An Analysis of the Visual Representation of Redox Reactions in Secondary Chemistry Textbooks from Different Chinese Communities
Abstract
:1. Introduction
- Concerning visual representations (macroscopic, microscopic and symbolic)
- Are these present in Chinese chemistry textbooks related to redox reactions?
- How are they used?
- How are the different representational levels represented and related to one another?
- Are there any indicators in the visual representations in the textbooks of a modern chemistry curriculum that use everyday life and societal illustrations to contextualize chemistry learning?
2. Materials and Methods
3. Results
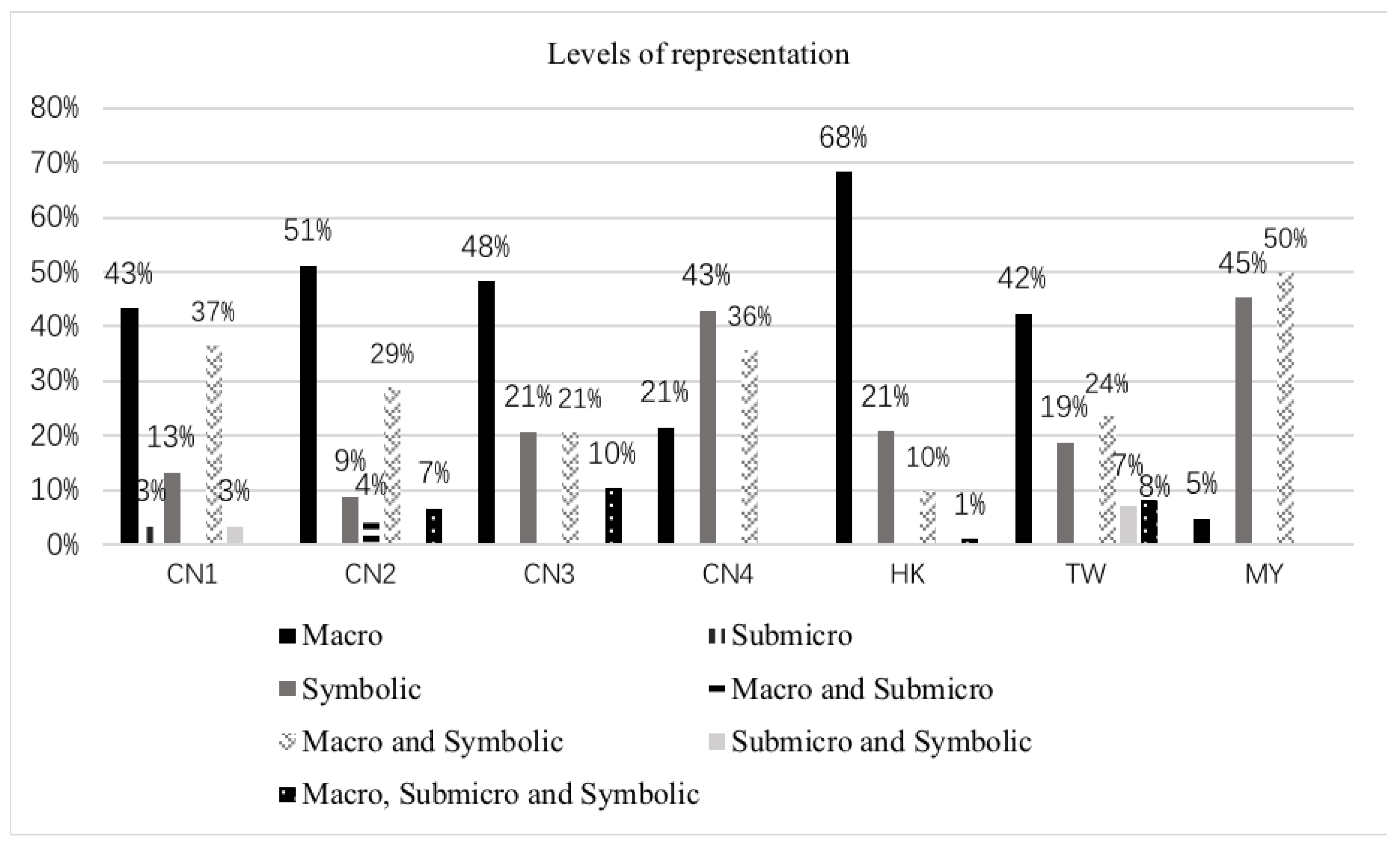
3.1. Levels of Representation
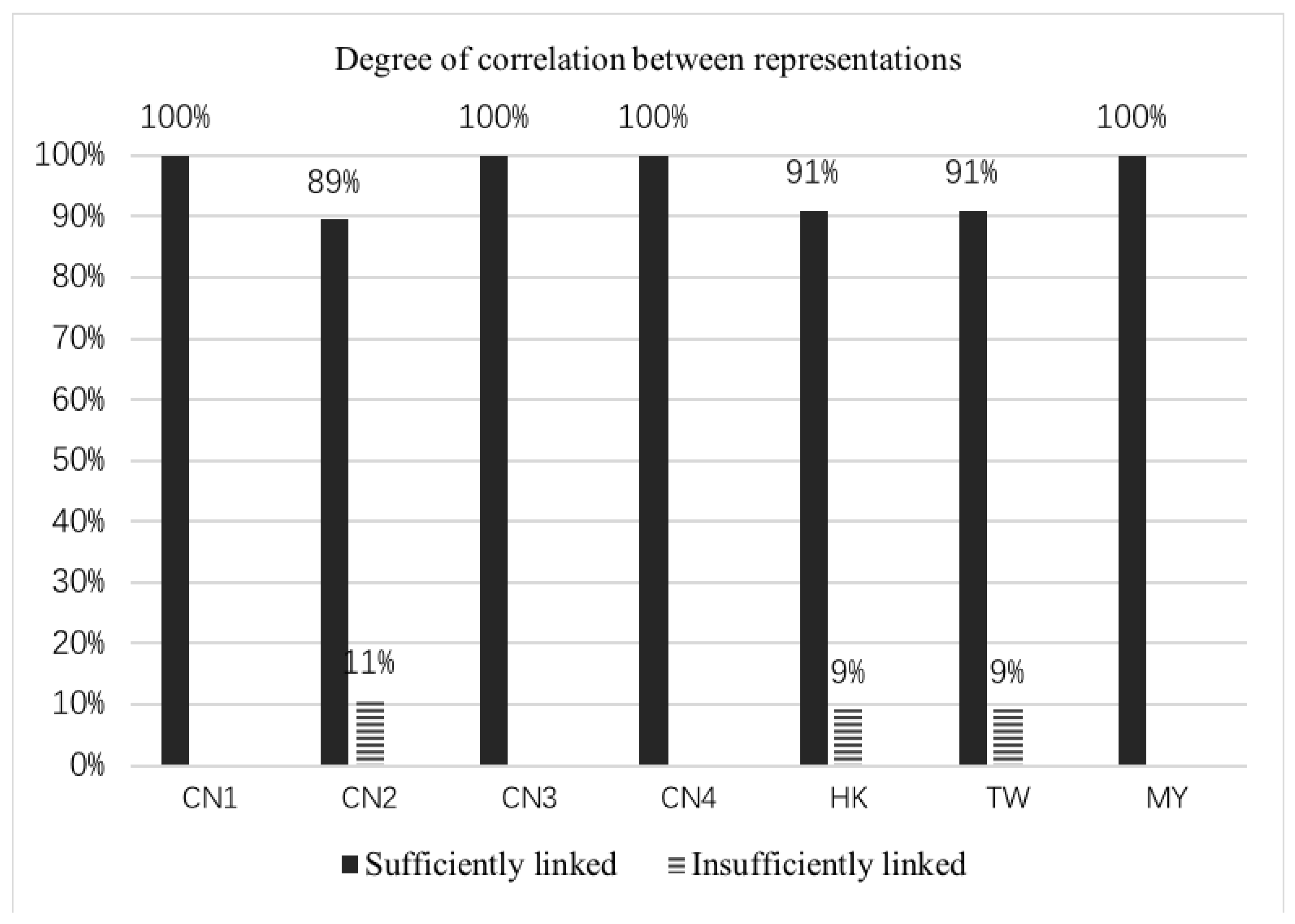
3.2. Multiple Visual Representations and Degree of Correlation between Representations
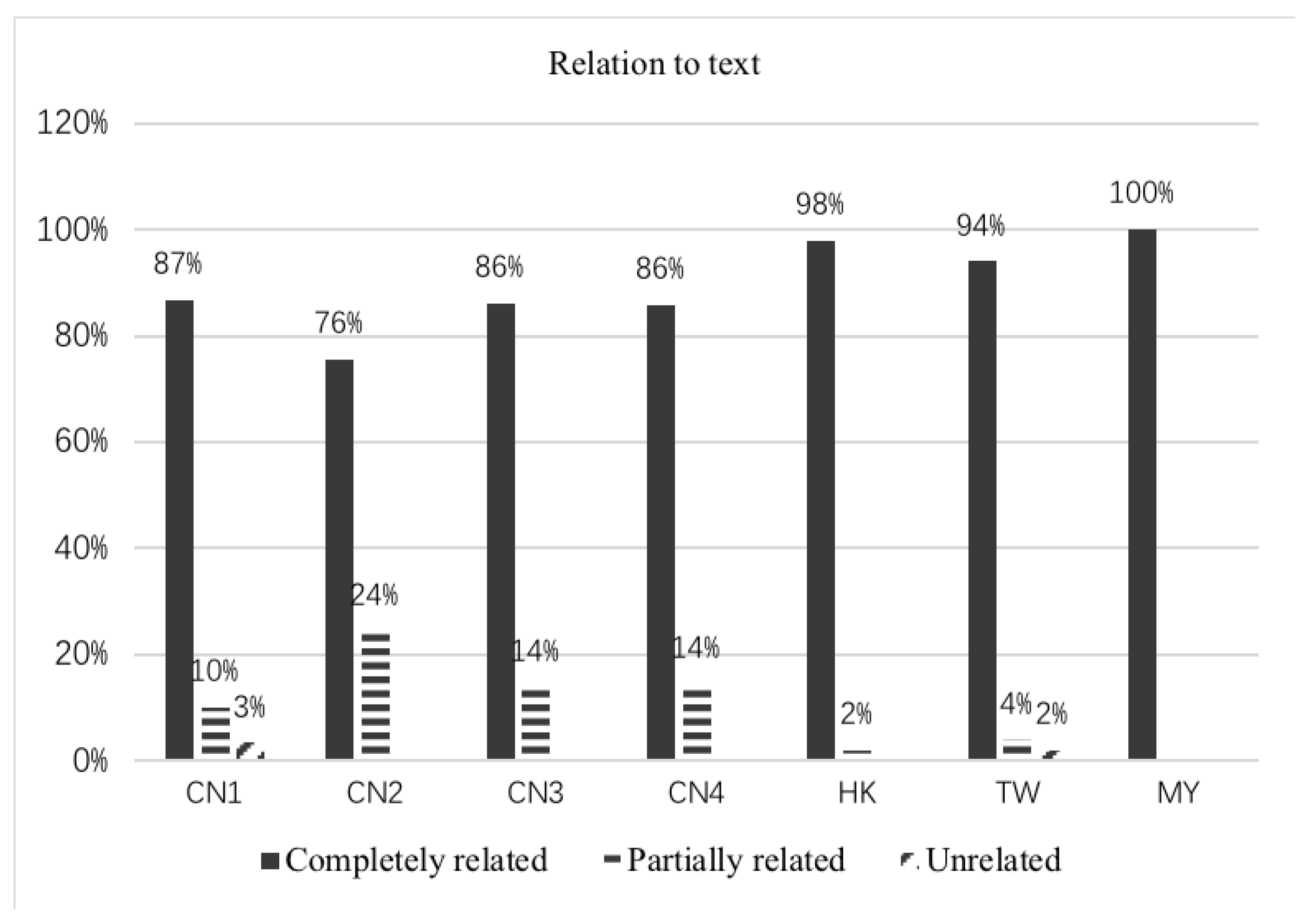
3.3. Relation to Text
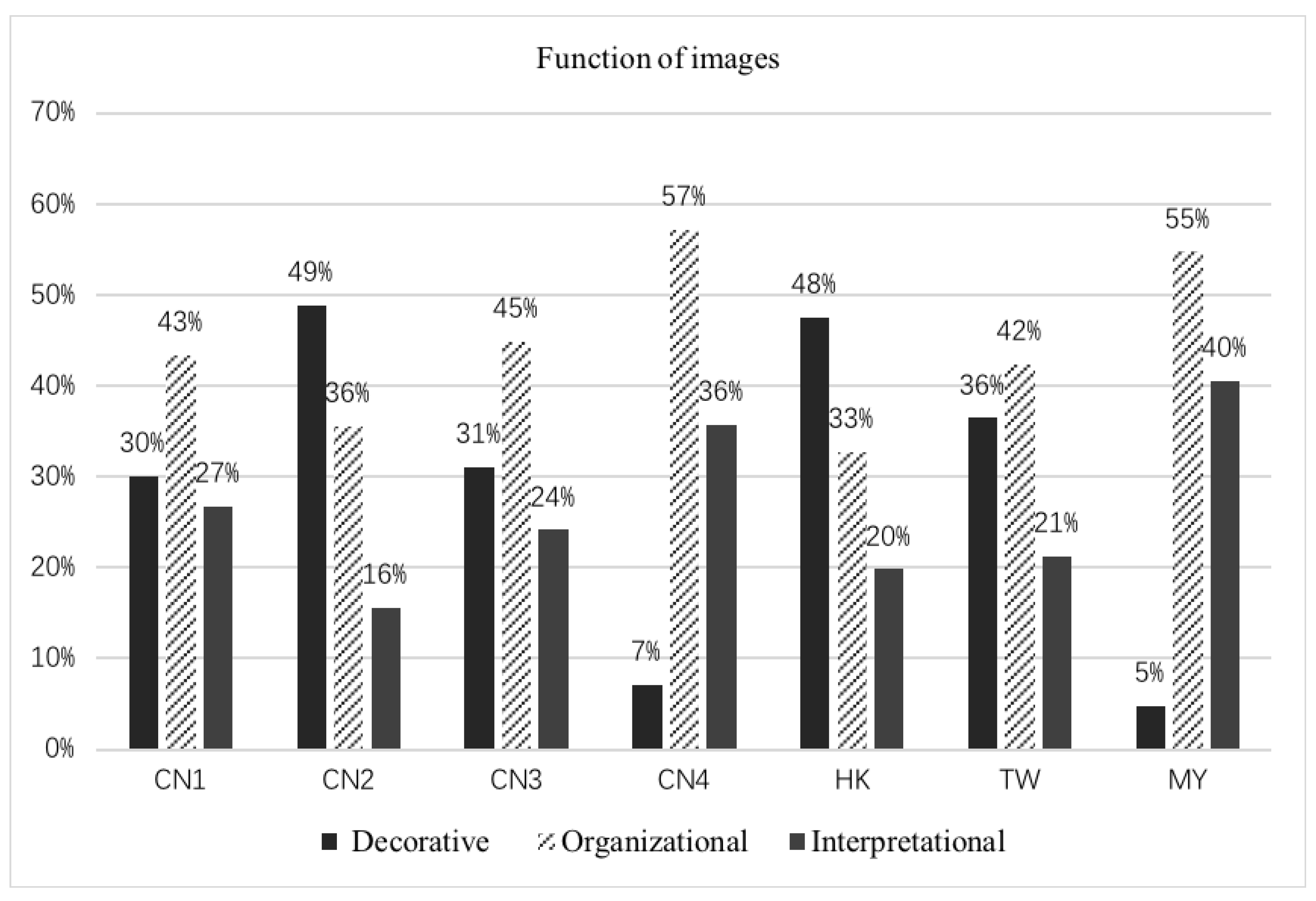
3.4. Function of Images
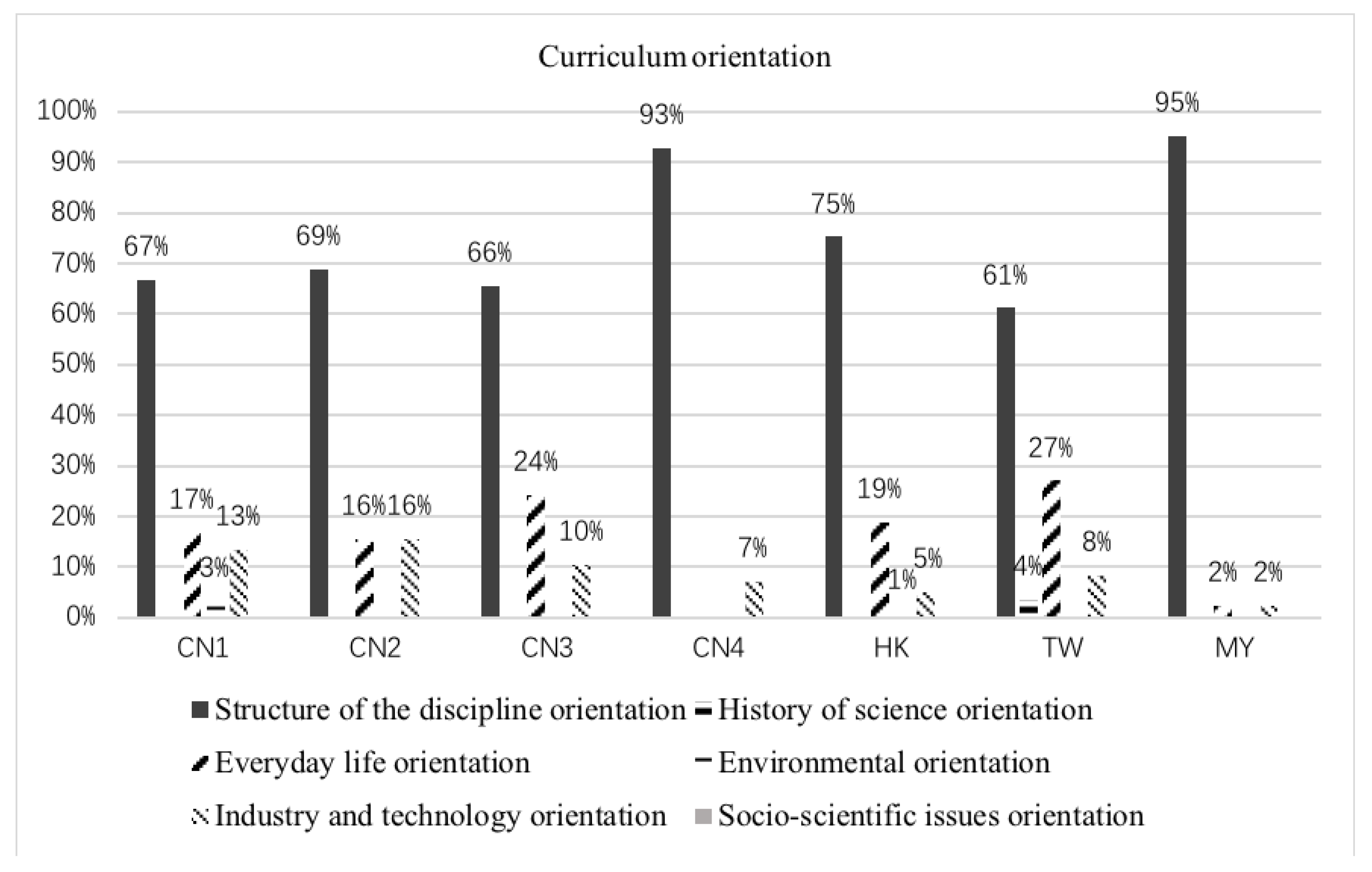
3.5. Curriculum Orientation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnstone, A.H. Why is science difficult to learn? Things are seldom what they seem. J. Comput. Assist. Learn. 1991, 7, 75–83. [Google Scholar] [CrossRef]
- Upahi, J.E.; Ramnarain, U. Representations of chemical phenomena in secondary school chemistry textbooks. Chem. Educ. Res. Pract. 2019, 20, 146–159. [Google Scholar] [CrossRef]
- Gabel, D. Improving teaching and learning through chemistry education research: A look to the future. J. Chem. Educ. 1999, 76, 548. [Google Scholar] [CrossRef]
- Talanquer, V. Macro, submicro, and symbolic: the many faces of the chemistry “triplet”. Int. J. Sci. Educ. 2011, 33, 179–195. [Google Scholar] [CrossRef]
- Taber, K.S. Revisiting the chemistry triplet: Drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education. Chem. Educ. Res. Pract. 2013, 14, 156–168. [Google Scholar] [CrossRef]
- Sjöström, J.; Talanquer, V. Humanizing chemistry education: from simple contextualization to multifaceted problematization. J. Chem. Educ. 2014, 91, 1125–1131. [Google Scholar] [CrossRef]
- Mahaffy, P. The future shape of chemistry education. Chem. Educ. Res. Pract. 2004, 5, 229–245. [Google Scholar] [CrossRef]
- Österlund, L.-L.; Berg, A.; Ekborg, M. Redox models in chemistry textbooks for the upper secondary school: Friend or foe? Chem. Educ. Res. Pract. 2010, 11, 182–192. [Google Scholar] [CrossRef]
