Molecular Remodeling of the Sperm Proteome Following Varicocele Sclero-Embolization: Implications for Semen Quality Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Semen Analysis
2.3. Proteomic Analysis
2.4. Western Blot
2.5. Statistical and Bioinformatics Analysis
3. Results
3.1. Patient Characteristics and Semen Analysis
3.2. Proteome Analysis
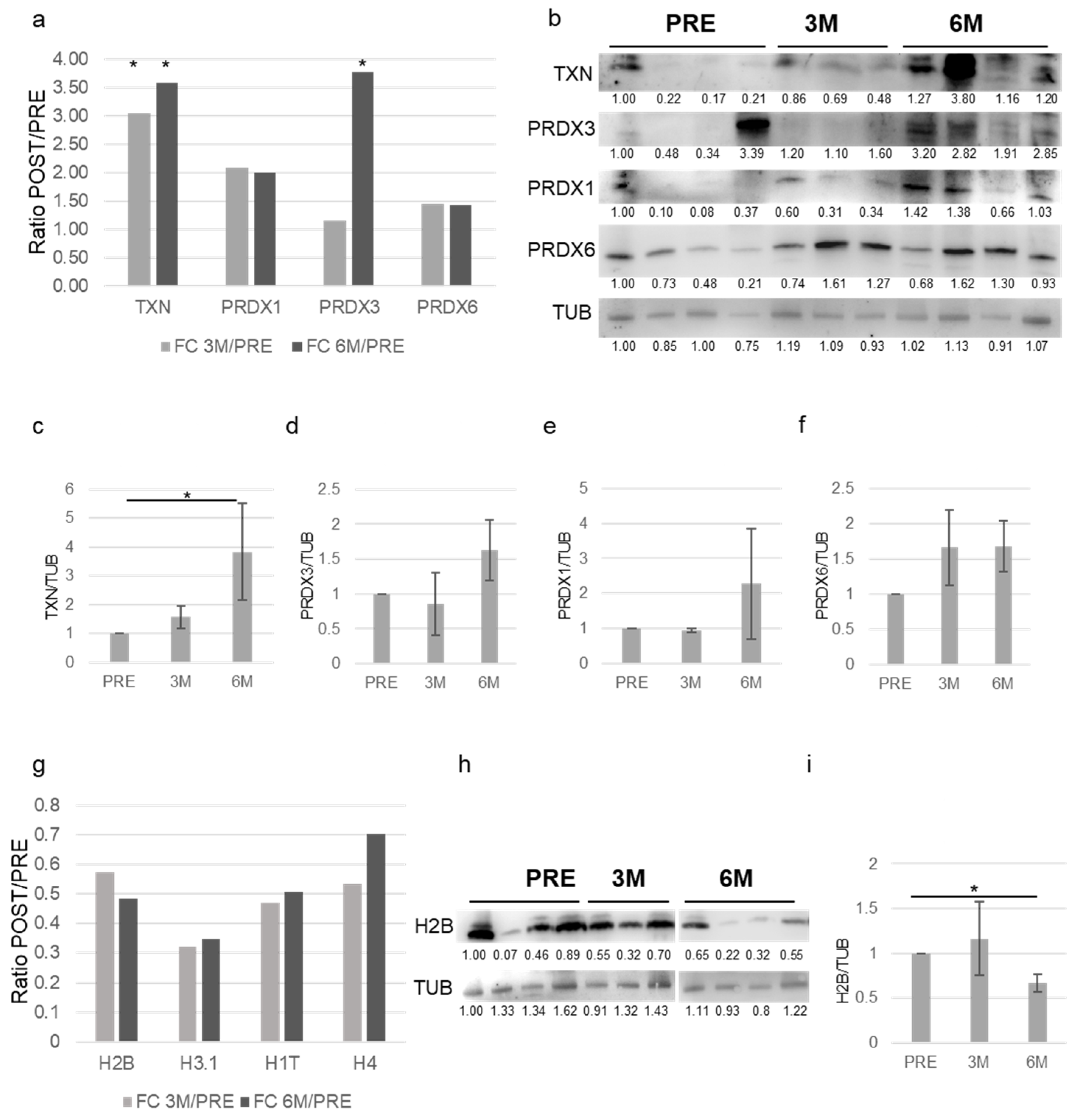
3.3. Validation of the Proteomics Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiba, K.; Fujisawa, M. Clinical Outcomes of Varicocele Repair in Infertile Men: A Review. World J. Men’s Health 2016, 34, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Sharma, R. Insight into oxidative stress in varicocele-associated male infertility: Part 1. Nat. Rev. Urol. 2012, 9, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Arya, D.; Thakur, M.; Thakur, R. Varicocele-associated male infertility: Cellular and molecular perspectives of pathophysiology. Andrology 2022, 10, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, R.I.; Agüero, G.; Ceballos, C.; Echeverría, S.; Hotaling, J.M. Varicoceles: Prevalence and pathogenesis in adult men. Fertil. Steril. 2017, 107, 1321–1329. [Google Scholar] [CrossRef]
- Pasqualotto, F.F.; Nelson, M.; Crippa, F.; Lucon, A.M.; Hallak, J.; Cedenho, A.P.; Borges, E.; Andrade, R.M. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil. Steril. 2008, 89, 629–634. [Google Scholar] [CrossRef]
- Abd-Elmoaty, M.A.; Abdelkawy, M.A.; El-Sherbiny, M.; El-Sayed, M.A.; Shokeir, A.A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil. Steril. 2010, 94, 1336–1339. [Google Scholar] [CrossRef]
- Salonia, A.; Akbal, C.; Hatzichristou, D.; Khera, M.; Le, T. EAU Guidelines on Sexual and Reproductive Health; European Association of Urology: Arnhem, The Netherlands, 2024; Available online: https://uroweb.org/guideline/sexual-and-reproductive-health/ (accessed on 31 May 2023).
