Systems Biology of Recombinant 2G12 and 353/11 mAb Production in CHO-K1 Cell Lines at Phosphoproteome Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Sample Preparation
2.2. Protein In-Solution Digestion and Peptide Desalting
2.3. Phosphopeptide Enrichment
2.4. LC-MS/MS Analysis
2.5. Data Processing LC-MS/MS
2.6. Bioinformatics Analysis
2.7. Computing Resources
3. Results and Discussion
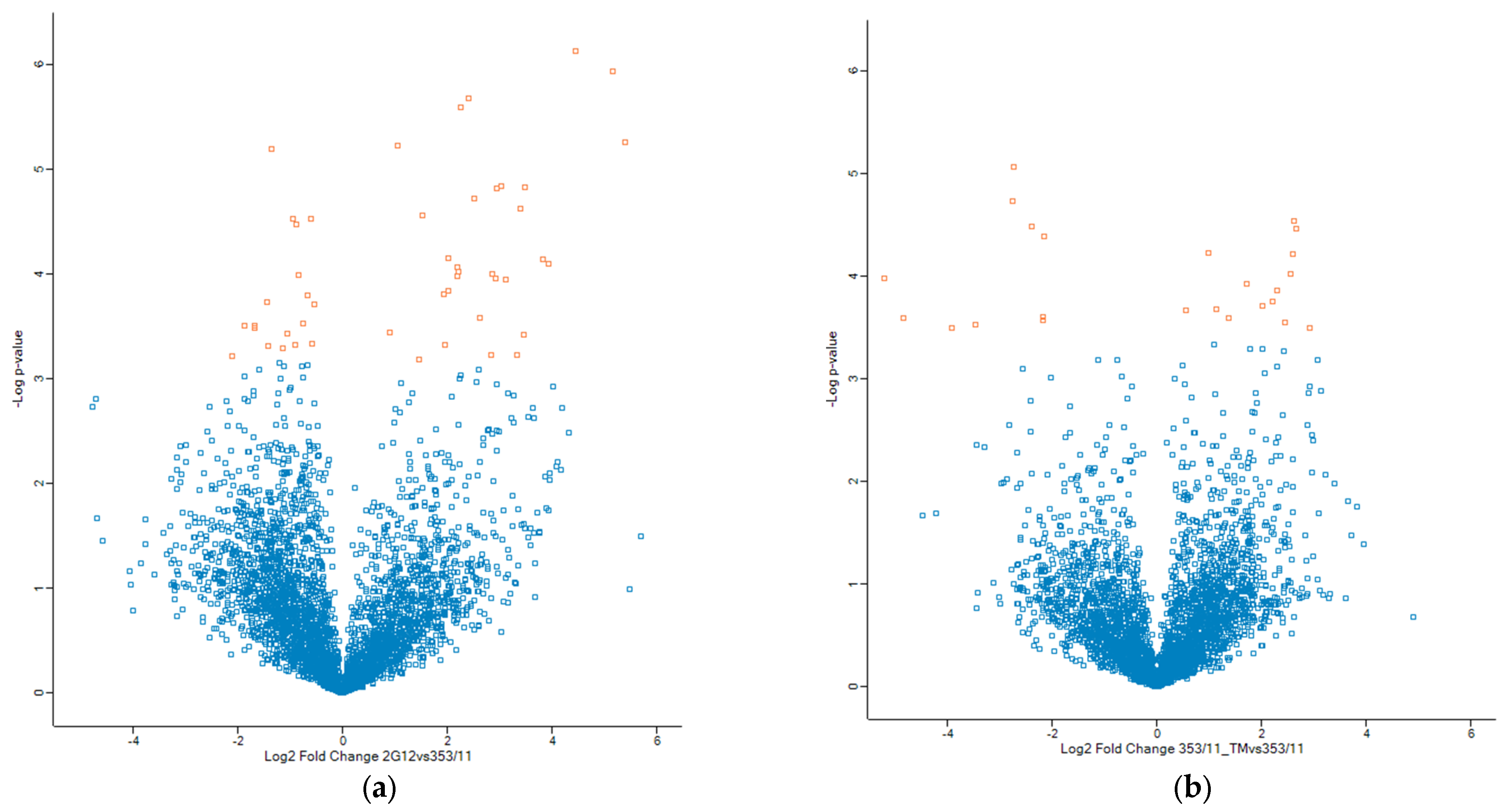
3.1. Quantative Phosphoprotemics Profiling Reveals Diferences
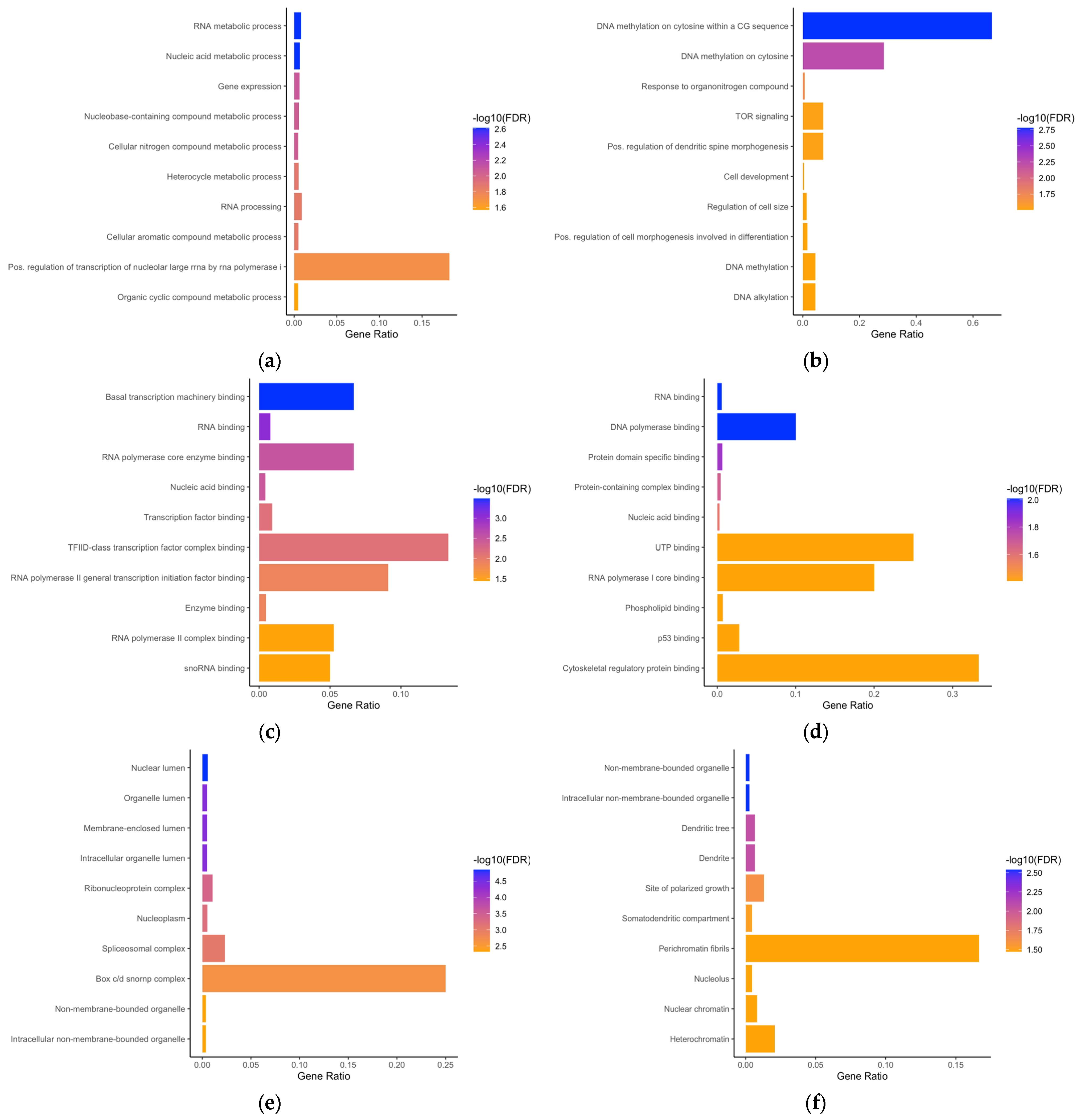
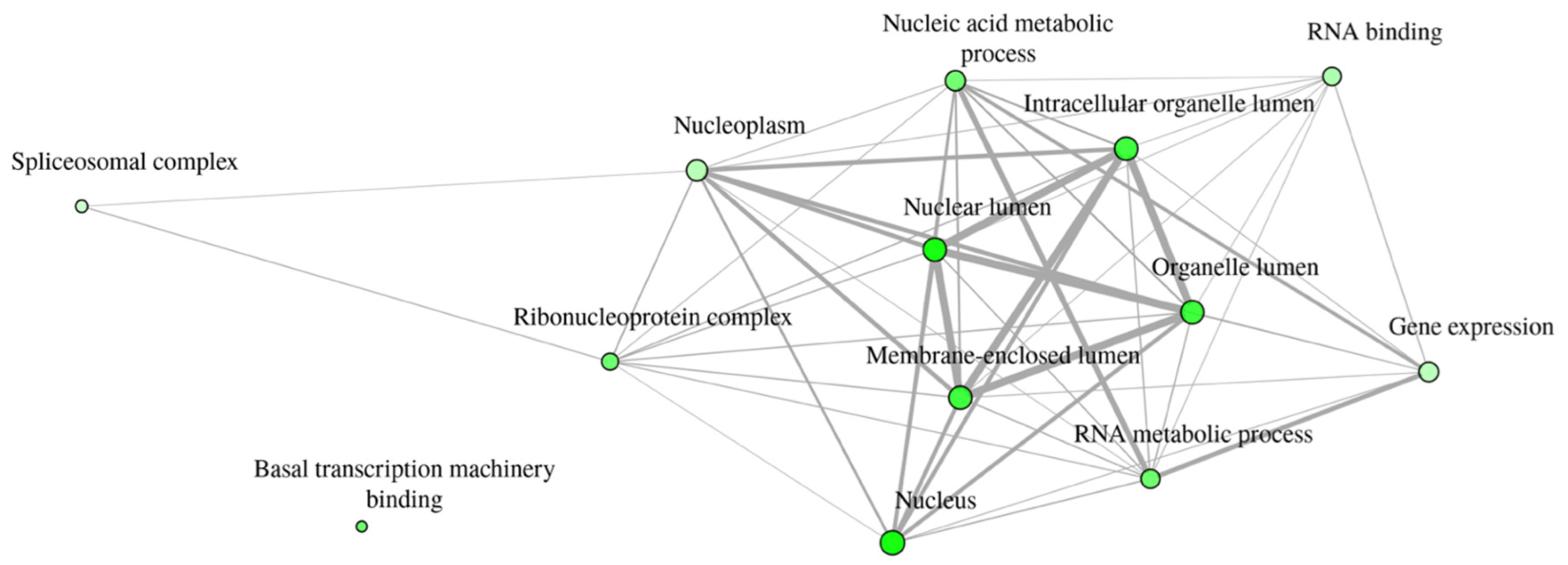
3.2. Gene Ontology Enrichment Analyses
3.3. Dynamics of Phosphorylation in the Two Different CHO Cell Lines That Produce the Antibodies
3.4. Function of Ribosomal Proteins, DNA Methylation, and Regulation of Chromatin Affected by Tunicamycin
3.5. Identification of Phosphorylated Key Proteins Under TM as an Extrinsic Stress Factor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, G.; Walsh, E. Biopharmaceutical benchmarks 2022. Nat. Biotechnol. 2022, 40, 1722–1760. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef]
- Baycin-Hizal, D.; Tabb, D.L.; Chaerkady, R.; Chen, L.; Lewis, N.E.; Nagarajan, H.; Sarkaria, V.; Kumar, A.; Wolozny, D.; Colao, J.; et al. Proteomic analysis of Chinese hamster ovary cells. J. Proteome Res. 2012, 11, 5265–5276. [Google Scholar] [CrossRef] [PubMed]
- Meleady, P.; Doolan, P.; Henry, M.; Barron, N.; Keenan, J.; O’Sullivan, F.; Clarke, C.; Gammell, P.; Melville, M.W.; Leonard, M.; et al. Sustained productivity in recombinant Chinese hamster ovary (CHO) cell lines: Proteome analysis of the molecular basis for a process-related phenotype. BMC Biotechnol. 2011, 11, 78. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, S.; Bones, J.; Ray, S.; Cha, S.; Karger, B.L.; Li, J.J.; Wilson, L.; Hinckle, G.; Rossomando, A. A quantitative proteomic analysis of cellular responses to high glucose media in Chinese hamster ovary cells. Biotechnol. Prog. 2015, 31, 1026–1038. [Google Scholar] [CrossRef]
- Xu, N.; Ma, C.; Ou, J.; Sun, W.W.; Zhou, L.; Hu, H.; Liu, X.M. Comparative Proteomic Analysis of Three Chinese Hamster Ovary (CHO) Host Cells. Biochem. Eng. J. 2017, 124, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Sommeregger, W.; Mayrhofer, P.; Steinfellner, W.; Reinhart, D.; Henry, M.; Clynes, M.; Meleady, P.; Kunert, R. Proteomic differences in recombinant CHO cells producing two similar antibody fragments. Biotechnol. Bioeng. 2016, 113, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Heffner, K.M.; Hizal, D.B.; Yerganian, G.S.; Kumar, A.; Can, O.; O’Meally, R.; Cole, R.; Chaerkady, R.; Wu, H.; Bowen, M.A.; et al. Lessons from the Hamster: Cricetulus griseus Tissue and CHO Cell Line Proteome Comparison. J. Proteome Res. 2017, 16, 3672–3687. [Google Scholar] [CrossRef]
- Lakshmanan, M.; Kok, Y.J.; Lee, A.P.; Kyriakopoulos, S.; Lim, H.L.; Teo, G.; Poh, S.L.; Tang, W.Q.; Hong, J.; Tan, A.H.; et al. Multi-omics profiling of CHO parental hosts reveals cell line-specific variations in bioprocessing traits. Biotechnol. Bioeng. 2019, 116, 2117–2129. [Google Scholar] [CrossRef]
- Kaushik, P.; Curell, R.V.; Henry, M.; Barron, N.; Meleady, P. LC-MS/MS-based quantitative proteomic and phosphoproteomic analysis of CHO-K1 cells adapted to growth in glutamine-free media. Biotechnol. Lett. 2020, 42, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Romanova, N.; Schelletter, L.; Hoffrogge, R.; Noll, T. Hyperosmolality in CHO cell culture: Effects on the proteome. Appl. Microbiol. Biotechnol. 2022, 106, 2569–2586. [Google Scholar] [CrossRef]
