M. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 511. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, D.H.; Go, H.K.; Youn, J.; Kim, H.K.; Jin, R.C.; Miller, R.B.; Kim, D.H.; Cho, B.S.; Yi, Y.W. Reproducible Large-Scale Isolation of Exosomes from Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Application in Acute Kidney Injury. Int. J. Mol. Sci. 2020, 21, 4774. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, R.; Sasaki, T.; Takahashi, Y.; Yamauchi, Y. Proteomic profiling of tissue extracellular vesicles (EVs) identifies tissue-specific EV markers and predicts the accessibility of tissue EVs to the circulation. bioRxiv 2025. preprint. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Ali, M.M.; Naquiallah, D.; Qureshi, M.; Mirza, M.I.; Hassan, C.; Masrur, M.; Bianco, F.M.; Frederick, P.; Cristoforo, G.P.; Gangemi, A.; et al. DNA methylation profile of genes involved in inflammation and autoimmunity correlates with vascular function in morbidly obese adults. Epigenetics 2021, 17, 93–109. [Google Scholar] [CrossRef]
- Connolly, K.D.; Wadey, R.M.; Mathew, D.; Johnson, E.; Rees, D.A.; James, P.E. Evidence for Adipocyte-Derived Extracellular Vesicles in the Human Circulation. Endocrinology 2018, 159, 3259–3267. [Google Scholar] [CrossRef]
- Zhou, H.H.; Tang, Y.L.; Xu, T.H.; Cheng, B. C-reactive protein: Structure, function, regulation, and role in clinical diseases. Front. Immunol. 2024, 15, 1425168. [Google Scholar] [CrossRef] [PubMed]
- King, B.C.; Blom, A.M. Complement in metabolic disease: Metaflammation and a two-edged sword. Semin. Immunopathol. 2021, 43, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Lago-Baameiro, N.; Camino, T.; Vazquez-Duran, A.; Sueiro, A.; Couto, I.; Santos, F.; Baltar, J.; Falcon-Perez, J.M.; Pardo, M. Intra and inter-organ communication through extracellular vesicles in obesity: Functional role of obesesomes and steatosomes. J. Transl. Med. 2025, 23, 207. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.P.; Fandl, H.K.; Hijmans, J.G.; Berry, A.R.; Cardenas, H.L.; Stockelman, K.A.; DeSouza, N.M.; Treuth, J.W.; Greiner, J.J.; Park, A.J.; et al. Effects of circulating endothelial microvesicles isolated from adults with obesity on endothelial cell inflammation, apoptosis, and nitric oxide production. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E38–E49. [Google Scholar] [CrossRef]
- Hattori, Y.; Suzuki, M.; Hattori, S.; Kasai, K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia 2003, 46, 1543–1549. [Google Scholar] [CrossRef]
- Fesus, G.; Dubrovska, G.; Gorzelniak, K.; Kluge, R.; Huang, Y.; Luft, F.C.; Gollasch, M. Adiponectin is a novel humoral vasodilator. Cardiovasc. Res. 2007, 75, 719–727. [Google Scholar] [CrossRef]
- Cohen, K.E.; Katunaric, B.; Schulz, M.E.; SenthilKumar, G.; Young, M.S.; Mace, J.E.; Freed, J.K. Role of Adiponectin Receptor 1 in Promoting Nitric Oxide-Mediated Flow-Induced Dilation in the Human Microvasculature. Front. Pharmacol. 2022, 13, 875900. [Google Scholar] [CrossRef]
- Sabaratnam, R.; Svenningsen, P. Adipocyte-Endothelium Crosstalk in Obesity. Front. Endocrinol. 2021, 12, 681290. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Q.; Chen, W.; Gao, Y.; Ye, J.; Chen, Y.; Wang, T.; Gao, L.; Liu, Y.; Yang, Y. New advances of adiponectin in regulating obesity and related metabolic syndromes. J. Pharm. Anal. 2024, 14, 100913. [Google Scholar] [CrossRef]
- Magalhaes, J.; Eira, J.; Liz, M.A. The role of transthyretin in cell biology: Impact on human pathophysiology. Cell Mol. Life Sci. 2021, 78, 6105–6117. [Google Scholar] [CrossRef]
- Liz, M.A.; Coelho, T.; Bellotti, V.; Fernandez-Arias, M.I.; Mallaina, P.; Obici, L. A Narrative Review of the Role of Transthyretin in Health and Disease. Neurol. Ther. 2020, 9, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sleddering, M.A.; Markvoort, A.J.; Dharuri, H.K.; Jeyakar, S.; Snel, M.; Juhasz, P.; Lynch, M.; Hines, W.; Li, X.; Jazet, I.M.; et al. Proteomic analysis in type 2 diabetes patients before and after a very low calorie diet reveals potential disease state and intervention specific biomarkers. PLoS ONE 2014, 9, e112835. [Google Scholar] [CrossRef]