- Grande, G.; Chianese, A.; Calogero, A.E.; Ciancia, E.; Candini, G.; Cito, G.; Tiziano, G.; La Marca, A.; Calabrese, A. Comprehensive diagnostic and therapeutic approach to male factor infertility aimed at natural fertility: A multicentric retrospective cohort study. Andrology 2025, 13, 252–261. [Google Scholar] [CrossRef]
- Mirilas, P.; Mentessidou, A. Microsurgical Subinguinal Varicocelectomy in Children, Adolescents, and Adults: Surgical Anatomy and Anatomically Justified Technique. J. Androl. 2012, 33, 1505–1513. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Z.; Lu, G.; Zhang, Z.; Li, J.; Zhang, H.; Yao, J.; Shi, B.; Zhang, Y.; Wang, X. Comparing Endovascular and Surgical Treatments for Varicocele: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2022, 33, 1310–1320. [Google Scholar] [CrossRef]
- Franco, A.; Belluomini, M.; Colletto, G.; Fumagalli, M. Varicocele: To Treat or Not to Treat? J. Clin. Med. 2023, 12, 4062. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Liu, J.; Zhao, L.; Zhang, J.; Wu, X.; Han, X.; Li, X.; Chen, L. Relationship between Varicocele and Sperm DNA Damage and the Effect of Varicocele Repair: A Meta-Analysis. Reprod. Biomed. Online 2012, 25, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Joo, H.S.; Kim, S.C.; Hwang, T.K.; Ko, K.; Jeong, J.S.; Lee, J.K. Clinical Significance of Subclinical Varicocelectomy in Male Infertility: Systematic Review and Meta-Analysis. Andrologia 2016, 48, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Schauer, I.; Seitz, G.; Kratzik, C.; Schubert, H.; Becker, C.; Weidner, W. Impact of Varicocelectomy on Sperm Parameters: A Meta-Analysis. J. Urol. 2012, 188, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Frattini, A.; La Marca, A.; Marra, G.; Di Maggio, M.; Ferrante, S.; De Lorenzi, F.; Calogero, A.E. Acquired Male Hypogonadism in the Post-Genomic Era—A Narrative Review. Life 2023, 13, 1854. [Google Scholar] [CrossRef]
