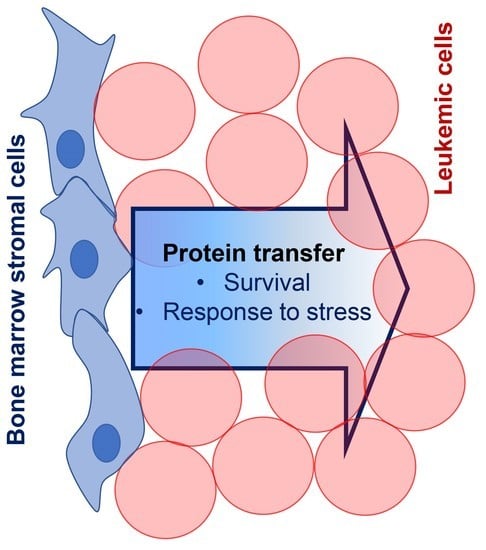
Shotgun Proteomics of Co-Cultured Leukemic and Bone Marrow Stromal Cells from Different Species as a Preliminary Approach to Detect Intercellular Protein Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
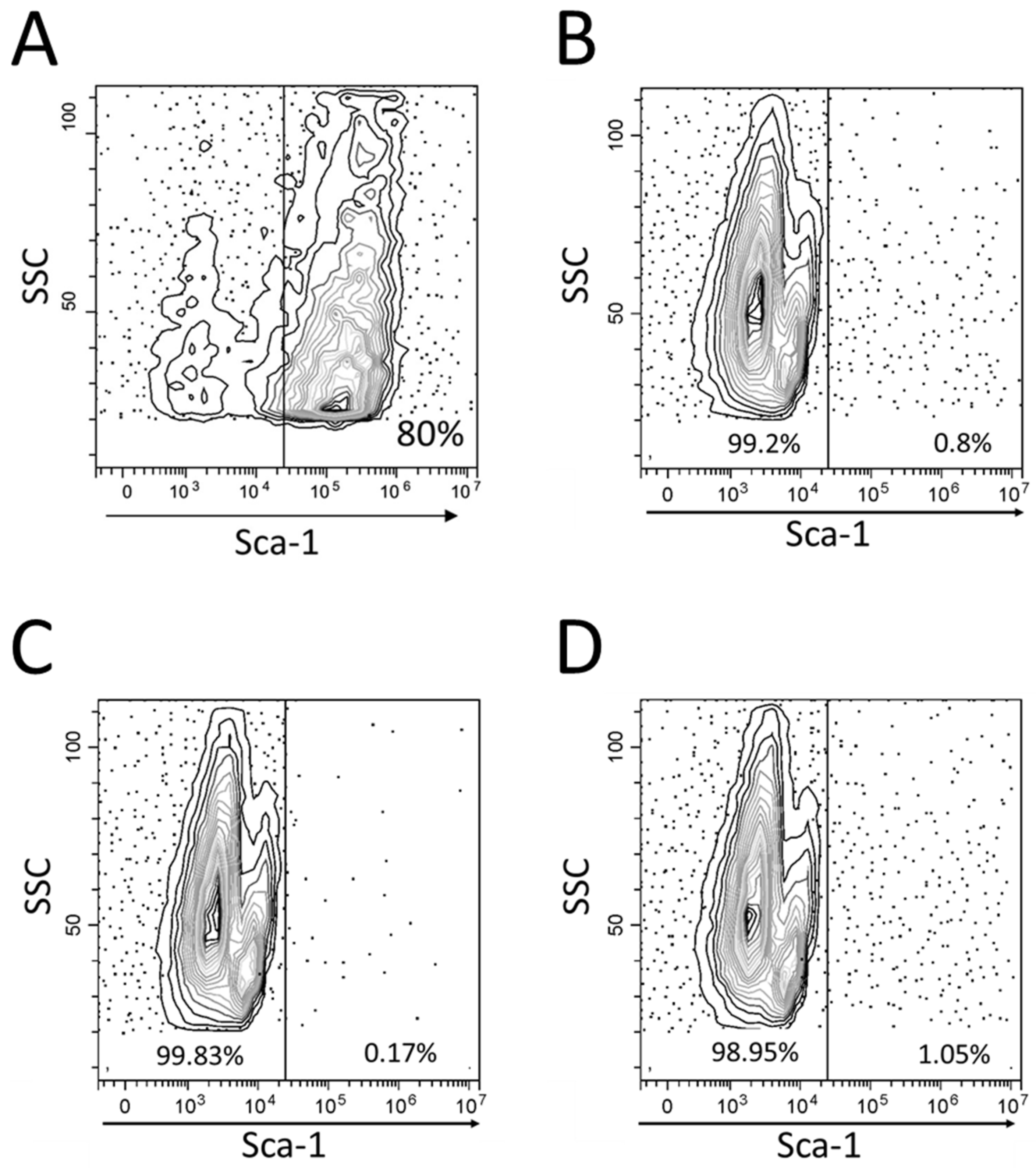
2.2. Flow Cytometry
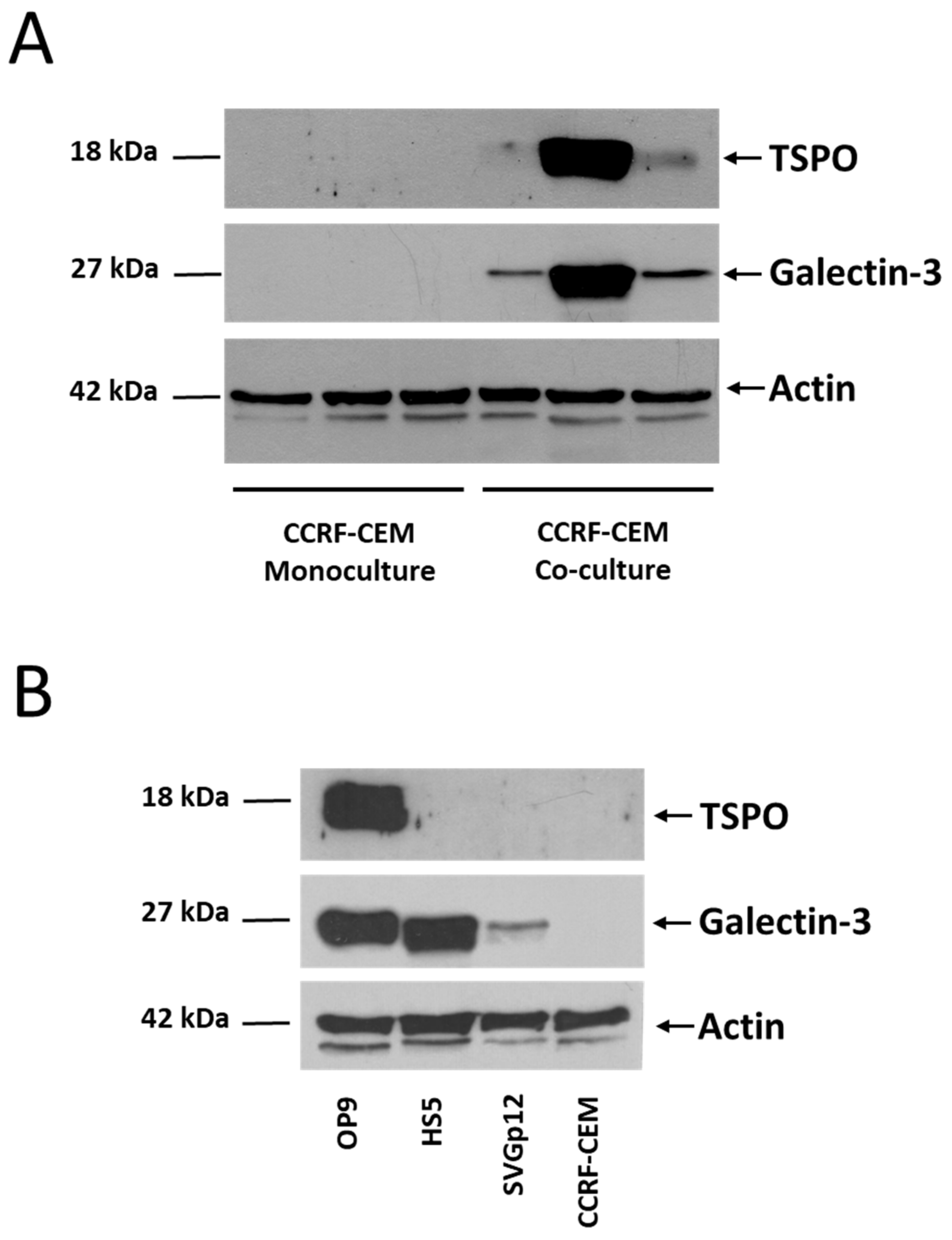
2.3. Western Blotting
2.4. Enzymatic Activity
2.5. Proteomic Analysis
2.5.1. Protein Digestion and Fractionation
2.5.2. Liquid Chromatography and Mass Spectrometry Analysis
2.5.3. Protein Identification and Relative Quantification
2.6. Statistical Analysis
3. Results
3.1. Leukemic Cells Acquired Significant Resistance to Vincristine, Methotrexate, and Hydrogen Peroxide when Co-Cultured with BM Stromal Cells
3.2. Shotgun Proteomic Analyses of Human Leukemic Cells Co-Cultured with Mouse Stromal Cells May Reveal the Intercellular Transfer of Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malvezzi, M.; Santucci, C.; Alicandro, G.; Carioli, G.; Boffetta, P.; Ribeiro, K.B.; Levi, F.; La Vecchia, C.; Negri, E.; Bertuccio, P. Childhood cancer mortality trends in the Americas and Australasia: An update to 2017. Cancer 2021, 127, 3445–3456. [Google Scholar] [CrossRef]
- Reinhardt, D.; Antoniou, E.; Waack, K. Pediatric Acute Myeloid Leukemia-Past, Present, and Future. J. Clin. Med. 2022, 11, 504. [Google Scholar] [CrossRef]
- DuVall, A.S.; Sheade, J.; Anderson, D.; Yates, S.J.; Stock, W. Updates in the Management of Relapsed and Refractory Acute Lymphoblastic Leukemia: An Urgent Plea for New Treatments Is Being Answered! JCO Oncol. Pract. 2022, 18, 479–487. [Google Scholar] [CrossRef]
- Oh, B.L.Z.; Lee, S.H.R.; Yeoh, A.E.J. Curing the Curable: Managing Low-Risk Acute Lymphoblastic Leukemia in Resource Limited Countries. J. Clin. Med. 2021, 10, 4728. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Lonetti, A.; Evangelisti, C.; Buontempo, F.; Orsini, E.; Evangelisti, C.; Cappellini, A.; Neri, L.M.; McCubrey, J.A.; Martelli, A.M. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: From biology to therapeutic targeting. Biochim. Biophys. Acta 2016, 1863, 449–463. [Google Scholar] [CrossRef]