- Alemi, M.; Oliveira, A.; Tavares, S.C.; Vieira, J.R.; Alves, M.G.; Oliveira, P.F.; Cardoso, I. Exploring the Physiological Role of Transthyretin in Glucose Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 6073. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, C.; Minniti, M.; Susini, V.; Caponi, L.; Panichella, G.; Castiglione, V.; Aimo, A.; Emdin, M.; Vergaro, G.; Franzini, M. The Journey of Human Transthyretin: Synthesis, Structure Stability, and Catabolism. Biomedicines 2022, 10, 1906. [Google Scholar] [CrossRef]
- Folli, C.; Favilla, R.; Berni, R. The interaction between retinol-binding protein and transthyretin analyzed by fluorescence anisotropy. Methods Mol. Biol. 2010, 652, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Human Protein Atlas. TTR Gene Summary-RNA Expression. Available online: https://www.proteinatlas.org/ENSG00000118271-TTR/summary/rna (accessed on 30 July 2025).
- Cai, H.; Li, M.; Sun, X.; Plath, M.; Li, C.; Lan, X.; Lei, C.; Huang, Y.; Bai, Y.; Qi, X.; et al. Global Transcriptome Analysis During Adipogenic Differentiation and Involvement of Transthyretin Gene in Adipogenesis in Cattle. Front. Genet. 2018, 9, 463. [Google Scholar] [CrossRef]
- Paulsson Rokke, H.; Sadat Gousheh, N.; Westermark, P.; Suhr, O.B.; Anan, I.; Ihse, E.; Pilebro, B.; Wixner, J. Abdominal fat pad biopsies exhibit good diagnostic accuracy in patients with suspected transthyretin amyloidosis. Orphanet J. Rare Dis. 2020, 15, 278. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Adipose Tissue-Endothelial Cell Interactions in Obesity-Induced Endothelial Dysfunction. Front. Cardiovasc. Med. 2021, 8, 681581. [Google Scholar] [CrossRef]
- Willis, C.M.; Nicaise, A.M.; Menoret, A.; Ryu, J.K.; Mendiola, A.S.; Jellison, E.R.; Givogri, M.I.; Han, D.K.; Bongarzone, E.R.; Akassoglou, K.; et al. Extracellular vesicle fibrinogen induces encephalitogenic CD8+ T cells in a mouse model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10488–10493. [Google Scholar] [CrossRef]