- Sulaj, E.; Schwaigerlehner, L.; Sandell, F.L.; Dohm, J.C.; Marzban, G.; Kunert, R. Quantitative proteomics reveals cellular responses to individual mAb expression and tunicamycin in CHO cells. Appl. Microbiol. Biotechnol. 2024, 108, 381. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Huhn, S.; Nelson, L.; Betenbaugh, M.; Du, Z. Significant impact of mTORC1 and ATF4 pathways in CHO cell recombinant protein production induced by CDK4/6 inhibitor. Biotechnol. Bioeng. 2022, 119, 1189–1206. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Heinrich, C.; Jabs, W.; Kaspar-Schonefeld, S.; Schmidt, A.; Rodrigues de Carvalho, N.; Albaum, S.P.; Baessmann, C.; Noll, T.; Hoffrogge, R. Label-free protein quantification of sodium butyrate treated CHO cells by ESI-UHR-TOF-MS. J. Biotechnol. 2017, 257, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Gallagher, C.; Kelly, R.M.; Frye, C.C.; Osborne, M.D.; Brady, C.P.; Barron, N.; Clynes, M.; Meleady, P. Clonal variation in productivity and proteolytic clipping of an Fc-fusion protein in CHO cells: Proteomic analysis suggests a role for defective protein folding and the UPR. J. Biotechnol. 2018, 281, 21–30. [Google Scholar] [CrossRef]
- Perez-Rodriguez, S.; Wulff, T.; Voldborg, B.G.; Altamirano, C.; Trujillo-Roldan, M.A.; Valdez-Cruz, N.A. Compartmentalized Proteomic Profiling Outlines the Crucial Role of the Classical Secretory Pathway during Recombinant Protein Production in Chinese Hamster Ovary Cells. ACS Omega 2021, 6, 12439–12458. [Google Scholar] [CrossRef] [PubMed]
- Bryan, L.; Henry, M.; Kelly, R.M.; Frye, C.C.; Osborne, M.D.; Clynes, M.; Meleady, P. Mapping the molecular basis for growth related phenotypes in industrial producer CHO cell lines using differential proteomic analysis. BMC Biotechnol. 2021, 21, 43. [Google Scholar] [CrossRef]
- Landry, C.R.; Levy, E.D.; Michnick, S.W. Weak functional constraints on phosphoproteomes. Trends Genet. 2009, 25, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Schelletter, L.; Albaum, S.; Walter, S.; Noll, T.; Hoffrogge, R. Clonal variations in CHO IGF signaling investigated by SILAC-based phosphoproteomics and LFQ-MS. Appl. Microbiol. Biotechnol. 2019, 103, 8127–8143. [Google Scholar] [CrossRef]
- Kaushik, P.; Henry, M.; Clynes, M.; Meleady, P. The Expression Pattern of the Phosphoproteome Is Significantly Changed During the Growth Phases of Recombinant CHO Cell Culture. Biotechnol. J. 2018, 13, e1700221. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Power, M.; Kaushik, P.; Coleman, O.; Clynes, M.; Meleady, P. Differential Phosphoproteomic Analysis of Recombinant Chinese Hamster Ovary Cells Following Temperature Shift. J. Proteome Res. 2017, 16, 2339–2358. [Google Scholar] [CrossRef]
- Bryan, L.; Henry, M.; Kelly, R.M.; Lloyd, M.; Frye, C.C.; Osborne, M.D.; Clynes, M.; Meleady, P. Global phosphoproteomic study of high/low specific productivity industrially relevant mAb producing recombinant CHO cell lines. Curr. Res. Biotechnol. 2021, 3, 49–56. [Google Scholar] [CrossRef]