- Tabe, Y.; Konopleva, M. Advances in understanding the leukaemia microenvironment. Br. J. Haematol. 2014, 164, 767–778. [Google Scholar] [CrossRef]
- Delahaye, M.C.; Salem, K.-I.; Pelletier, J.; Aurrand-Lions, M.; Mancini, S.J.C. Toward Therapeutic Targeting of Bone Marrow Leukemic Niche Protective Signals in B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2021, 10, 606540. [Google Scholar] [CrossRef]
- Griessinger, E.; Moschoi, R.; Biondani, G.; Peyron, J.-F. Mitochondrial Transfer in the Leukemia Microenvironment. Trends Cancer 2017, 3, 828–839. [Google Scholar] [CrossRef]
- Kolba, M.D.; Dudka, W.; Zaręba-Kozioł, M.; Kominek, A.; Ronchi, P.; Turos, L.; Chroscicki, P.; Wlodarczyk, J.; Schwab, Y.; Klejman, A.; et al. Tunneling nanotube-mediated intercellular vesicle and protein transfer in the stroma-provided imatinib resistance in chronic myeloid leukemia cells. Cell Death Dis. 2019, 10, 817. [Google Scholar] [CrossRef]
- Polak, R.; de Rooij, B.; Pieters, R.; den Boer, M.L. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 2015, 126, 2404–2414. [Google Scholar] [CrossRef]
- Moschoi, R.; Imbert, V.; Nebout, M.; Chiche, J.; Mary, D.; Prebet, T.; Saland, E.; Castellano, R.; Pouyet, L.; Collette, Y.; et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood 2016, 128, 253–264. [Google Scholar] [CrossRef]
- Fei, F.; Joo, E.J.; Tarighat, S.S.; Schiffer, I.; Paz, H.; Fabbri, M.; Abdel-Azim, H.; Groffen, J.; Heisterkamp, N. B-cell precursor acute lymphoblastic leukemia and stromal cells communicate through Galectin-3. Oncotarget 2015, 6, 11378–11394. [Google Scholar] [CrossRef]
- Fei, F.; Abdel-Azim, H.; Lim, M.; Arutyunyan, A.; von Itzstein, M.; Groffen, J.; Heisterkamp, N. Galectin-3 in pre-B acute lymphoblastic leukemia. Leukemia 2013, 27, 2385–2388. [Google Scholar] [CrossRef]
- Tarighat, S.S.; Fei, F.; Joo, E.J.; Abdel-Azim, H.; Yang, L.; Geng, H.; Bum-Erdene, K.; Grice, I.D.; von Itzstein, M.; Blanchard, H.; et al. Overcoming Microenvironment-Mediated Chemoprotection through Stromal Galectin-3 Inhibition in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 12167. [Google Scholar] [CrossRef]