- Musale, V.; Wasserman, D.H.; Kang, L. Extracellular matrix remodelling in obesity and metabolic disorders. Life Metab. 2023, 2, 4. [Google Scholar] [CrossRef]
- Fyfe-Desmarais, G.; Desmarais, F.; Rassart, E.; Mounier, C. Apolipoprotein D in Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1027. [Google Scholar] [CrossRef]
- Dassati, S.; Waldner, A.; Schweigreiter, R. Apolipoprotein D takes center stage in the stress response of the aging and degenerative brain. Neurobiol. Aging 2014, 35, 1632–1642. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Do Carmo, S.; Lora, J.M.; Torres-Schumann, S.; Vogel, M.; Allhorn, M.; Gonzalez, C.; Bastiani, M.J.; Rassart, E.; Sanchez, D. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell 2008, 7, 506–515. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Peroxiredoxin 2: An Important Element of the Antioxidant Defense of the Erythrocyte. Antioxidants 2023, 12, 1012. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.J.; Chae, U.; Seong, J.; Lee, H.S.; Lee, S.R.; Lee, S.; Lee, D.S. Peroxiredoxin 2 mediates insulin sensitivity of skeletal muscles through regulation of protein tyrosine phosphatase oxidation. Int. J. Biochem. Cell Biol. 2018, 99, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, D.A.; Heber, A.; Capin, D.; Kreutz, T.; Opitz, D.; Lenzen, E.; Bloch, W.; Brixius, K.; Brinkmann, C. Training increases peroxiredoxin 2 contents in the erythrocytes of overweight/obese men suffering from type 2 diabetes. Wien. Med. Wochenschr. 2011, 161, 511–518. [Google Scholar] [CrossRef]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediators Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef]