- Basheer, A.; Hugerat, M.; Kortam, N.; Hofstein, A. The effectiveness of teachers’ use of demonstrations for enhancing students’ understanding of and attitudes to learning the oxidation reduction concept. Eurasia J. Math. Sci. Tech. Educ. 2017, 13, 555–570. [Google Scholar] [CrossRef]
- Barke, H.D. Two ideas of the redox reaction: Misconceptions and their challenge in chemistry education. Afr. J. Chem. Educ. 2012, 2, 32–50. [Google Scholar]
- Finley, F.N.; Stewart, J.; Yarroch, W.L. Teachers’ perceptions of important and difficult science content. Sci. Educ. 1982, 66, 531–538. [Google Scholar] [CrossRef]
- Obomanu, B.; Onuoha, C. Students conceptual difficulties in electrochemistry in senior secondary schools. J. Emerg. Trends Educ. Res. Pol. Stud. 2012, 3, 99. [Google Scholar]
- Paik, S.H.; Kim, S.; Kim, K. Suggestion of a viewpoint change for the classification criteria of redox reactions. J. Chem. Educ. 2017, 94, 563–568. [Google Scholar] [CrossRef]
- Schmidt, H.J.; Volke, D. Shift of meaning and students’ alternative concepts. Int. J. Sci. Educ. 2003, 25, 1409–1424. [Google Scholar] [CrossRef]
- De Jong, O.; Treagust, D.F. The teaching and learning of electrochemistry. In Chemical Education: Towards Research-Based Practice; Gilbert, J.K., De Jong, O., Justi, R., Treagust, D.F., Van Driel, J.H., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 317–337. [Google Scholar]
- Shibley, I.A., Jr.; Amaral, K.E.; Aurentz, D.J.; McCaully, R.J. Oxidation and reduction reactions in organic chemistry. J. Chem. Educ. 2010, 87, 1351–1354. [Google Scholar] [CrossRef]
- Osman, K.; Lee, T.T. Impact of interactive multimedia module with pedagogical agents on students’ understanding and motivation in the learning of electrochemistry. Int. J. Sci. Math. Educ. 2014, 12, 395–421. [Google Scholar] [CrossRef]
- Eilks, I.; Witteck, T.; Pietzner, V. The role and potential dangers of visualisation when learning about sub-microscopic explanations in chemistry education. Cent. Educ. Policy Stud. J. 2012, 2, 125–145. [Google Scholar]
- Ramnarain, U.; Joseph, A. Learning difficulties experienced by grade 12 South African students in the chemical representation of phenomena. Chem. Educ. Res. Pract. 2012, 13, 462–470. [Google Scholar] [CrossRef]
- Valanides, N.; Nicolaidou, A.; Eilks, I. Twelfth grade students’ understanding of oxidation and combustion: Using action research to improve teachers’ practical knowledge and teaching practice. Res. Sci. Tech. Educ. 2003, 21, 159–175. [Google Scholar] [CrossRef]
- Gilbert, J.K. On the nature of “context” in chemical education. Int. J. Sci. Educ. 2006, 28, 957–976. [Google Scholar] [CrossRef]