- Burt, R.; Dey, A.; Aref, S.; Aguiar, M.; Akarca, A.; Bailey, K.; Day, W.; Hooper, S.; Kirkwood, A.; Kirschner, K.; et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019, 134, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Wang, B.; Chen, P.; Zheng, X.; Hou, D.; Wang, X.; Zhang, B.; Chen, L.; Li, D.; Lin, X.; et al. Metformin sensitizes AML cells to chemotherapy through blocking mitochondrial transfer from stromal cells to AML cells. Cancer Lett. 2022, 532, 215582. [Google Scholar] [CrossRef]
- Ebinger, S.; Özdemir, E.Z.; Ziegenhain, C.; Tiedt, S.; Castro Alves, C.; Grunert, M.; Dworzak, M.; Lutz, C.; Turati, V.A.; Enver, T.; et al. Characterization of Rare, Dormant, and Therapy-Resistant Cells in Acute Lymphoblastic Leukemia. Cancer Cell 2016, 30, 849–862. [Google Scholar] [CrossRef]
- Kuek, V.; Hughes, A.M.; Kotecha, R.S.; Cheung, L.C. Therapeutic Targeting of the Leukaemia Microenvironment. Int. J. Mol. Sci. 2021, 22, 6888. [Google Scholar] [CrossRef]
- Balandrán, J.C.; Dávila-Velderrain, J.; Sandoval-Cabrera, A.; Zamora-Herrera, G.; Terán-Cerqueda, V.; García-Stivalet, L.A.; Limón-Flores, J.A.; Armenta-Castro, E.; Rodríguez-Martínez, A.; Leon-Chavez, B.A.; et al. Patient-Derived Bone Marrow Spheroids Reveal Leukemia-Initiating Cells Supported by Mesenchymal Hypoxic Niches in Pediatric B-ALL. Front. Immunol. 2021, 12, 746492. [Google Scholar] [CrossRef]
- Velázquez-Avila, M.; Balandrán, J.C.; Ramírez-Ramírez, D.; Velázquez-Avila, M.; Sandoval, A.; Felipe-López, A.; Nava, P.; Alvarado-Moreno, J.A.; Dozal, D.; Prieto-Chávez, J.L.; et al. High cortactin expression in B-cell acute lymphoblastic leukemia is associated with increased transendothelial migration and bone marrow relapse. Leukemia 2019, 33, 1337–1348. [Google Scholar] [CrossRef]
- Balandrán, J.C.; Purizaca, J.; Enciso, J.; Dozal, D.; Sandoval, A.; Jiménez-Hernández, E.; Alemán-Lazarini, L.; Perez-Koldenkova, V.; Quintela-Núñez del Prado, H.; Rios de los Ríos, J.; et al. Pro-inflammatory-Related Loss of CXCL12 Niche Promotes Acute Lymphoblastic Leukemic Progression at the Expense of Normal Lymphopoiesis. Front. Immunol. 2017, 7, 666. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.C.; Yates, J.R., 3rd. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef]