- Johnson, L.N. The regulation of protein phosphorylation. Biochem. Soc. Trans. 2009, 37, 627–641. [Google Scholar] [CrossRef]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, R.R.; Nieves, E.; Weiss, L.M. The Methods Employed in Mass Spectrometric Analysis of Posttranslational Modifications (PTMs) and Protein-Protein Interactions (PPIs). Adv. Exp. Med. Biol. 2019, 1140, 169–198. [Google Scholar] [CrossRef]
- Cohen, P. The regulation of protein function by multisite phosphorylation—A 25 year update. Trends Biochem. Sci. 2000, 25, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.J.; Cohen, P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem. Sci. 1993, 18, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, T.E.; Jorgensen, T.J.; Jensen, O.N.; Larsen, M.R. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat. Protoc. 2006, 1, 1929–1935. [Google Scholar] [CrossRef]
- Levene, P.A.; Alsberg, C.L. The cleavage products of vitellin. J. Biol. Chem. 1906, 2, 127–133. [Google Scholar] [CrossRef]
- Mecham, D.K.; Olcott, H.S. An egg yolk protein containing 10% phosphorus. Fed. Proc. 1948, 7, 173. [Google Scholar] [PubMed]
- Burnett, G.; Kennedy, E.P. The enzymatic phosphorylation of proteins. J. Biol. Chem. 1954, 211, 969–980. [Google Scholar] [CrossRef]
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.K.; Quinn, A.M.; Hunter, T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 1988, 241, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Gunawardena, J. Multisite protein phosphorylation makes a good threshold but can be a poor switch. Proc. Natl. Acad. Sci. USA 2005, 102, 14617–14622. [Google Scholar] [CrossRef]
- Roach, P.J. Multisite and hierarchal protein phosphorylation. J. Biol. Chem. 1991, 266, 14139–14142. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.R.; Veenstra, T.D. Characterization of Phosphorylated Proteins Using Mass Spectrometry. Curr. Protein Pept. Sci. 2021, 22, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Leykam, J.; Andrews, P.C.; Gage, D.A.; Allison, J. An approach to locate phosphorylation sites in a phosphoprotein: Mass mapping by combining specific enzymatic degradation with matrix-assisted laser desorption/ionization mass spectrometry. Anal. Biochem. 1994, 219, 9–20. [Google Scholar] [CrossRef]
- Yip, T.T.; Hutchens, T.W. Mapping and sequence-specific identification of phosphopeptides in unfractionated protein digest mixtures by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. FEBS Lett. 1992, 308, 149–153. [Google Scholar] [CrossRef]
- Stensballe, A.; Andersen, S.; Jensen, O.N. Characterization of phosphoproteins from electrophoretic gels by nanoscale Fe(III) affinity chromatography with off-line mass spectrometry analysis. Proteomics 2001, 1, 207–222. [Google Scholar] [CrossRef]
- Mann, M.; Ong, S.E.; Gronborg, M.; Steen, H.; Jensen, O.N.; Pandey, A. Analysis of protein phosphorylation using mass spectrometry: Deciphering the phosphoproteome. Trends Biotechnol. 2002, 20, 261–268. [Google Scholar] [CrossRef]
- Delom, F.; Chevet, E. Phosphoprotein analysis: From proteins to proteomes. Proteome Sci. 2006, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Riley, N.M.; Coon, J.J. Phosphoproteomics in the Age of Rapid and Deep Proteome Profiling. Anal. Chem. 2016, 88, 74–94. [Google Scholar] [CrossRef] [PubMed]