- Milardi, D.; Kalogris, S.; Reale, F.; Di Francesco, F.; Venturini, E.; Piacente, G.; Bovi, G.; D’Onofrio, L. Novel Biomarkers of Androgen Deficiency From Seminal Plasma Profiling Using High-Resolution Mass Spectrometry. J. Clin. Endocrinol. Metab. 2014, 99, 2214–2221. [Google Scholar] [CrossRef][Green Version]
- Grande, G.; Calogero, A.E.; Porrello, G.; Di Maggio, M.; Bastianelli, G.; Marra, G. Semen Proteomics Reveals the Impact of Enterococcus faecalis on Male Fertility. Protein Pept. Lett. 2018, 25, 182–188. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.; Sikka, S.; Tiwari, P.; Barbagallo, F.; Muti, N. Major Protein Alterations in Spermatozoa from Infertile Men with Unilateral Varicocele. Reprod. Biol. Endocrinol. 2015, 13, 29. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.; Sikka, S.; Tiwari, P.; Barbagallo, F.; Muti, N. Differential Proteomic Profiling of Spermatozoal Proteins of Infertile Men with Unilateral or Bilateral Varicocele. Urology 2015, 86, 237–244. [Google Scholar] [CrossRef]
- Camargo, M.; Casanova, R.; Gualdrón, A.; Rodríguez, I.; Sánchez, J.; García, M.; Fernández, J. Unbiased Label-Free Quantitative Proteomic Profiling and Enriched Proteomic Pathways in the Seminal Plasma of Adult Men Before and After Varicocelectomy. Hum. Reprod. 2013, 28, 112–123. [Google Scholar] [CrossRef]
- Del Giudice, P.T.; Fabbri, R.; Pellegrini, P.; Franchi, D.; Bertini, L.; Ricci, G.; Bonini, P.; Donato, F.; Del Balzo, U.; Scarpini, M. Changes in the Seminal Plasma Proteome of Adolescents Before and After Varicocelectomy. Fertil. Steril. 2013, 99, 1192–1199. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A. Proteomic Profiling of Seminal Plasma Proteins from Varicocele Patients. World J. Men’s Health 2021, 39, 112–121. [Google Scholar] [CrossRef]
- Hosseinifar, H.; Fathi, M.; Khani, P.; Aliyari, M.; Rajabzadeh, A. Study of the Effect of Varicocelectomy on Sperm Protein Expression in Patients with Varicocele and Poor Sperm Quality via Two-Dimensional Gel Electrophoresis. J. Assist. Reprod. Genet. 2014, 31, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Tenzer, S. Label-Free Quantification in Ion Mobility–Enhanced Data-Independent Acquisition Proteomics. Nat. Protoc. 2016, 11, 795–812. [Google Scholar] [CrossRef]