- Rechavi, O.; Kalman, M.; Fang, Y.; Vernitsky, H.; Jacob-Hirsch, J.; Foster, L.J.; Kloog, Y.; Goldstein, I. Trans-SILAC: Sorting out the non-cell-autonomous proteome. Nat. Methods 2010, 7, 923–927. [Google Scholar] [CrossRef]
- Turos-Korgul, L.; Kolba, M.D.; Chroscicki, P.; Zieminska, A.; Piwocka, K. Tunneling Nanotubes Facilitate Intercellular Protein Transfer and Cell Networks Function. Front. Cell Dev. Biol. 2022, 10, 915117. [Google Scholar] [CrossRef]
- Garcia-Hernandez, A.; Reyes-Uribe, E.; Arce-Salinas, C.; de la Cruz-Lopez, K.-G.; Manzo-Merino, J.; Guzman-Ortiz, A.-L.; Quezada, H.; Cortes-Reynosa, P.; Breton-Mora, F.; Elizalde-Acosta, I.; et al. Extracellular vesicles from blood of breast cancer women induce angiogenic processes in HUVECs. Tissue Cell 2022, 76, 101814. [Google Scholar] [CrossRef]
- Calleja, L.F.; Yoval-Sánchez, B.; Hernández-Esquivel, L.; Gallardo-Pérez, J.C.; Sosa-Garrocho, M.; Marín-Hernández, Á.; Jasso-Chávez, R.; Macías-Silva, M.; Salud Rodríguez-Zavala, J. Activation of ALDH1A1 by omeprazole reduces cell oxidative stress damage. FEBS J. 2021, 288, 4064–4080. [Google Scholar] [CrossRef]
- Marín-Hernández, A.; López-Ramírez, S.Y.; Del Mazo-Monsalvo, I.; Gallardo-Pérez, J.C.; Rodríguez-Enríquez, S.; Moreno-Sánchez, R.; Saavedra, E. Modeling cancer glycolysis under hypoglycemia, and the role played by the differential expression of glycolytic isoforms. FEBS J. 2014, 281, 3325–3345. [Google Scholar] [CrossRef]
- Doellinger, J.; Schneider, A.; Hoeller, M.; Lasch, P. Sample Preparation by Easy Extraction and Digestion (SPEED)—A Universal, Rapid, and Detergent-free Protocol for Proteomics Based on Acid Extraction. Mol. Cell. Proteomics. 2020, 19, 209–222. [Google Scholar] [CrossRef]