- Needham, E.J.; Parker, B.L.; Burykin, T.; James, D.E.; Humphrey, S.J. Illuminating the dark phosphoproteome. Sci. Signal 2019, 12, eaau8645. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Mashalidis, E.H.; Kuk, A.C.Y.; Yamamoto, K.; Kaeser, B.; Ichikawa, S.; Lee, S.Y. GlcNAc-1-P-transferase-tunicamycin complex structure reveals basis for inhibition of N-glycosylation. Nat. Struct. Mol. Biol. 2018, 25, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu. Rev. Biochem. 1987, 56, 497–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; He, Z.; Deng, J.; Zhang, Z.; Liu, L.; Ye, W.; Liu, S. Tunicamycin induces ER stress and inhibits tumorigenesis of head and neck cancer cells by inhibiting N-glycosylation. Am. J. Transl. Res. 2020, 12, 541–550. [Google Scholar]
- Guha, P.; Kaptan, E.; Gade, P.; Kalvakolanu, D.V.; Ahmed, H. Tunicamycin induced endoplasmic reticulum stress promotes apoptosis of prostate cancer cells by activating mTORC1. Oncotarget 2017, 8, 68191–68207. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Ke, Z.J.; Comer, A.L.; Xu, M.; Frank, J.A.; Zhang, Z.; Shi, X.; Luo, J. Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol. Appl. Pharmacol. 2015, 283, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar] [CrossRef]
- Wu, R.; Dephoure, N.; Haas, W.; Huttlin, E.L.; Zhai, B.; Sowa, M.E.; Gygi, S.P. Correct interpretation of comprehensive phosphorylation dynamics requires normalization by protein expression changes. Mol. Cell Proteom. 2011, 10, M111 009654. [Google Scholar] [CrossRef] [PubMed]
- Schwaigerlehner, L.; Mayrhofer, P.; Diem, M.; Steinfellner, W.; Fenech, E.; Oostenbrink, C.; Kunert, R. Germinality does not necessarily define mAb expression and thermal stability. Appl. Microbiol. Biotechnol. 2019, 103, 7505–7518. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Reinhart, D.; Castan, A.; Kunert, R. Rapid development of clone-specific, high-performing perfusion media from established feed supplements. Biotechnol. Prog. 2020, 36, e2933. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, P.; Castan, A.; Kunert, R. Shake tube perfusion cell cultures are suitable tools for the prediction of limiting substrate, CSPR, bleeding strategy, growth and productivity behavior. J. Chem. Technol. Biotechnol. 2021, 96, 2930–2939. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Reinhart, D.; Castan, A.; Kunert, R. Monitoring of heat- and light exposure of cell culture media by RAMAN spectroscopy: Towards an analytical tool for cell culture media quality control. Biochem. Eng. J. 2021, 166, 107845. [Google Scholar] [CrossRef]
- Reinhart, D.; Damjanovic, L.; Castan, A.; Ernst, W.; Kunert, R. Differential gene expression of a feed-spiked super-producing CHO cell line. J. Biotechnol. 2018, 285, 23–37. [Google Scholar] [CrossRef]
- Selvaprakash, K.; Sideri, C.K.; Henry, M.; Efeoglu, E.; Ryan, D.; Meleady, P. Characterization of the Ubiquitin-Modified Proteome of Recombinant Chinese Hamster Ovary Cells in Response to Endoplasmic Reticulum Stress. Biotechnol. J. 2024, 19, e202400413. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Cox, J. Perseus: A Bioinformatics Platform for Integrative Analysis of Proteomics Data in Cancer Research. Methods Mol. Biol. 2018, 1711, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Morrison, D.K.; Cutler, R.E. The complexity of Raf-1 regulation. Curr. Opin. Cell Biol. 1997, 9, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; D’Souza, R.C.; Tyanova, S.; Schaab, C.; Wisniewski, J.R.; Cox, J.; Mann, M. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 2014, 8, 1583–1594. [Google Scholar] [CrossRef]