- Pintó, R.; Ametller, J. Students’ difficulties in reading images. Comparing results from four national research groups. Int. J. Sci. Educ. 2002, 24, 333–341. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Koulaidis, V.; Sklaveniti, S. Towards an analysis of visual images in school science textbooks and press articles about science and technology. Res. Sci. Educ. 2003, 33, 189–216. [Google Scholar] [CrossRef]
- Eilks, I. Experiences and reflections about teaching atomic structure in a jigsaw classroom in lower secondary school chemistry lesson. J. Chem. Educ. 2005, 82, 313. [Google Scholar] [CrossRef]
- Gkitzia, V.; Salta, K.; Tzougraki, C. Development and application of suitable criteria for the evaluation of chemical representations in school textbooks. Chem. Educ. Res. Pract. 2011, 12, 5–14. [Google Scholar] [CrossRef]
- Stylianidou, F. Analysis of science textbook pictures about energy and pupils’ readings of them. Int. J. Sci. Educ. 2002, 24, 257–283. [Google Scholar] [CrossRef]
- Eilks, I. Teacher pathways through the particulate nature of matter in lower secondary school chemistry: Continuous switching between different models or a coherent conceptual structure? In Concepts of Matter in Science Education; Tsaparlis, G., Sevian, H., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 213–230. [Google Scholar]
- Stuckey, M.; Hofstein, A.; Mamlok-Naaman, R.; Eilks, I. The meaning of ‘relevance’ in science education and its implications for the science curriculum. Stud. Sci. Educ. 2013, 49, 1–34. [Google Scholar] [CrossRef]
- Holman, J. Resources or courses? Contrasting approaches to the introduction of industry and technology to the secondary curriculum. Sch. Sci. Rev. 1987, 68, 432–438. [Google Scholar]
- Raved, L.; Assarafs, O.B.Z. Attitudes towards science learning among 10th-Grade students: A qualitative look. Int. J. Sci. Educ. 2011, 33, 1219–1243. [Google Scholar] [CrossRef]
- Wu, S.P.W.; Rau, M.A. Effectiveness and efficiency of adding drawing prompts to an interactive educational technology when learning with visual representations. Learn. Instr. 2018, 55, 93–104. [Google Scholar] [CrossRef]
- Ainsworth, S. DeFT: A conceptual framework for considering learning with multiple representations. Learn. Instr. 2006, 16, 183–198. [Google Scholar] [CrossRef]
- Rau, M.A.; Bowman, H.E.; Moore, J.W. An adaptive collaboration script for learning with multiple visual representations in chemistry. Comput. Educ. 2017, 109, 38–55. [Google Scholar] [CrossRef]