- Kim, H.; Dan, K.; Shin, H.; Lee, J.; Wang, J.I.; Han, D. An efficient method for high-pH peptide fractionation based on C18 StageTips for in-depth proteome profiling. Anal. Methods 2019, 11, 4693–4698. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Garrido, S.M.; Appelbaum, F.R.; Willman, C.L.; Banker, D.E. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5). Exp. Hematol. 2001, 29, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Konoplev, S.; Hu, W.; Zaritskey, A.Y.; Afanasiev, B.V.; Andreeff, M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia 2002, 16, 1713–1724. [Google Scholar] [CrossRef]
- Zeng, Z.; Samudio, I.J.; Munsell, M.; An, J.; Huang, Z.; Estey, E.; Andreeff, M.; Konopleva, M. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol. Cancer Ther. 2006, 5, 3113–3121. [Google Scholar] [CrossRef]
- Marlein, C.R.; Zaitseva, L.; Piddock, R.E.; Robinson, S.D.; Edwards, D.R.; Shafat, M.S.; Zhou, Z.; Lawes, M.; Bowles, K.M.; Rushworth, S.A. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood 2017, 130, 1649–1660. [Google Scholar] [CrossRef]
- Millard, S.M.; Heng, O.; Opperman, K.S.; Sehgal, A.; Irvine, K.M.; Kaur, S.; Sandrock, C.J.; Wu, A.C.; Magor, G.W.; Batoon, L.; et al. Fragmentation of tissue-resident macrophages during isolation confounds analysis of single-cell preparations from mouse hematopoietic tissues. Cell Rep. 2021, 37, 110058. [Google Scholar] [CrossRef]
- Paraguassú-Braga, F.H.; Borojevic, R.; Bouzas, L.F.; Barcinski, M.A.; Bonomo, A. Bone marrow stroma inhibits proliferation and apoptosis in leukemic cells through gap junction-mediated cell communication. Cell Death Differ. 2003, 10, 1101–1108. [Google Scholar] [CrossRef]
- Kumar, B.; Garcia, M.; Weng, L.; Jung, X.; Murakami, J.L.; Hu, X.; McDonald, T.; Lin, A.; Kumar, A.R.; DiGiusto, D.L.; et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia 2018, 32, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Miwa, N.; Uebi, T.; Kawamura, S. S100-annexin complexes--biology of conditional association. FEBS J. 2008, 275, 4945–4955. [Google Scholar] [CrossRef]