- Ramasamy, P.; Vandermarliere, E.; Vranken, W.F.; Martens, L. Panoramic Perspective on Human Phosphosites. J. Proteome Res. 2022, 21, 1894–1915. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.; Sefton, B.M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl. Acad. Sci. USA 1980, 77, 1311–1315. [Google Scholar] [CrossRef]
- Salazar, C.; Hofer, T. Multisite protein phosphorylation—From molecular mechanisms to kinetic models. FEBS J. 2009, 276, 3177–3198. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.W.; Amode, M.R.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2024. Nucleic Acids Res. 2024, 52, D891–D899. [Google Scholar] [CrossRef]
- Misteli, T. RNA splicing: What has phosphorylation got to do with it? Curr. Biol. 1999, 9, R198–R200. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.; Zhang, G.; Wang, L.; Zhu, Z.; Zhang, W.; Huang, H.; Gao, R. Heterogeneous nuclear ribonucleoprotein K promotes the progression of lung cancer by inhibiting the p53-dependent signaling pathway. Thorac. Cancer 2022, 13, 1311–1321. [Google Scholar] [CrossRef]
- Mikula, M.; Bomsztyk, K. Direct recruitment of ERK cascade components to inducible genes is regulated by heterogeneous nuclear ribonucleoprotein (hnRNP) K. J. Biol. Chem. 2011, 286, 9763–9775. [Google Scholar] [CrossRef]
- Meyuhas, O. Ribosomal Protein S6 Phosphorylation: Four Decades of Research. Int. Rev. Cell Mol. Biol. 2015, 320, 41–73. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.W.; You, K.S.; Park, J.S.; Lee, S.G.; Seong, Y.S. Ribosomal Protein S6: A Potential Therapeutic Target against Cancer? Int. J. Mol. Sci. 2021, 23, 48. [Google Scholar] [CrossRef]
- Bohlen, J.; Roiuk, M.; Teleman, A.A. Phosphorylation of ribosomal protein S6 differentially affects mRNA translation based on ORF length. Nucleic Acids Res. 2021, 49, 13062–13074. [Google Scholar] [CrossRef] [PubMed]
- Ruvinsky, I.; Sharon, N.; Lerer, T.; Cohen, H.; Stolovich-Rain, M.; Nir, T.; Dor, Y.; Zisman, P.; Meyuhas, O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes. Dev. 2005, 19, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Pende, M.; Kozma, S.C.; Jaquet, M.; Oorschot, V.; Burcelin, R.; Le Marchand-Brustel, Y.; Klumperman, J.; Thorens, B.; Thomas, G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 2000, 408, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Ruvinsky, I.; Meyuhas, O. Ribosomal protein S6 phosphorylation: From protein synthesis to cell size. Trends Biochem. Sci. 2006, 31, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Frost, V.; Morley, S.J.; Mercep, L.; Meyer, T.; Fabbro, D.; Ferrari, S. The phosphodiesterase inhibitor SQ 20006 selectively blocks mitogen activation of p70S6k and transition to S phase of the cell division cycle without affecting the steady state phosphorylation of eIF-4E. J. Biol. Chem. 1995, 270, 26698–26706. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Williams, M.; Terada, N.; Alessi, D.R.; Proud, C.G. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001, 20, 4370–4379. [Google Scholar] [CrossRef]
- Krieg, J.; Hofsteenge, J.; Thomas, G. Identification of the 40 S ribosomal protein S6 phosphorylation sites induced by cycloheximide. J. Biol. Chem. 1988, 263, 11473–11477. [Google Scholar] [CrossRef]