- Kiss, M.G.; Binder, C.J. The multifaceted impact of complement on atherosclerosis. Atherosclerosis 2022, 351, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, F.; Rus, H. The role of complement activation in atherosclerosis. Immunol. Res. 2004, 30, 73–80. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Yang, S.; Chen, Y. Comparison of Plasma Exosome Proteomes Between Obese and Non-Obese Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2023, 16, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Kranendonk, M.E.; Visseren, F.L.; van Balkom, B.W.; Nolte-‘t Hoen, E.N.; van Herwaarden, J.A.; de Jager, W.; Schipper, H.S.; Brenkman, A.B.; Verhaar, M.C.; Wauben, M.H.; et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity 2014, 22, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef] [PubMed]
- Suren Garg, S.; Kushwaha, K.; Dubey, R.; Gupta, J. Association between obesity, inflammation and insulin resistance: Insights into signaling pathways and therapeutic interventions. Diabetes Res. Clin. Pract. 2023, 200, 110691. [Google Scholar] [CrossRef]
- Riuzzi, F.; Chiappalupi, S.; Arcuri, C.; Giambanco, I.; Sorci, G.; Donato, R. S100 proteins in obesity: Liaisons dangereuses. Cell Mol. Life Sci. 2020, 77, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.K.; Balasubramanyam, J.; Balakumar, M.; Deepa, M.; Anjana, R.M.; Abhijit, S.; Kaviya, A.; Velmurugan, K.; Miranda, P.; Balasubramanyam, M.; et al. Altered Circulating Levels of Retinol Binding Protein 4 and Transthyretin in Relation to Insulin Resistance, Obesity, and Glucose Intolerance in Asian Indians. Endocr. Pract. 2015, 21, 861–869. [Google Scholar] [CrossRef]
- Pfutzner, A.; Schondorf, T.; Hanefeld, M.; Forst, T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: Effects of insulin-sensitizing treatment with pioglitazone. J. Diabetes Sci. Technol. 2010, 4, 706–716. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, J.; Cui, X.; Xia, Y.; Gao, H.; Wang, X.; Cheng, M. S100A9 promotes inflammatory response in diabetic nonalcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2022, 618, 127–132. [Google Scholar] [CrossRef]