- Minas, A.; Angelopoulou, R.; Sifakis, S.; Spermoulas, K.; Matalliotakis, M.; Kalampokas, T.; Tsatsakis, A.; Mamoulakis, C. Insight into Inflammation Involvement in Varicocele: A Narrative Review. Am. J. Reprod. Immunol. 2023, 90, e13786. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.A.; Allamaneni, S.S. Relationship between Oxidative Stress, Varicocele and Infertility: A Meta-Analysis. Reprod. Biomed. Online 2006, 12, 630–633. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Raina, K. Impact of Varicocele Repair on Semen Parameters in Infertile Men: A Systematic Review and Meta-Analysis. World J. Men’s Health 2023, 41, 320–337. [Google Scholar] [CrossRef]
- Baazeem, A.; Belzile, E.; Ciampi, A.; Dohle, G.; Jarvi, K.; Salonia, A.; Weidner, W.; Zini, A. Varicocele and Male Factor Infertility Treatment: A New Meta-Analysis and Review of the Role of Varicocele Repair. Eur. Urol. 2011, 60, 796–808. [Google Scholar] [CrossRef]
- Tiseo, B.C.; Esteves, S.C. Summary Evidence on the Effects of Varicocele Treatment on Improving Natural Fertility in Subfertile Men. Asian J. Androl. 2016, 18, 229–233. [Google Scholar] [CrossRef]
- Finelli, R.; Darbandi, S.; Pushparaj, P.N.; Henkel, R.; Ko, E.; Agarwal, A. In Silico Sperm Proteome Analysis to Investigate DNA Repair Mechanisms in Varicocele Patients. Front. Endocrinol. 2021, 12, 757592. [Google Scholar] [CrossRef]
- Finetti, F.; Cassioli, C.; Cendron, L.; Fanelli, G.; Bernardi, A.; Bazzoni, R.; Magni, M.; Baldari, C.T. The Intraflagellar Transport Protein IFT20 Recruits ATG16L1 to Early Endosomes to Promote Autophagosome Formation in T Cells. Front. Cell Dev. Biol. 2021, 9, 634003. [Google Scholar] [CrossRef] [PubMed]