- Buchan, J.R.; Parker, R. Eukaryotic stress granules: The ins and outs of translation. Mol. Cell 2009, 36, 932–941. [Google Scholar] [CrossRef]
- Takahara, T.; Maeda, T. Transient sequestration of TORC1 into stress granules during heat stress. Mol. Cell 2012, 47, 242–252. [Google Scholar] [CrossRef]
- Harrod, A.; Lane, K.A.; Downs, J.A. The role of the SWI/SNF chromatin remodelling complex in the response to DNA double strand breaks. DNA Repair 2020, 93, 102919. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Ang, J.Y.J.; Lee, A.Y.; Cao, Q.; Li, K.Y.; Yip, K.Y.; Leung, D.C.Y. G9a Plays Distinct Roles in Maintaining DNA Methylation, Retrotransposon Silencing, and Chromatin Looping. Cell Rep. 2020, 33, 108315. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sugimoto, K.; Nozaki, M.; Ueda, J.; Ohta, T.; Ohki, M.; Fukuda, M.; Takeda, N.; Niida, H.; Kato, H.; et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002, 16, 1779–1791. [Google Scholar] [CrossRef]
- Poulard, C.; Noureddine, L.M.; Pruvost, L.; Le Romancer, M. Structure, Activity, and Function of the Protein Lysine Methyltransferase G9a. Life 2021, 11, 1082. [Google Scholar] [CrossRef]
- Epsztejn-Litman, S.; Feldman, N.; Abu-Remaileh, M.; Shufaro, Y.; Gerson, A.; Ueda, J.; Deplus, R.; Fuks, F.; Shinkai, Y.; Cedar, H.; et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nat. Struct. Mol. Biol. 2008, 15, 1176–1183. [Google Scholar] [CrossRef]
- Alver, B.H.; Kim, K.H.; Lu, P.; Wang, X.; Manchester, H.E.; Wang, W.; Haswell, J.R.; Park, P.J.; Roberts, C.W. The SWI/SNF chromatin remodelling complex is required for maintenance of lineage specific enhancers. Nat. Commun. 2017, 8, 14648. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.P.; Dutta, A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002, 71, 333–374. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Ficarro, S.B.; Jiang, W. Essential role of phosphorylation of MCM2 by Cdc7/Dbf4 in the initiation of DNA replication in mammalian cells. Mol. Biol. Cell 2006, 17, 4459–4472. [Google Scholar] [CrossRef]
- Ryazanov, A.G.; Shestakova, E.A.; Natapov, P.G. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature 1988, 334, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Liu, J.; Zhou, L.; Zhang, C.; Xu, L.; Qin, Q.; Zhan, L.; Lu, J.; Cheng, H.; et al. Eukaryotic elongation factor 2 kinase confers tolerance to stress conditions in cancer cells. Cell Stress. Chaperones 2015, 20, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.W.; Moore, C.E.; Wang, X.; Proud, C.G. Eukaryotic elongation factor 2 kinase, an unusual enzyme with multiple roles. Adv. Biol. Regul. 2014, 55, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Loerch, S.; Kunder, N.; Stanowick, A.D.; Lou, T.F.; Campbell, Z.T. Functionally distinct roles for eEF2K in the control of ribosome availability and p-body abundance. Nat. Commun. 2021, 12, 6789. [Google Scholar] [CrossRef]
- Proud, C.G. Peptide-chain elongation in eukaryotes. Mol. Biol. Rep. 1994, 19, 161–170. [Google Scholar] [CrossRef]
- Wang, X.; Regufe da Mota, S.; Liu, R.; Moore, C.E.; Xie, J.; Lanucara, F.; Agarwala, U.; Pyr Dit Ruys, S.; Vertommen, D.; Rider, M.H.; et al. Eukaryotic elongation factor 2 kinase activity is controlled by multiple inputs from oncogenic signaling. Mol. Cell Biol. 2014, 34, 4088–4103. [Google Scholar] [CrossRef] [PubMed]