- Mulholland, M.; Depuydt, M.A.C.; Jakobsson, G.; Ljungcrantz, I.; Grentzmann, A.; To, F.; Bengtsson, E.; Jaensson Gyllenback, E.; Gronberg, C.; Rattik, S.; et al. Interleukin-1 receptor accessory protein blockade limits the development of atherosclerosis and reduces plaque inflammation. Cardiovasc. Res. 2024, 120, 581–595. [Google Scholar] [CrossRef]
- Yan, B.; Luo, L.; Liu, L.; Wang, Z.; Chen, R.; Wu, Y.; Xiao, X. Serpin family proteins as potential biomarkers and therapeutic drugs in stroke: A systematic review and meta-analysis on clinical/preclinical studies. CNS Neurosci. Ther. 2023, 29, 1738–1749. [Google Scholar] [CrossRef]
- Huntington, J.A. Serpin structure, function and dysfunction. J. Thromb. Haemost. 2011, 9 (Suppl. S1), 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Averill, M.M.; Barnhart, S.; Becker, L.; Li, X.; Heinecke, J.W.; Leboeuf, R.C.; Hamerman, J.A.; Sorg, C.; Kerkhoff, C.; Bornfeldt, K.E. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: Implications for atherosclerosis and adipose tissue inflammation. Circulation 2011, 123, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, S.; Scherer, P.E. Exploring adipose tissue-derived extracellular vesicles in inter-organ crosstalk: Implications for metabolic regulation and adipose tissue function. Cell Rep. 2025, 44, 115732. [Google Scholar] [CrossRef]
- Rome, S.; Blandin, A.; Le Lay, S. Adipocyte-Derived Extracellular Vesicles: State of the Art. Int. J. Mol. Sci. 2021, 22, 1788. [Google Scholar] [CrossRef]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708.e13. [Google Scholar] [CrossRef]
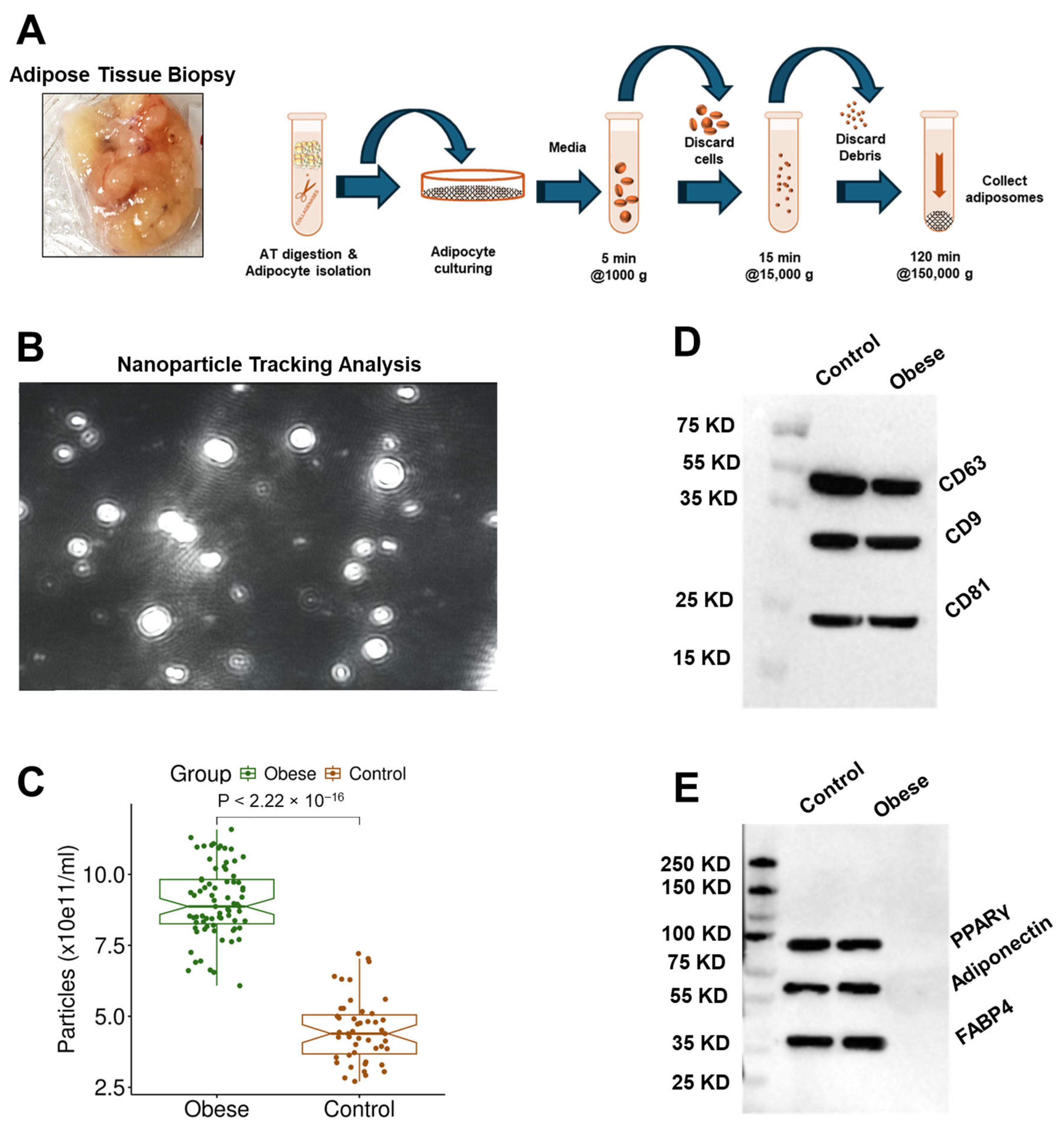
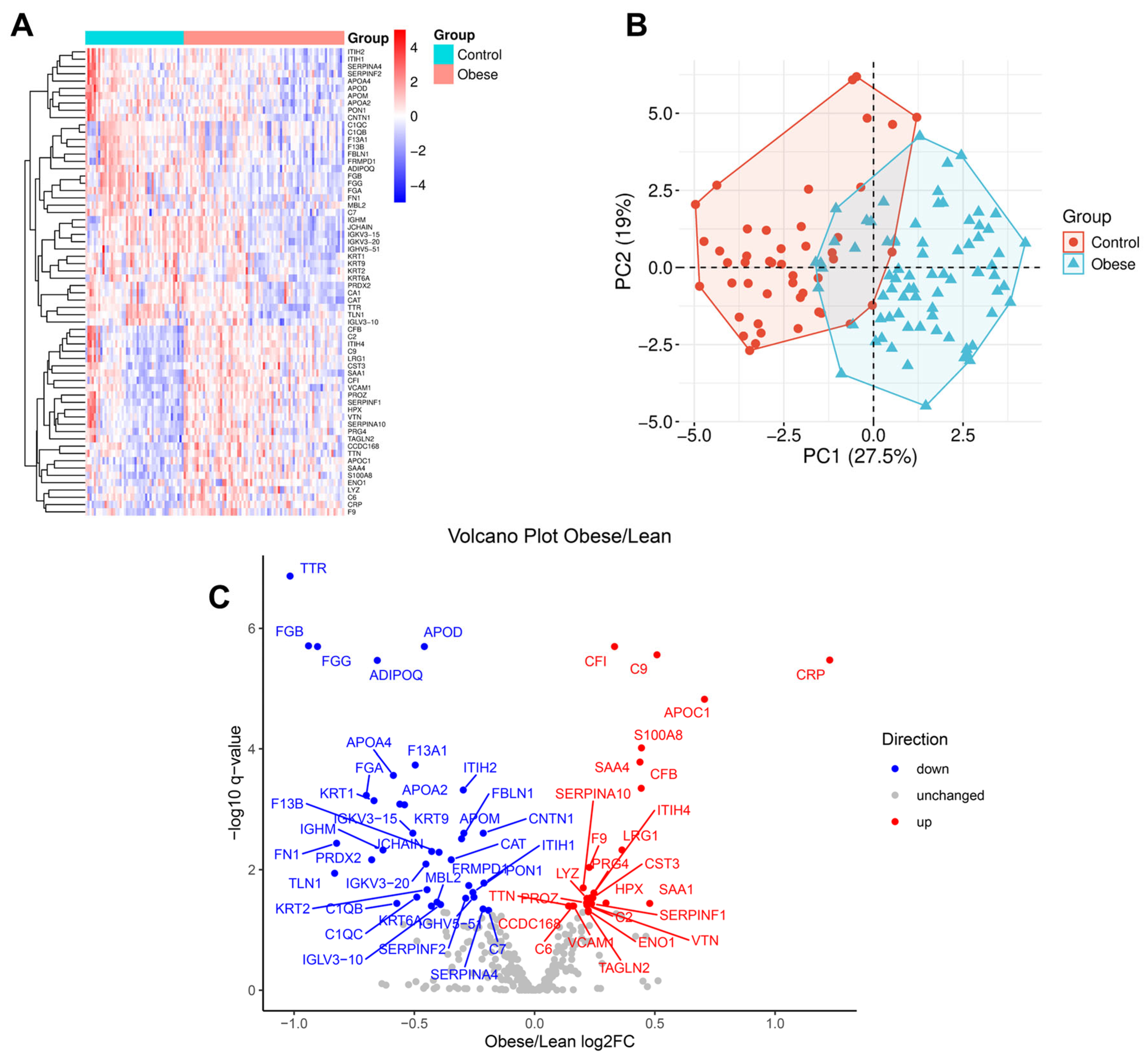
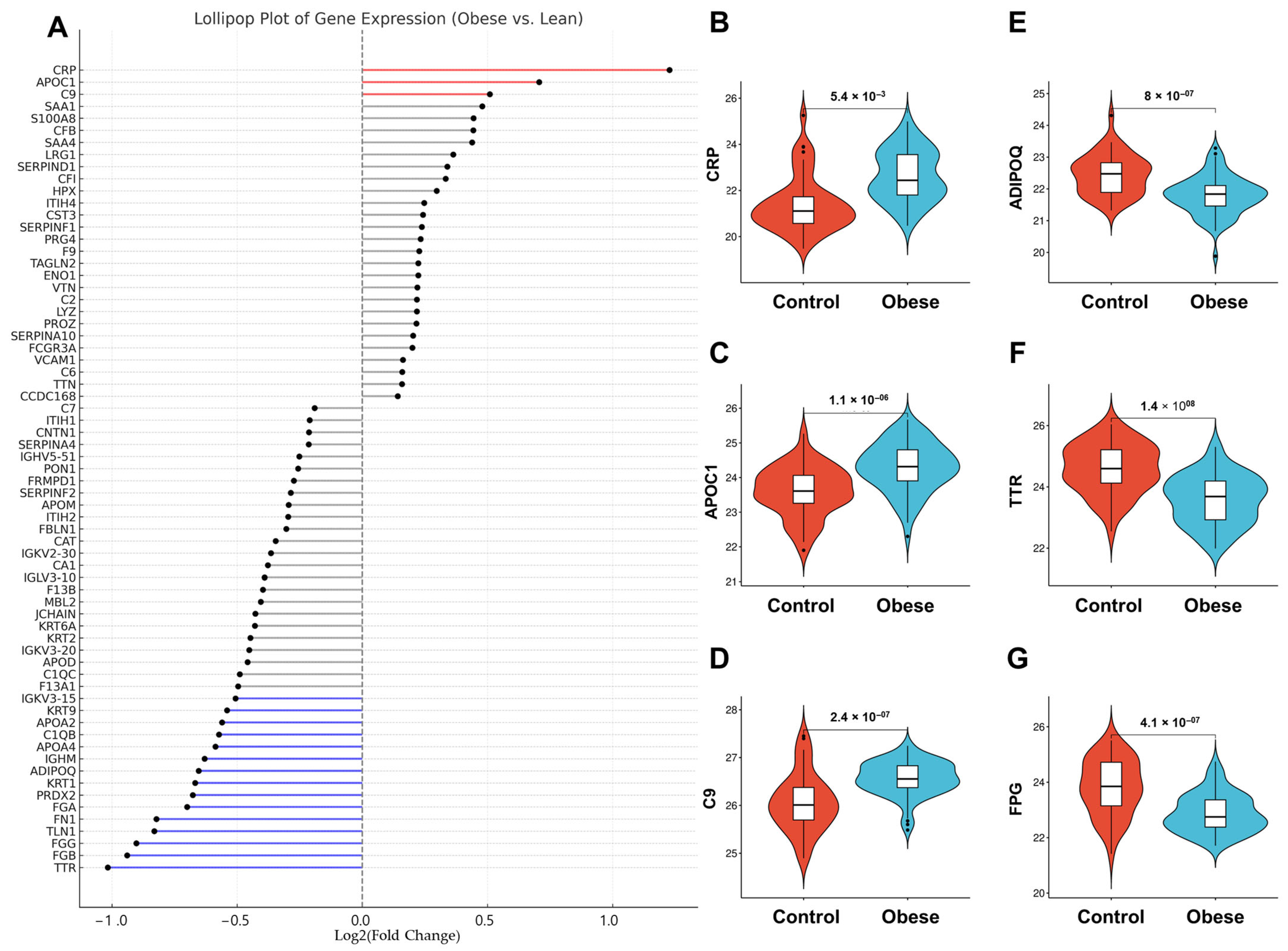
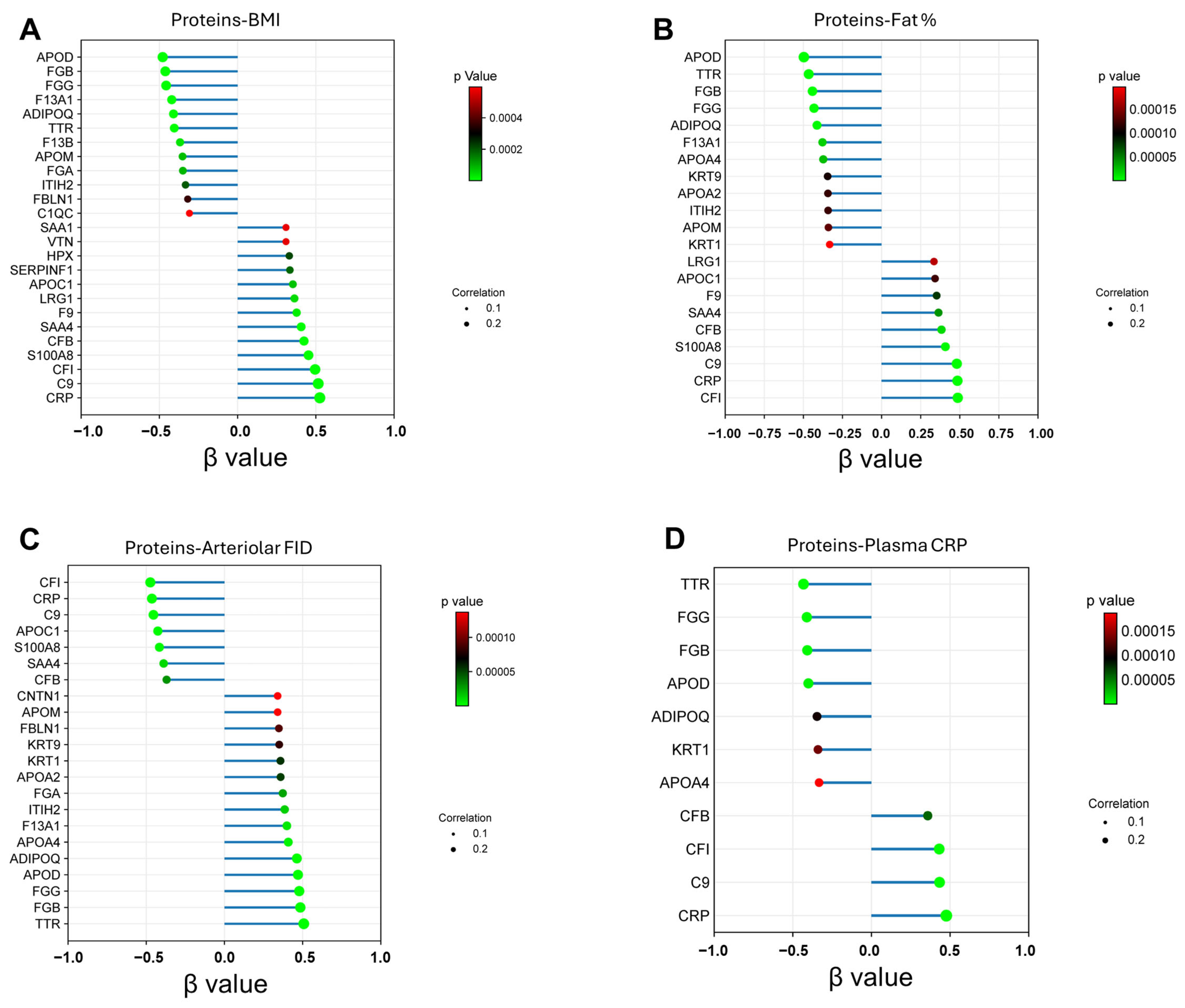
| Obese (n = 75) | Control (n = 47) | p-Value | |
|---|---|---|---|
| Age (years) | 37.21 ± 7.69 | 34.61 ± 8.23 | 0.0792 |
| BMI (kg/m2) | 48.53 ± 7.12 | 25.70 ± 2.99 | <0.01 |
| Total Fat percent (%) | 52.26 ± 6.80 | 21.37 ± 3.51 | <0.01 |
| Visceral fat mass, kg | 2.17 ± 0.69 | 0.77 ± 0.32 | <0.01 |
| Heart rate (b/min) | 81.40 ± 12.35 | 75.85 ± 12.59 | 0.0198 |