- Eilks, I.; Rauch, F.; Ralle, B.; Hofstein, A. How to allocate the chemistry curriculum between science and society. In Teaching Chemistry—A Studybook; Eilks, I., Hofstein, A., Eds.; Sense Publisher: Rotterdam, The Netherlands, 2013; pp. 1–36. [Google Scholar]
- Martinez-Gracia, M.V.; Gil-Quylez, M.J.; Osada, J. Genetic engineering: A matter that requires further refinement in Spanish secondary school textbooks. Int. J. Sci. Educ. 2003, 25, 1147–1168. [Google Scholar] [CrossRef]
- Devetak, I.; Vogrinc, J. The criteria for evaluating the quality of the science textbooks. In Critical Analysis of Science Textbooks: Evaluating Instructional Effectiveness; Khine, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 3–15. [Google Scholar]
- Souza, K.A.F.D.; Porto, P.A. Chemistry and chemical education through text and image: Analysis of twentieth century textbooks used in Brazilian context. Sci. Educ. 2012, 21, 705–727. [Google Scholar] [CrossRef]
- Cheng, K.L.; Wong, S.L. Nature of science as portrayed in the physics official curricula and textbooks in Hong Kong and on the mainland of the People’s Republic of China. In Topics and Trends in Current Science Education; Bruguière, C., Tiberghien, A., Clément, P., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 519–534. [Google Scholar]
- Wang, L.; Tang, J.J.; Zhang, R.H.; Hu, J.H.; Zhi, Y.; Wei, R. Investigation of the use of new chemistry textbooks of senior high school and the influential factors. J. Educ. Stud. 2015, 11, 77–86. [Google Scholar]
- Dagher, Z.R.; Erduran, S. Reconceptualizing the nature of science for science education. Sci. Educ. 2016, 25, 147–164. [Google Scholar] [CrossRef]
- Van Den Akker, J. The science curriculum: Between ideals and outcomes. In International Handbook of Science Education; Fraser, B.J., Tobin, K.G., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 421–447. [Google Scholar]
- Lee, V.R. Adaptations and continuities in the use and design of visual representations in US middle school science textbooks. Int. J. Sci. Educ. 2010, 32, 1099–1126. [Google Scholar] [CrossRef]
- Khaddoor, R.; Al-Amoush, S.; Eilks, I. A comparative analysis of the intended curriculum and its presentation in 10th grade chemistry textbooks from seven Arabic countries. Chem. Educ. Res. Pract. 2017, 18, 375–385. [Google Scholar] [CrossRef]
- Calado, F.M.; Scharfenberg, F.-J.; Bogner, F.X. To what extent do biology textbooks contribute to scientific literacy? Criteria for analysing science-technology-society-environment issues. Educ. Sci. 2015, 5, 255–280. [Google Scholar] [CrossRef]