- Redpath, N.T.; Foulstone, E.J.; Proud, C.G. Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J. 1996, 15, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, M.; Casado, P.; Akhtar, N.; Alvarez-Teijeiro, S.; Rajeeve, V.; Cutillas, P.R. eEF2K Activity Determines Synergy to Cotreatment of Cancer Cells With PI3K and MEK Inhibitors. Mol. Cell Proteom. 2022, 21, 100240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ren, X.; Zhang, Y.; Shan, Y.; Huber-Keener, K.J.; Zhang, L.; Kimball, S.R.; Harvey, H.; Jefferson, L.S.; Yang, J.M. Integrated regulation of autophagy and apoptosis by EEF2K controls cellular fate and modulates the efficacy of curcumin and velcade against tumor cells. Autophagy 2013, 9, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, U.; Nilsson, A.; Nygard, O. Functional properties of phosphorylated elongation factor 2. Eur. J. Biochem. 1990, 191, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Proud, C.G. Eukaryotic elongation factor 2 kinase as a drug target in cancer, and in cardiovascular and neurodegenerative diseases. Acta Pharmacol. Sin. 2016, 37, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Kim, S.; Kim, K.; Yeom, J.; Park, S.; Kim, I. Euchromatin histone methyltransferase II (EHMT2) regulates the expression of ras-related GTP binding C (RRAGC) protein. BMB Rep. 2020, 53, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhu, Q.; Lu, X.; Du, Y.; Cao, L.; Shen, C.; Hou, T.; Li, M.; Li, Z.; Liu, C.; et al. G9a coordinates with the RPA complex to promote DNA damage repair and cell survival. Proc. Natl. Acad. Sci. USA 2017, 114, E6054–E6063. [Google Scholar] [CrossRef]
- Chen, H.; He, A.; Li, H.; Chen, H.; Xie, H.; Luo, L.; Huang, Y.; Chen, J.; Guan, J.; He, Q.; et al. TSSK4 upregulation in alveolar epithelial type-II cells facilitates pulmonary fibrosis through HSP90-AKT signaling restriction and AT-II apoptosis. Cell Death Dis. 2021, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Hsu, C.L.; Wang, Y.C.; Ishihama, Y.; Ku, W.C.; Huang, H.C.; Juan, H.F. Temporal Phosphoproteome Dynamics Induced by an ATP Synthase Inhibitor Citreoviridin. Mol. Cell Proteom. 2015, 14, 3284–3298. [Google Scholar] [CrossRef] [PubMed]
- Paez-Ribes, M.; Gonzalez-Gualda, E.; Doherty, G.J.; Munoz-Espin, D. Targeting senescent cells in translational medicine. EMBO Mol. Med. 2019, 11, e10234. [Google Scholar] [CrossRef]
- Kim, A.H.; Khursigara, G.; Sun, X.; Franke, T.F.; Chao, M.V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell Biol. 2001, 21, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Godel, M.; Temerinac, D.; Grahammer, F.; Hartleben, B.; Kretz, O.; Riederer, B.M.; Propst, F.; Kohl, S.; Huber, T.B. Microtubule Associated Protein 1b (MAP1B) Is a Marker of the Microtubular Cytoskeleton in Podocytes but Is Not Essential for the Function of the Kidney Filtration Barrier in Mice. PLoS ONE 2015, 10, e0140116. [Google Scholar] [CrossRef]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Laks, D.R.; Oses-Prieto, J.A.; Alvarado, A.G.; Nakashima, J.; Chand, S.; Azzam, D.B.; Gholkar, A.A.; Sperry, J.; Ludwig, K.; Condro, M.C.; et al. A molecular cascade modulates MAP1B and confers resistance to mTOR inhibition in human glioblastoma. Neuro Oncol. 2018, 20, 764–775. [Google Scholar] [CrossRef]