- Belletti, B.; Baldassarre, G. Stathmin: A Protein with Many Tasks. New Biomarkers and Potential Targets in Cancer. Expert Opin. Ther. Targets 2011, 15, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, E.; Evrard, B.; Coulaud, D.; Perrard, M.-H.; Durand, P.; Jegou, B. Proteome Analysis of Rat Spermatogonia: Reinvestigation of Stathmin Spatiotemporal Expression within the Testis. Mol. Reprod. Dev. 2001, 60, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kostetskii, I.; Tang, W.; Sapiro, R.; Wei, Z.; Patel, A.M.; Bennett, J.; Gerton, G.L.; Moss, S.B.; Radice, G.L. Dual Functions of the Intraflagellar Transport Protein IFT20 in Spermiogenesis: The Formation of Sperm Flagella and Removal of the Cytoplasm by Autophagy. bioRxiv 2016. [Google Scholar] [CrossRef]
- Moreno, R.D.; Vega, M.; Zurita, A.R. The Emerging Role of Matrix Metalloproteases of the ADAM Family in Male Germ Cell Apoptosis. Spermatogenesis 2011, 1, 253–262. [Google Scholar] [CrossRef]
- Shalini, S.; Bansal, M.P. Role of Selenium in Regulation of Spermatogenesis: Involvement of Activator Protein 1. BioFactors 2005, 23, 151–162. [Google Scholar] [CrossRef]
- Newton, T.D.; Kwon, S.; Chang, C.; Kerr, J.; Pluth, M.D. Hydrolysis-Based Small Molecule Hydrogen Selenide (H2Se) Donors for Intracellular H2Se Delivery. J. Am. Chem. Soc. 2021, 143, 18559–18567. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.; Meng, Q.; Han, H.; et al. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Scarlata, E. Oxidative Stress and Reproductive Function: Protection of Mammalian Spermatozoa against Oxidative Stress. Reproduction 2022, 164, F1–F14. [Google Scholar] [CrossRef]
- Afsari, M.; Fesahat, F.; Talebi, A.R.; Agarwal, A.; Henkel, R.; Zare, F.; Gül, M.; Iraci, N.; Cannarella, R.; Makki, M.; et al. ANXA2, SP17, SERPINA5, PRDX2 Genes, and Sperm DNA Fragmentation Differentially Represented in Male Partners of Infertile Couples with Normal and Abnormal Sperm Parameters. Andrologia 2022, 54, e14556. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Wang, P.; Li, S.; Li, L.; Zhang, S.; Wei, H. Semen Protein CRISP3 Promotes Reproductive Performance of Boars through Immunomodulation. Int. J. Mol. Sci. 2024, 25, 2264. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.; Scorilas, A.; Soosaipillai, A.; Earle, T.; Mullen, B.; Diamandis, E.P. Association between Kallikrein-Related Peptidases (KLKs) and Macroscopic Indicators of Semen Analysis: Their Relation to Sperm Motility. Biol. Chem. 2009, 390, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Sharif, F.; Ashour, M.; Abuwarda, H.; Ismail, S.; Salem, N.; Suleiman, R.; Hassouna, N. Antioxidant Genes Variants and Their Association with Sperm DNA Fragmentation. Biochem. Genet. 2024, 62, 79–90. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Toshimori, K. Organization and Modifications of Sperm Acrosomal Molecules during Spermatogenesis and Epididymal Maturation. Microsc. Res. Tech. 2003, 61, 39–45. [Google Scholar] [CrossRef]
- Tulsiani, D.R.P.; Abou-Haila, A.; Loeser, C.R.; Pereira, B.M. Biological and Functional Significance of the Sperm Acrosome and Acrosomal Enzymes in Mammalian Fertilization. Exp. Cell Res. 1998, 240, 151–164. [Google Scholar] [CrossRef]
- Deppe, M.; Földes, A.; Willms, D.; Bergmann, M.; Waberski, D. Effect of Protease Inhibitors on the Acrosome Reaction and Sperm–Zona Pellucida Binding in Bovine Sperm. Reprod. Domest. Anim. 2008, 43, 348–353. [Google Scholar] [CrossRef]
- Mulla, K.F.E.; Rehman, R.; Qureshi, A.A. The Effects of Smoking and Varicocele on Human Sperm Acrosin Activity and the Acrosome Reaction. Hum. Reprod. 1995, 10, 1208–1212. [Google Scholar] [CrossRef]
- Esteves, S.C.; Roque, M.; Bedoschi, G.M.; Agarwal, A. Sperm DNA Fragmentation Testing: Summary Evidence and Clinical Practice Recommendations. Andrologia 2021, 53, e13874. [Google Scholar] [CrossRef]
- Liang, J.; Wen, X.; Zeng, Y.; Sun, L.; Li, T.; Zhang, J.; Ma, J.; Yang, J. Comparison of Proteomic Profiles from the Testicular Tissue of Males with Impaired and Normal Spermatogenesis. Syst. Biol. Reprod. Med. 2021, 67, 145–156. [Google Scholar] [CrossRef]