- Livni-Alcasid, G.; Haskel-Ittah, M.; Yarden, A. As symbol as that: Inconsistencies in symbol systems of alleles in textbooks, and students’ justifications for them. Educ. Sci. 2018, 8, 110. [Google Scholar] [CrossRef]
- Department of Statistics Malaysia. Current Population Estimates, Malaysia, 2016–2017. Available online: www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=155&bul_id=a1d1UTFZazd5ajJiRWFHNDduOXFFQT09&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09 (accessed on 5 January 2018).
- Liu, H. Retrospect and prospect of university entrance examination. Educ. Res. 2007, 11, 19–24. [Google Scholar]
- Hong Kong Education Bureau, Recommended Textbook List. Available online: https://cd.edb.gov.hk/rtl/search.asp (accessed on 3 January 2019).
- National Academy for Educational Research, Project of the Implementation of 12-Year Basic Education. Available online: www.naer.edu.tw/files/15-1000-7944,c639-1.php?Lang=zh-tw (accessed on 5 December 2017).
- Dong Zong, The background of Dong Zong’s Establishment. Available online: www.dongzong.my/aboutus.php (accessed on 5 January 2018).
- Carney, R.N.; Levin, J.R. Pictorial illustrations still improve students’ learning from text. Educ. Psych. Rev. 2002, 14, 5–26. [Google Scholar] [CrossRef]
- Nyachwaya, J.M.; Gillaspie, M. Features of representations in general chemistry textbooks: A peek through the lens of the cognitive load theory. Chem. Educ. Res. Pract. 2015, 17, 58–71. [Google Scholar] [CrossRef]
- Roseman, J.E.; Stern, L.; Koppal, M. A method for analyzing the coherence of high school biology textbooks. J. Res. Sci. Teach. 2010, 47, 47–70. [Google Scholar] [CrossRef]
- Treagust, D.F.; Chittleborough, G.; Mamiala, T. The role of submicroscopic and symbolic representations in chemical explanations. Int. J. Sci. Educ. 2003, 25, 1353–1368. [Google Scholar] [CrossRef]
- Berliner, D.C. The development of expertise in pedagogy. Charles W. Hunt Memorial Lecture presented at the annual meeting of the American Association of Colleges for Teacher Education, New Orleans, LA, USA, 17–20 February 1988. [Google Scholar]
- Kozma, R.; Russell, J. Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. J. Res. Sci. Teach. 1997, 34, 949–968. [Google Scholar] [CrossRef]
- Mahaffy, P. The human element: Chemistry education’s contribution to our global future. In Chemistry Education; García-Martínez, J., Serrano-Torregrosas, E., Eds.; Wiley-VCH: Darmstadt, Germany, 2011; pp. 131–157. [Google Scholar]