| Systolic blood pressure (mmHg) | 132.12 ± 17.71 | 119.26 ± 11.51 | <0.01 |
| Diastolic blood pressure (mmHg) | 79.31 ± 9.82 | 75.30 ± 8.34 | 0.0185 |
| Fasting insulin (µIU/mL) | 15.89 ± 6.15 | 8.49 ± 1.21 | <0.01 |
| Fasting glucose (mg/dL) | 105.20 ± 29.81 | 94.02 ± 11.43 | 0.0043 |
| HOMA-IR | 4.41 ± 2.82 | 1.96 ± 0.52 | <0.01 |
| HbA1C (%) | 6.16 ± 1.35 | 5.38 ± 0.33 | <0.01 |
| Total cholesterol (mg/dL) | 165.51 ± 31.73 | 147.89 ± 32.15 | 0.0041 |
| LDL (mg/dL) | 95.64 ± 26.93 | 82.24 ± 18.03 | 0.0014 |
| HDL (mg/dL) | 43.81 ± 10.28 | 55.39 ± 17.20 | 0.0001 |
| Triglycerides (mg/dL) | 127.77 ± 64.01 | 89.65 ± 26.28 | <0.01 |
| Nitric oxide (µmol/L) | 3.28 ± 1.54 | 6.41 ± 2.43 | <0.01 |
| Alkaline phosphatase (U/L) | 80.64 ± 18.04 | 68.87 ± 21.52 | 0.0026 |
| AST (U/L) | 19.44 ± 12.75 | 17.78 ± 5.87 | 0.3340 |
| ALT (U/L) | 22.12 ± 19.81 | 19.83 ± 8.83 | 0.3854 |
| Total protein (g/dL) | 7.22 ± 0.44 | 7.11 ± 0.52 | 0.2221 |
| Albumin (g/dL) | 4.13 ± 0.36 | 4.44 ± 0.41 | <0.01 |
| Hemoglobin (g/dL) | 12.47 ± 1.29 | 13.93 ± 1.67 | <0.01 |
| Brachial FMD (%) | 5.78 ± 3.44 | 17.43 ± 7.34 | <0.01 |
| Arteriolar FID (%) | 48.32 ± 1.94 | 76.11 ± 3.21 | <0.01 |
| Leptin (ng/mL) | 33.93 ± 12.63 | 10.30 ± 5.93 | <0.01 |
| Adiponectin (µg/mL) | 5.88 ± 1.87 | 14.43 ± 7.98 | <0.01 |
| Leptin/adiponectin ratio | 6.35 ± 3.43 | 1.06 ± 1.24 | <0.01 |
| IL6 (pg/mL) | 22.01 ± 12.54 | 5.34 ± 2.56 | <0.01 |
| CRP (mg/L) | 3.89 ± 1.51 | 0.67 ± 0.18 | <0.01 |
| Hypertensive | 34 (45.3%) | 0 (0%) | <0.01 |
| Diabetic | 20 (26.7%) | 0 (0%) | <0.01 |
| Insulin resistant | 36 (48.0%) | 0 (0%) | <0.01 |
| Dyslipidemia | 34 (45.3%) | 0 (0%) | <0.01 |
| Hepatic steatosis | 43 (57.3%) | 0 (0%) | <0.01 |
| Impaired Vascular Function | 46 (61.3%) | 0 (0%) | <0.01 |
| Systemic inflammation | 49 (65.3%) | 12 (26.1%) | <0.01 |
| Target Molecules of Significant Difference | Regulators | Diseases and Functions | Consistency Score | Nodal Total | Known Regulator-Disease/Function Relationship |
|---|---|---|---|---|---|