- Scupoli, M.T.; Perbellini, O.; Krampera, M.; Vinante, F.; Cioffi, F.; Pizzolo, G. Interleukin 7 requirement for survival of T-cell acute lymphoblastic leukemia and human thymocytes on bone marrow stroma. Haematologica 2007, 92, 264–266. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.-W.; Yu, S.-W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep. 2020, 53, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bonsack, F.; Sukumari-Ramesh, S. TSPO: An Evolutionarily Conserved Protein with Elusive Functions. Int. J. Mol. Sci. 2018, 19, 1694. [Google Scholar] [CrossRef]
- Milenkovic, V.M.; Slim, D.; Bader, S.; Koch, V.; Heinl, E.-S.; Alvarez-Carbonell, D.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. CRISPR-Cas9 Mediated TSPO Gene Knockout alters Respiration and Cellular Metabolism in Human Primary Microglia Cells. Int. J. Mol. Sci. 2019, 20, 3359. [Google Scholar] [CrossRef] [PubMed]
- Carayon, P.; Portier, M.; Dussossoy, D.; Bord, A.; Petitprêtre, G.; Canat, X.; Fur, G.L.; Casellas, P. Involvement of Peripheral Benzodiazepine Receptors in the Protection of Hematopoietic Cells Against Oxygen Radical Damage. Blood 1996, 87, 3170–3178. [Google Scholar] [CrossRef]
- Stoebner, P.E.; Carayon, P.; Casellas, P.; Portier, M.; Lavabre-Bertrand, T.; Cuq, P.; Cano, J.P.; Meynadier, J.; Meunier, L. Transient protection by peripheral benzodiazepine receptors during the early events of ultraviolet light-induced apoptosis. Cell Death Differ. 2001, 8, 747–753. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhao, L.; Ma, W.; Rodriguez, I.R.; Fariss, R.N.; Wong, W.T. Macroglia-Microglia Interactions via TSPO Signaling Regulates Microglial Activation in the Mouse Retina. J. Neurosci. 2014, 34, 3793–3806. [Google Scholar] [CrossRef]
- De Rosa, A.; Zappavigna, S.; Villa, M.R.; Improta, S.; Cesario, E.; Mastrullo, L.; Caraglia, M.; Stiuso, P. Prognostic role of translocator protein and oxidative stress markers in chronic lymphocytic leukemia patients treated with bendamustine plus rituximab. Oncol. Lett. 2015, 9, 1327–1332. [Google Scholar] [CrossRef]
- Mermelekas, G.; Vlahou, A.; Zoidakis, J. SRM/MRM targeted proteomics as a tool for biomarker validation and absolute quantification in human urine. Expert Rev. Mol. Diagn. 2015, 15, 1441–1454. [Google Scholar] [CrossRef]