- Hammoud, S.; Emery, B.; Carrell, D. Altered protamine expression and diminished spermatogenesis: What is the link? Hum. Reprod. Update 2007, 13, 313–327. [Google Scholar] [CrossRef]
- Miller, D.; Brinkworth, M.; Iles, D. Paternal DNA Packaging in Spermatozoa: More than the Sum of Its Parts? DNA, Histones, Protamines and Epigenetics. Reproduction 2010, 139, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Wang, M.; Zhang, X.; Ren, J.; Yuan, Y.; Sha, J.; Guo, X. Multi-Omics Approaches for Revealing the Epigenetic Regulation of Histone H3.1 during Spermatogonial Stem Cell Differentiation In Vitro. Int. J. Mol. Sci. 2023, 24, 3314. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, Y.; Yang, Y.; Xu, L. Structural Insights into Instability of the Nucleosome Driven by Histone Variant H3T. Biochem. Biophys. Res. Commun. 2024, 727, 150307. [Google Scholar] [CrossRef]
- Montellier, E.; Boussouar, F.; Rousseaux, S.; Zhang, K.; Buchou, T.; Fenaille, F.; Shiota, H.; Debernardi, A.; Héry, P.; Curtet, S.; et al. Chromatin-to-Nucleoprotamine Transition Is Controlled by the Histone H2B Variant TH2B. Genes Dev. 2013, 27, 1680–1692. [Google Scholar] [CrossRef]
- Razi, M.; Sadrkhanloo, R.A.; Malekinejad, H.; Sarafzadeh-Rezaei, F. Varicocele Time-dependently Affects DNA Integrity of Sperm Cells: Evidence for Lower In Vitro Fertilization Rate in Varicocele-Positive Rats. Int. J. Fertil. Steril. 2011, 5, 174–185. [Google Scholar] [PubMed] [PubMed Central]
- Oliva, R. Protamines and Male Infertility. Hum. Reprod. Update 2006, 12, 417–435. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, L.; Su, P.; Li, L.; Zhang, M.; Zhang, Y. Autophagy: A Multifaceted Player in the Fate of Sperm. Hum. Reprod. Update 2021, 28, 200–231. [Google Scholar] [CrossRef]
- Sadeghi, N.; Abdi, S.; Kianmehr, Z.; Ghanbari, M.; Soukhtanloo, M. Signs of ROS-Associated Autophagy in Testis and Sperm in a Rat Model of Varicocele. Oxid. Med. Cell. Longev. 2020, 2020, 5140383. [Google Scholar] [CrossRef]