| ADIPOQ, CAT, CRP, FGG, FN1, S100A8, SAA1, SERPINF1, TTR, VCAM1 | TNF | Apoptosis of endothelial cells, Apoptosis of muscle cells, Damage of endothelial tissue, Interaction of mononuclear leukocytes | 4.111 | 15 | 100% (4/4) |
| C2, CFB, CRP, FN1, VCAM1 | CG (complex), IL1 (family) | Cellular infiltration by leukocytes | 3.578 | 8 | 100% (2/2) |
| ADIPOQ, CRP, FGG, FN1, KRT1, S100A8, SAA1, SERPINF1, VCAM1 | TNF | Inflammatory response | −2.667 | 11 | 100% (1/1) |
| ADIPOQ, CRP, FGG, FN1, S100A8, SAA1, SERPINF1, VCAM1 | TNF | Activation of leukocytes | −3.536 | 10 | 100% (1/1) |
| ADIPOQ, CRP, FGG, FN1, S100A8, SAA1, SERPINF1, VCAM1 | TNF | Activation of myeloid cells | −3.889 | 10 | 100% (1/1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakab, M.S.; Asada, M.C.; Mirza, I.; Morsy, M.H.; Mostafa, A.; Bianco, F.M.; Ali, M.M.; Hassan, C.; Masrur, M.A.; Layden, B.T.; et al. Adiposome Proteomics Uncover Molecular Signatures of Cardiometabolic Risk in Obese Individuals. Proteomes 2025, 13, 39. https://doi.org/10.3390/proteomes13030039
Rakab MS, Asada MC, Mirza I, Morsy MH, Mostafa A, Bianco FM, Ali MM, Hassan C, Masrur MA, Layden BT, et al. Adiposome Proteomics Uncover Molecular Signatures of Cardiometabolic Risk in Obese Individuals. Proteomes. 2025; 13(3):39. https://doi.org/10.3390/proteomes13030039
Chicago/Turabian StyleRakab, Mohamed Saad, Monica C. Asada, Imaduddin Mirza, Mohammed H. Morsy, Amro Mostafa, Francesco M. Bianco, Mohamed M. Ali, Chandra Hassan, Mario A. Masrur, Brian T. Layden, and et al. 2025. "Adiposome Proteomics Uncover Molecular Signatures of Cardiometabolic Risk in Obese Individuals" Proteomes 13, no. 3: 39. https://doi.org/10.3390/proteomes13030039
APA StyleRakab, M. S., Asada, M. C., Mirza, I., Morsy, M. H., Mostafa, A., Bianco, F. M., Ali, M. M., Hassan, C., Masrur, M. A., Layden, B. T., & Mahmoud, A. M. (2025). Adiposome Proteomics Uncover Molecular Signatures of Cardiometabolic Risk in Obese Individuals. Proteomes, 13(3), 39. https://doi.org/10.3390/proteomes13030039