- Smith, L.M.; Agar, J.N.; Chamot-Rooke, J.; Danis, P.O.; Ge, Y.; Loo, J.A.; Paša-Tolić, L.; Tsybin, Y.O.; Kelleher, N.L. The Human Proteoform Project: Defining the human proteome. Sci. Adv. 2021, 7, eabk0734. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevárez-Ramírez, A.J.; Guzmán-Ortiz, A.L.; Cortes-Reynosa, P.; Perez-Salazar, E.; Jaimes-Ortega, G.A.; Valle-Rios, R.; Marín-Hernández, Á.; Rodríguez-Zavala, J.S.; Ruiz-May, E.; Castrejón-Flores, J.L.; et al. Shotgun Proteomics of Co-Cultured Leukemic and Bone Marrow Stromal Cells from Different Species as a Preliminary Approach to Detect Intercellular Protein Transfer. Proteomes 2023, 11, 15. https://doi.org/10.3390/proteomes11020015
Nevárez-Ramírez AJ, Guzmán-Ortiz AL, Cortes-Reynosa P, Perez-Salazar E, Jaimes-Ortega GA, Valle-Rios R, Marín-Hernández Á, Rodríguez-Zavala JS, Ruiz-May E, Castrejón-Flores JL, et al. Shotgun Proteomics of Co-Cultured Leukemic and Bone Marrow Stromal Cells from Different Species as a Preliminary Approach to Detect Intercellular Protein Transfer. Proteomes. 2023; 11(2):15. https://doi.org/10.3390/proteomes11020015
Chicago/Turabian StyleNevárez-Ramírez, Abraham Josué, Ana Laura Guzmán-Ortiz, Pedro Cortes-Reynosa, Eduardo Perez-Salazar, Gustavo Alberto Jaimes-Ortega, Ricardo Valle-Rios, Álvaro Marín-Hernández, José S. Rodríguez-Zavala, Eliel Ruiz-May, José Luis Castrejón-Flores, and et al. 2023. "Shotgun Proteomics of Co-Cultured Leukemic and Bone Marrow Stromal Cells from Different Species as a Preliminary Approach to Detect Intercellular Protein Transfer" Proteomes 11, no. 2: 15. https://doi.org/10.3390/proteomes11020015
APA StyleNevárez-Ramírez, A. J., Guzmán-Ortiz, A. L., Cortes-Reynosa, P., Perez-Salazar, E., Jaimes-Ortega, G. A., Valle-Rios, R., Marín-Hernández, Á., Rodríguez-Zavala, J. S., Ruiz-May, E., Castrejón-Flores, J. L., & Quezada, H. (2023). Shotgun Proteomics of Co-Cultured Leukemic and Bone Marrow Stromal Cells from Different Species as a Preliminary Approach to Detect Intercellular Protein Transfer. Proteomes, 11(2), 15. https://doi.org/10.3390/proteomes11020015