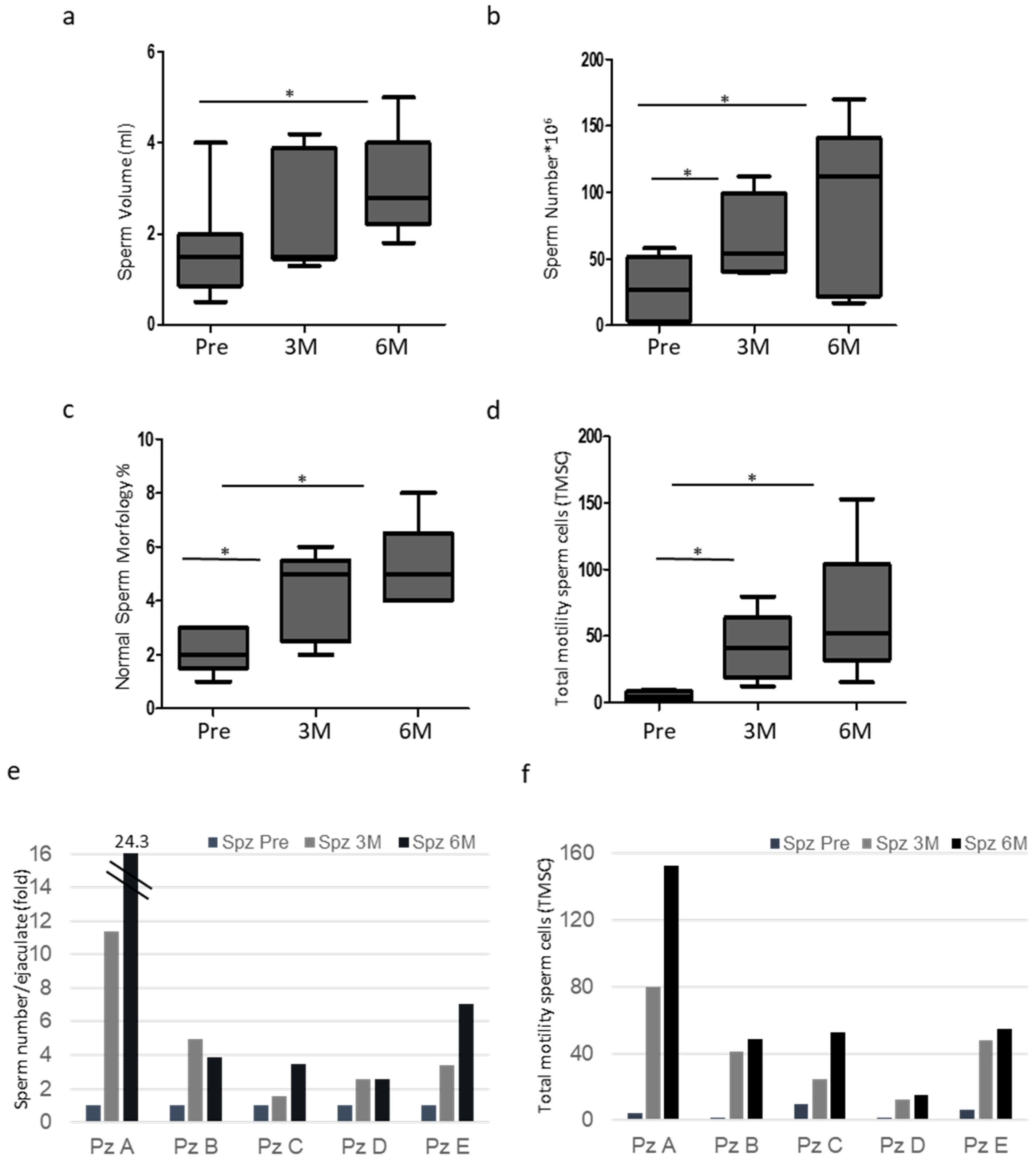
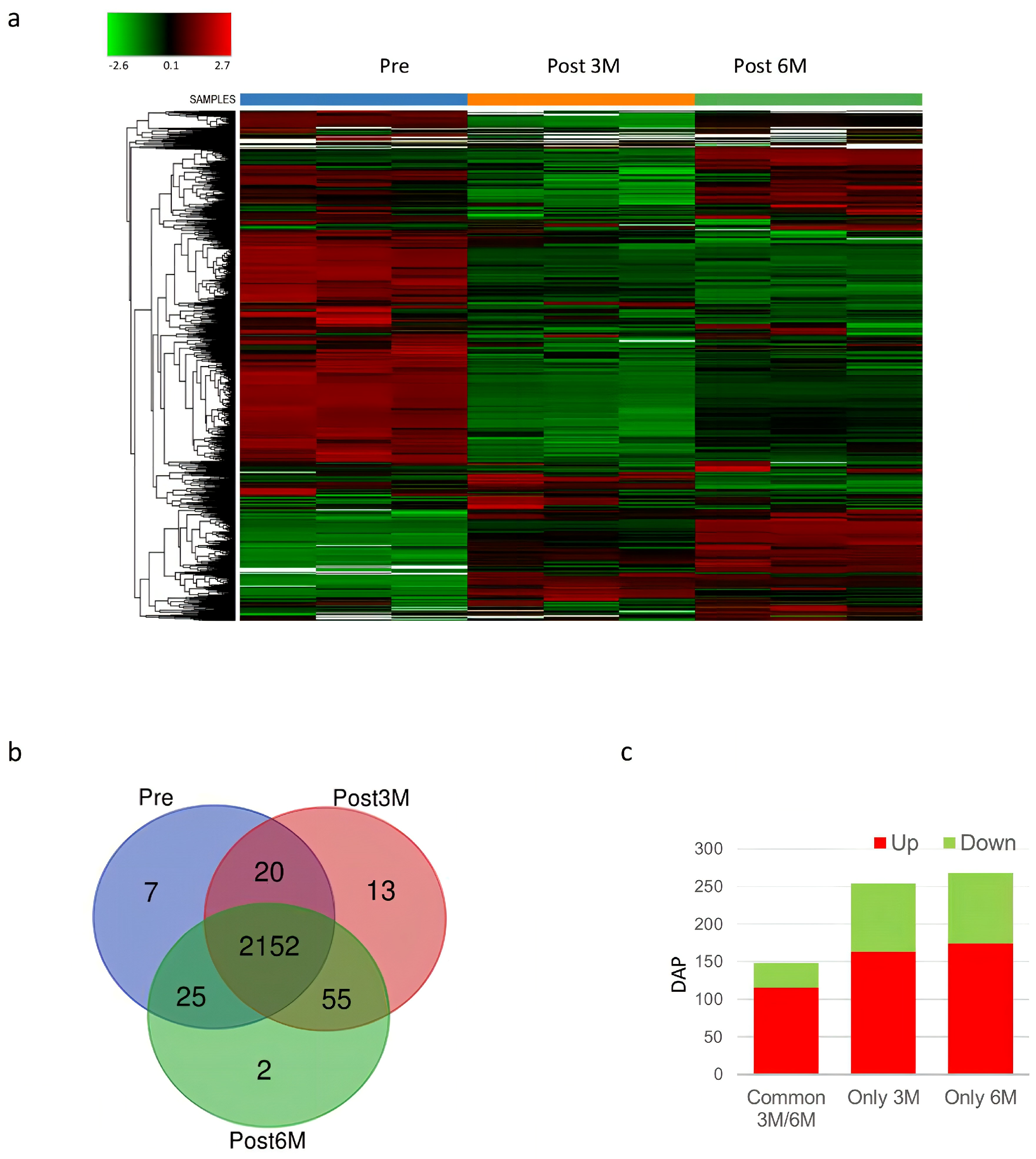
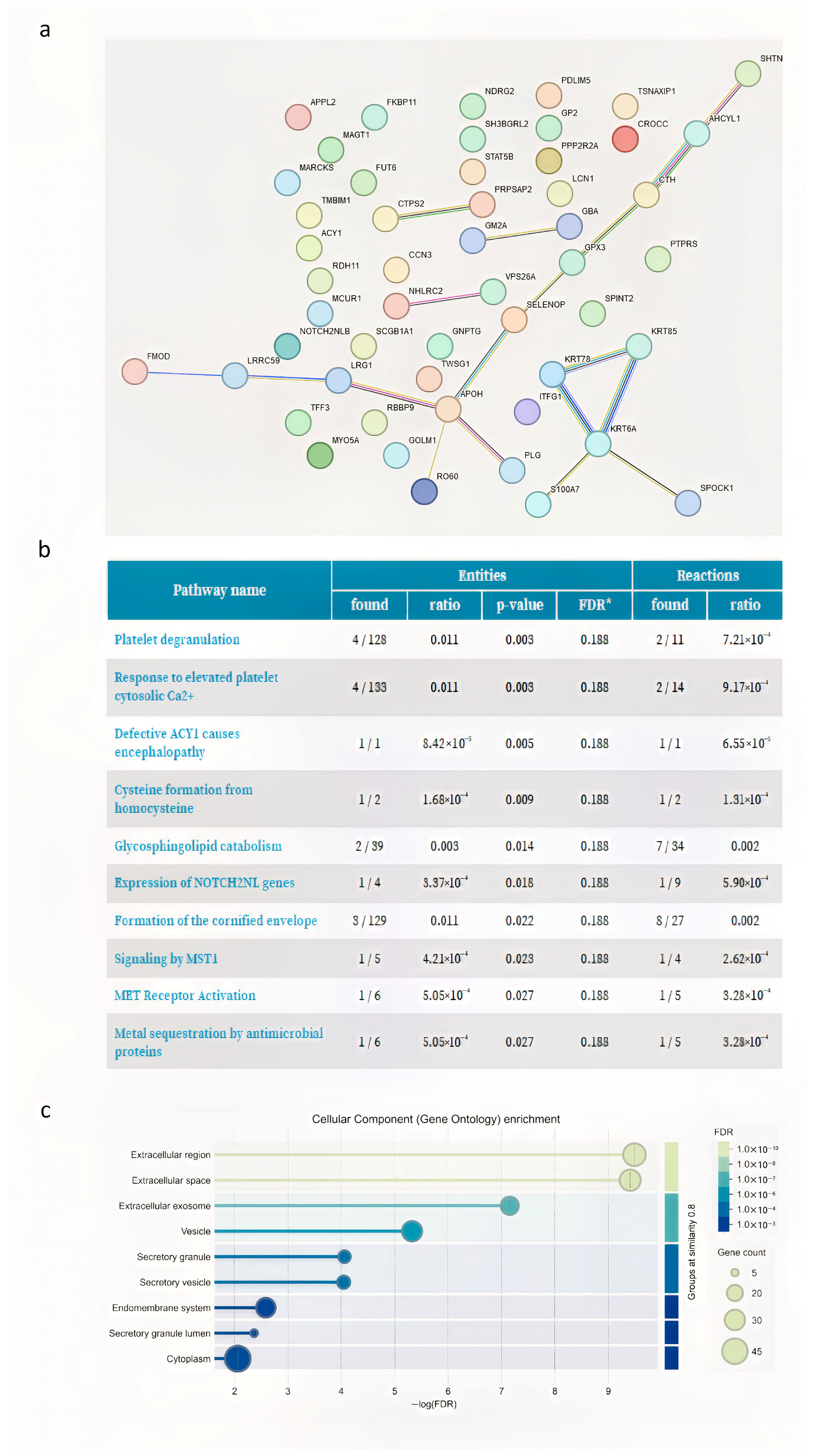
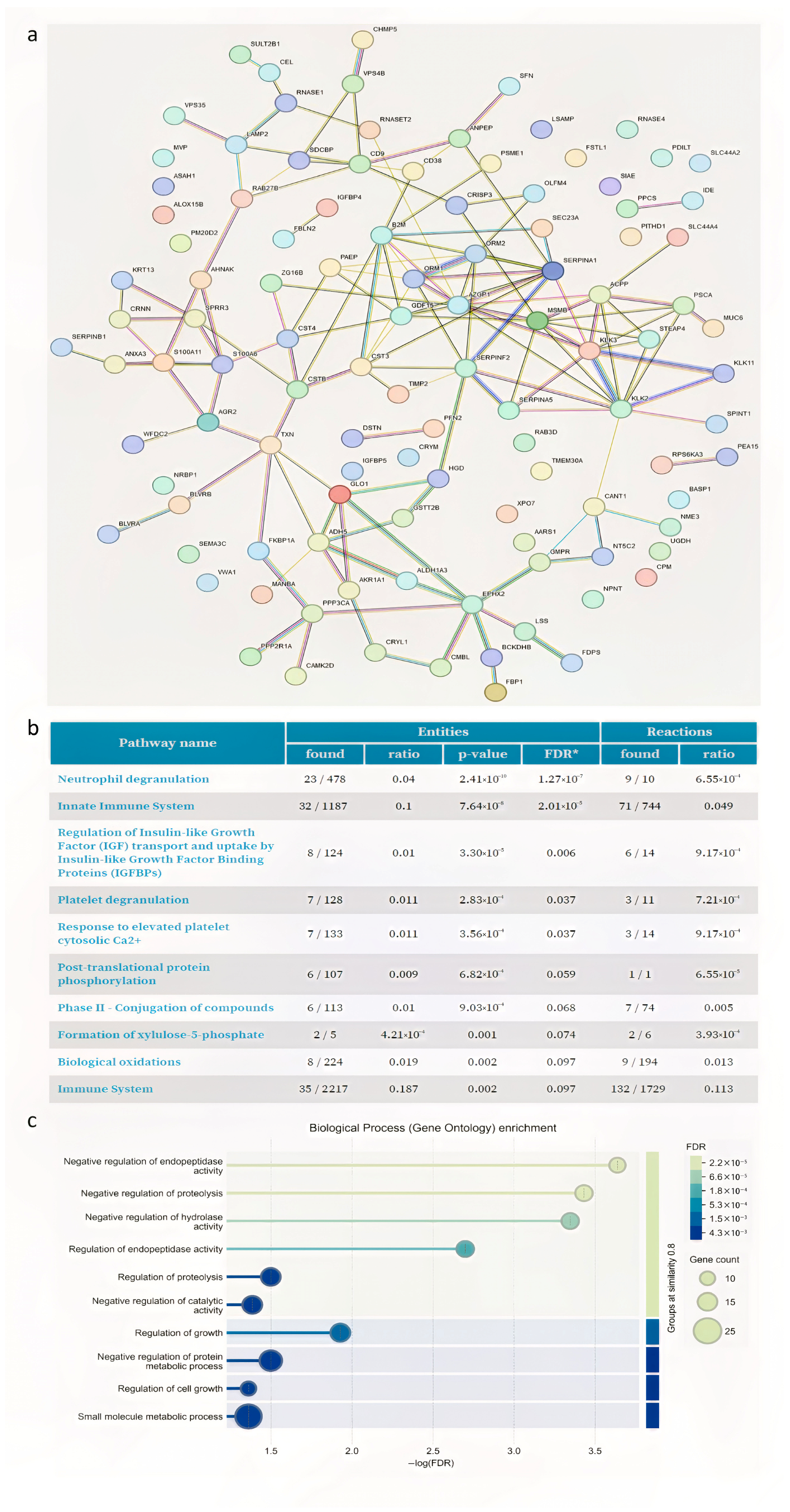

| Patients | Age | Varicocele Features |
|---|---|---|
| A | 41 | Left, Grade III |
| B | 39 | Left, Grade III |
| C | 32 | Left, Grade III |
| D | 23 | Left, Grade III |
| E | 43 | Left, Grade IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milardi, D.; Vergani, E.; Mancini, F.; Di Nicuolo, F.; Teveroni, E.; Vodola, E.P.; Oliva, A.; Grande, G.; Cina, A.; Iezzi, R.; et al. Molecular Remodeling of the Sperm Proteome Following Varicocele Sclero-Embolization: Implications for Semen Quality Improvement. Proteomes 2025, 13, 34. https://doi.org/10.3390/proteomes13030034
Milardi D, Vergani E, Mancini F, Di Nicuolo F, Teveroni E, Vodola EP, Oliva A, Grande G, Cina A, Iezzi R, et al. Molecular Remodeling of the Sperm Proteome Following Varicocele Sclero-Embolization: Implications for Semen Quality Improvement. Proteomes. 2025; 13(3):34. https://doi.org/10.3390/proteomes13030034
Chicago/Turabian StyleMilardi, Domenico, Edoardo Vergani, Francesca Mancini, Fiorella Di Nicuolo, Emanuela Teveroni, Emanuele Pierpaolo Vodola, Alessandro Oliva, Giuseppe Grande, Alessandro Cina, Roberto Iezzi, and et al. 2025. "Molecular Remodeling of the Sperm Proteome Following Varicocele Sclero-Embolization: Implications for Semen Quality Improvement" Proteomes 13, no. 3: 34. https://doi.org/10.3390/proteomes13030034
APA StyleMilardi, D., Vergani, E., Mancini, F., Di Nicuolo, F., Teveroni, E., Vodola, E. P., Oliva, A., Grande, G., Cina, A., Iezzi, R., Cicchinelli, M., Iavarone, F., Baroni, S., Ferlin, A., Urbani, A., & Pontecorvi, A. (2025). Molecular Remodeling of the Sperm Proteome Following Varicocele Sclero-Embolization: Implications for Semen Quality Improvement. Proteomes, 13(3), 34. https://doi.org/10.3390/proteomes13030034