- Cuban, L. The lure of curricular reform and its pitiful history. Phi Delta Kappan 1993, 75, 182–185. [Google Scholar]
- Russell, J.W.; Kozma, R.B.; Jones, T.; Wykof, J.; Marx, N.; Davis, J. Use of simultaneous-synchronised macroscopic, microscopic and symbolic representations to enhance the teaching and learning of chemical concepts. J. Chem. Educ. 1997, 74, 330–334. [Google Scholar] [CrossRef]
- Eilks, I.; Hofstein, A. Teaching Chemistry—A Studybook; Sense Publisher: Rotterdam, The Netherlands, 2013. [Google Scholar]
- Chiappetta, E.L.; Fillman, D.A. Analysis of five high school biology textbooks used in the United States for inclusion of the nature of science. Int. J. Sci. Educ. 2007, 29, 1847–1868. [Google Scholar] [CrossRef]
- Lavonen, J. Commentary: What we can learn from science education in Asian countries? In Science Education Research and Practice in Asia; Chiu, M.-H., Ed.; Springer: Singapore, 2016; pp. 215–221. [Google Scholar]
- Yore, L.D.; Shymansky, J.A.; Treagust, D.F. An international perspective on the people and events shaping science education in Taiwan—past, present, and future. In Science Education Research and Practices in Taiwan; Chiu, M.-H., Ed.; Springer: Singapore, 2016; pp. 397–419. [Google Scholar]
- De Jong, O. Comments on section 4: Thoughts on science curriculum reform and teacher learning in western countries and Taiwan. In Science Education Research and Practices in Taiwan; Chiu, M.-H., Ed.; Springer: Singapore, 2016; pp. 387–394. [Google Scholar]
- Nurul-Awanis, A.; Hazlina, A.; Yoke-Ma, L.; Zariyawati, M. Malaysian education system reform: Educationists’ perspectives. In Proceedings of the International Conference on Social Science, Economics and Art 2011, Kuala Lumpur, Malaysia, 14–15 January 2011. [Google Scholar]
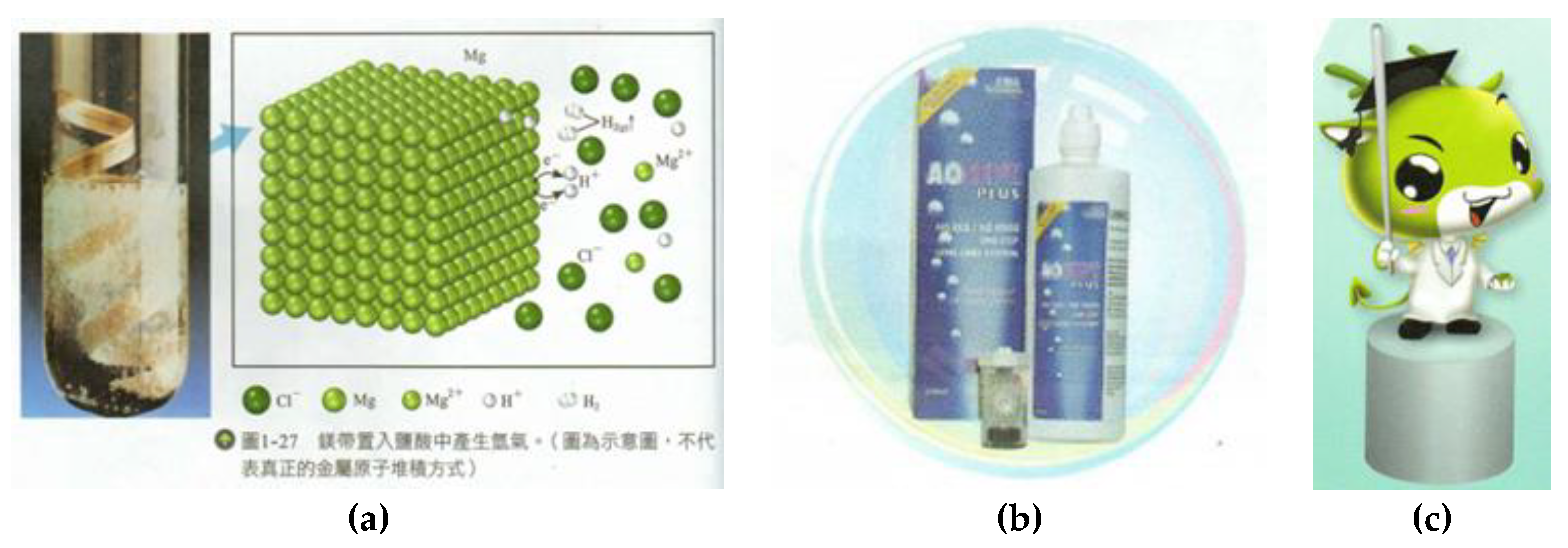
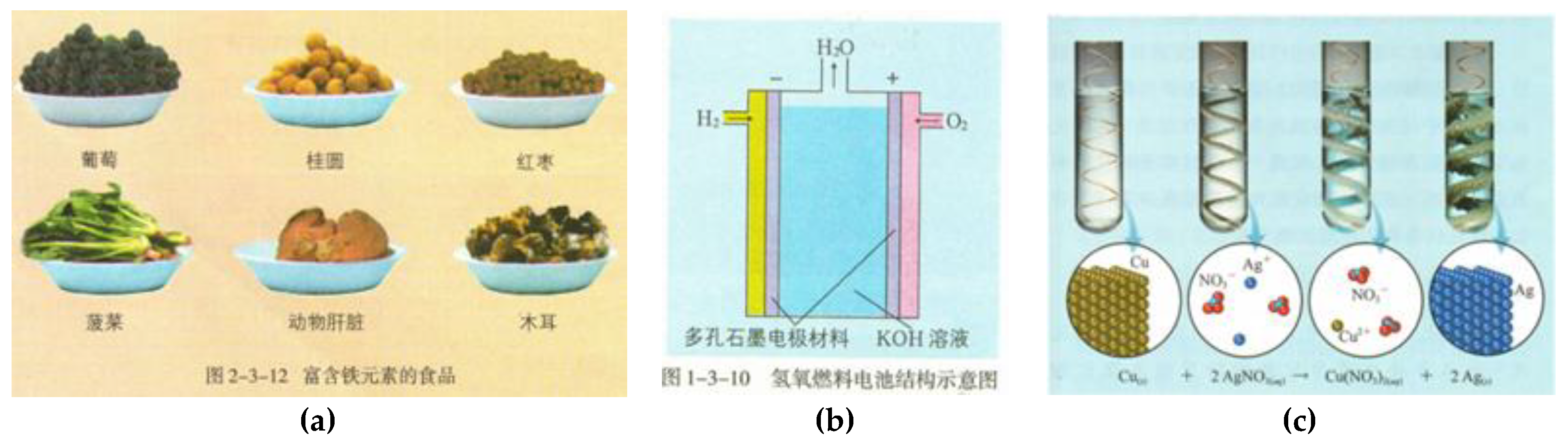
| Textbook | Reference |
|---|---|
| CN1 | Song, X. Q. (Ed.). (2007). Chemistry 1 (3rd ed.). Beijing: People Education Press. |
| Song, X. Q. (Ed.). (2007). Chemical reaction mechanism (3rd ed.). Beijing: People Education Press. | |
| CN2 | Wang, L. (Ed.). (2007a). Chemistry 1 (3rd ed.). Shandong: Shandong Science and Technology Press. |
| Wang, L. (Ed.). (2011). Chemical reaction mechanisms (4th ed.). Shandong: Shandong Science and Technology Press. | |
| CN3 | Wang, Z. H. (2014). Chemistry 1 (6th ed.). Nanjing: Jiangsu Education Press. |
| Wang, Z. H. (2014). Chemical reaction mechanisms (5th ed.). Nanjing: Jiangsu Education Press. | |