- Goold, R.G.; Owen, R.; Gordon-Weeks, P.R. Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J. Cell Sci. 1999, 112 Pt 19, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Freeberg, M.A.; Han, T.; Kamath, A.; Yao, Y.; Fukuda, T.; Suzuki, T.; Kim, J.K.; Inoki, K. LARP1 functions as a molecular switch for mTORC1-mediated translation of an essential class of mRNAs. Elife 2017, 6, e25237. [Google Scholar] [CrossRef]
- Zou, L.H.; Shang, Z.F.; Tan, W.; Liu, X.D.; Xu, Q.Z.; Song, M.; Wang, Y.; Guan, H.; Zhang, S.M.; Yu, L.; et al. TNKS1BP1 functions in DNA double-strand break repair though facilitating DNA-PKcs autophosphorylation dependent on PARP-1. Oncotarget 2015, 6, 7011–7022. [Google Scholar] [CrossRef]
- Seimiya, H.; Smith, S. The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182). J. Biol. Chem. 2002, 277, 14116–14126. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, B.; Xie, S.; Yan, J.; Yang, L.; Zhou, X.; Zeng, E. Biological Functions of TNKS1 and Its Relationship with Wnt/beta-Catenin Pathway in Astrocytoma. Onco Targets Ther. 2019, 12, 10841–10850. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Q.; Lv, J.; Sun, Y.; Feng, Z.; Zhang, M.; Zhang, F.; Xia, C.; Gao, Y.; Zhang, Z.; et al. High expression of NOLC1 as an independent prognostic factor for survival in patients with colorectal cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 15697–15712. [Google Scholar] [CrossRef] [PubMed]


| Protein | Protein Names | Gene Names | Sequence Window | Residue | Log2 Change 353/11_TM vs. 353/11 |
|---|---|---|---|---|---|
| G3IHK4 | Eukaryotic elongation factor 2 kinase | Eef2k | NYYSNLMKTECGSTGSPASSFHFKEAWKHAI | S73 | 2.31 |
| A0A8C2MXF1 | euchromatic histone lysine methyltransferase 2 (Ehmt2) | Ehmt2 | LGKVTSDAAKRRKLNSGSLSEDFGSARGSGD | S229 | 1.72 |
| A0A8C2MUK8 | Heat shock protein HSP 90-BETA | Hsp90ab1 | EDKDDEEKPKIEDVGSDEEDDSGKDKKKKTK | S255 | −3.46 |
| A0A8C2MBL3 | Microtubule-associated protein 1B; Map1b heavy chain; Map1 light chain Lc1 | Map1b | IKDVSDERLSPTKSPSLSPSPPSPIEKTPLG | S1255 | −5.19 |
| A0A8C2MCK2 | La-related protein 1 | Larp1 | ANKLFGAPEPSTIARSLPTTVPESPNYRNAR | S735 | 1.00 |
| EPSTIARSLPTTVPESPNYRNARTPRTPRTP | S743 | 1.00 | |||
| G3HRJ2 | 182 kDa tankyrase-1-binding protein | Tnks1bp1 | LSPSALKAKLRSRNRSAEEGEVTESKSSQKE | S1630 | −3.92 |
| A0A8C2LS20 | Nucleolar and coiled-body phosphoprotein 1 | Nolc1 | ANGTPASQNGKAGKESEEDEEEEETKMAVSK | S542 | −4.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaj, E.; Sandell, F.L.; Schwaigerlehner, L.; Marzban, G.; Dohm, J.C.; Kunert, R. Systems Biology of Recombinant 2G12 and 353/11 mAb Production in CHO-K1 Cell Lines at Phosphoproteome Level. Proteomes 2025, 13, 9. https://doi.org/10.3390/proteomes13010009
Sulaj E, Sandell FL, Schwaigerlehner L, Marzban G, Dohm JC, Kunert R. Systems Biology of Recombinant 2G12 and 353/11 mAb Production in CHO-K1 Cell Lines at Phosphoproteome Level. Proteomes. 2025; 13(1):9. https://doi.org/10.3390/proteomes13010009
Chicago/Turabian StyleSulaj, Eldi, Felix L. Sandell, Linda Schwaigerlehner, Gorji Marzban, Juliane C. Dohm, and Renate Kunert. 2025. "Systems Biology of Recombinant 2G12 and 353/11 mAb Production in CHO-K1 Cell Lines at Phosphoproteome Level" Proteomes 13, no. 1: 9. https://doi.org/10.3390/proteomes13010009
APA StyleSulaj, E., Sandell, F. L., Schwaigerlehner, L., Marzban, G., Dohm, J. C., & Kunert, R. (2025). Systems Biology of Recombinant 2G12 and 353/11 mAb Production in CHO-K1 Cell Lines at Phosphoproteome Level. Proteomes, 13(1), 9. https://doi.org/10.3390/proteomes13010009