| CN4 | Yao, Z. P. (Ed.). (2007). Chemistry (Volume 1) (1st ed.). Shanghai: Shanghai Scientific and Technical Publishers. |
| Yao, Z. P. (Ed.). (2008). Chemistry (Volume 3) (1st ed.). Shanghai: Shanghai Scientific and Technical Publishers. | |
| HK | Zhong, H. M. (2009). New 21st Century Chemistry 2B (1st ed.). Hong Kong: Jing Kung Educational Press. |
| TW | Huang, D. S. (Ed.). (2010). Basic Chemistry 1 (1st ed.). Taiwan: LungTeng Culture Lungteng Cultural. |
| Huang, D. S. (Ed.). (2011). Basic Chemistry 2 (1st ed.). Taiwan: LungTeng Culture Lungteng Cultural. | |
| Huang, D. S. (Ed.). (2012). Selective Chemistry 1 (1st ed.). Taiwan: LungTeng Culture Lungteng Cultural. | |
| MY | MICSS (1996). Upper secondary school chemistry (volume 1) (1st ed.). Malaysia: United Chinese School Committees’ Association of Malaysia. |
| MICSS (1997). Upper secondary school chemistry (volume 2) (1st ed.). Malaysia: United Chinese School Committees’ Association of Malaysia. |
| Category | Subcategory | Description |
|---|---|---|
| Levels of representation | Macro | Presents only observable and realistic aspects (M) |
| Submicro | Illustrates unobservable entities and abstract aspects (S) | |
| Symbolic | Uses symbols and codes of chemistry (S) | |
| Macro and submicro | Represents two levels: macro and submicro (M+S) | |
| Macro and symbolic | Represents two levels: macro and symbolic (M+S) | |
| Submicro and symbolic | Represents two levels: submicro and symbolic (S+S) | |
| Macro, submicro and symbolic | Represents the three levels: macro, submicro and symbolic (M+S+S) | |
| Degree of correlation between representations comprising multiple ones | Sufficiently linked | Equivalence of the surface features of the components is clearly indicated |
| Insufficiently linked | Equivalence of only some surface features is indicated clearly | |
| Unlinked | Includes subordinate representations that are placed next to one another and there is no indication of the equivalence of their surface features | |
| Relation to text | Completely related | Representation depicts the exact text content |
| Partially related | Representation depicts the subject or a familiar subject to the text, text does not direct the reader to the relationship between text and representation | |
| Unrelated | Representation is irrelevant to the text content. The text describes the content without mentioning the correspondence with the representation |
| Category | Subcategory | Description |
|---|---|---|
| Function of images | Decorative | Not relevant to the text—illustrations only help the reader enjoy the textbook by making it more attractive |
| Organizational | Illustrations help the reader organize information into a coherent structure and encourage more detailed processing of text; captions name the fact but do not provide extra information to the text | |
| Interpretational | Strong relationship to the content—illustrations explain and help the reader understand concepts and ideas in the text; captions name the fact and add extra information about the fact |
| Category | Subcategory | Description |
|---|---|---|
| Curriculum orientation | Structure of the discipline orientation | Illustrations represent scientific theories and facts and their relation to one another |
| History of science orientation | Illustrations represent scientific content as it emerged in the past or its historical development | |
| Everyday life orientation | Illustrations represent entities from everyday life | |
| Environmental orientation | Illustrations represent scientific content behind questions of environmental protection | |
| Industry and technology orientation | Illustrations represent chemical technology and its application in industry | |
| Socio-scientific issues orientation | Illustrations provoke the learning allowing the students to develop general educational skills to prepare them to become responsible citizens in future |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; de Goes, L.F.; Treagust, D.F.; Eilks, I. An Analysis of the Visual Representation of Redox Reactions in Secondary Chemistry Textbooks from Different Chinese Communities. Educ. Sci. 2019, 9, 42. https://doi.org/10.3390/educsci9010042
Chen X, de Goes LF, Treagust DF, Eilks I. An Analysis of the Visual Representation of Redox Reactions in Secondary Chemistry Textbooks from Different Chinese Communities. Education Sciences. 2019; 9(1):42. https://doi.org/10.3390/educsci9010042
Chicago/Turabian StyleChen, Xiaoge, Luciane F. de Goes, David F. Treagust, and Ingo Eilks. 2019. "An Analysis of the Visual Representation of Redox Reactions in Secondary Chemistry Textbooks from Different Chinese Communities" Education Sciences 9, no. 1: 42. https://doi.org/10.3390/educsci9010042
APA StyleChen, X., de Goes, L. F., Treagust, D. F., & Eilks, I. (2019). An Analysis of the Visual Representation of Redox Reactions in Secondary Chemistry Textbooks from Different Chinese Communities. Education Sciences, 9(1), 42. https://doi.org/10.3390/educsci9010042






