Abstract
Electrohydrodynamic processing (EHDP) has revolutionized nanotechnology since it is a simple method for developing microstructures and nanostructures from a wide range of polymer solutions using the application of high voltages. However, EHDP has scarcely been introduced into food engineering courses at any educational level, whereas it is fundamental for professionals in the discipline. The present educational article aims to introduce, for the first time, the basis of the EHDP technology and its management to students in postgraduate courses for food emulsion and related disciplines. To this end, the document reports the step-by-step preparation of zein solutions in aqueous ethanol with varying weight protein contents and the characterization of such solutions in terms of their density, viscosity, surface tension, and conductivity. Then, the methodology also describes the processability of the resultant zein solutions using EHDP. Process parameters, including applied voltage, flow-rate, and tip-to-collector distance, were studied to optimize proper deposition of zein materials in the form of a wide range of morphologies, from nanobeads to microfibers. The attained results were related to the zein solution properties and processing conditions, which can help to understand the effect of these parameters and exemplify the potential of this technology to develop novel ultrathin food hydrocolloid structures. Finally, the application of this methodology was assessed through online surveys taken during food engineering courses and findings indicate that postgraduate students appreciate the exposure provided by the experimental activities, particularly to achieve familiarity with food hydrocolloid solutions and scientific literacy in the EHDP technology.
1. Introduction
Electrohydrodynamic processing (EHDP) is one of the most accessed nanofabrication techniques during the last three decades. Although the electrohydrodynamic concept is not necessarily new, research on EHDP has increased exponentially due to both the increasing knowledge and interest in nanotechnology [1]. EHDP is a versatile top-down method that is based on the application of high voltages to polymer solutions. The basic setup consists of four different components: a syringe pump to force a controllable flow-rate of a polymer solution, a metal spinneret that usually consists of needles of varying diameters, a high-voltage power supply that applies high electrical fields to the tip of the spinneret, and a grounded metal collector on which the samples are deposited [2]. According to the process basis, the application of a voltage at the tip of the spinneret creates an electrical field between it and the grounded collector. The polymer solution is pumped through the metal spinneret and, when it receives the applied electrical field, it creates the so-called Taylor cone. Thus, the polymer is stretched and twisted, whereas the solvent quickly evaporates. At the end of the process, microstructures and nanostructures are deposited onto the grounded collector. EHDP can be operated in two basic methods: electrospraying, which leads to the production of micro- and nanoparticles, and electrospinning that, in turn, leads to the production of micro- and nanofibers. Furthermore, nozzle- and electrode-free pyro-electrohydrodynamic systems have been recently developed as an alternative to conventional configurations [3]. Over other nanotechnological processes, EHDP technologies show several advantages due to the fact that they are versatile, use benign solvents, and operate at ambient working conditions. In addition, it can be combined with other processes to develop advanced materials, such as, for instance, electroless deposition (ELD) techniques [4,5,6].
Due to their biological properties and sustainable sourcing in the field of food technology, materials processed by EHDP, created using naturally occurring polymers, such as proteins and polysaccharides, have attracted much attention [7]. Other applications span from biotechnology, filtration, or biomedicine to wound care applications and organ-on-chip technology [8]. Among various biopolymers, zein, the main storage protein in maize or corn, presents the advantages of being renewable and biodegradable. Technically, zein is not a single polypeptide; rather, it represents a mixture of several proteins or polypeptides of various molecular weights (MWs), which mainly vary in their solubilities [9]. This storage protein is considered a prolamine owing to its high content of hydrophobic amino acids, such as proline and glutamine, and is soluble in aqueous ethanol, which is a sustainable solvent for EHDP. In particular, a solution of 70–85% (wt/wt) ethanol is the most optimal solvent for processing zein using EHDP [10]. Other studies have also reported the use of deep eutectic solvents (DES) during EHDP of zein, which are widely recognized as non-volatile and non-hazardous solvents [11]. Although its imbalanced amino acid profile and low water solubility represents the major obstacles for its direct application in food consumption, it shows unique physicochemical properties for encapsulation purposes and sustainable food packaging applications [12].
Even though both electrospraying and electrospinning are currently very promising for the production of ultrathin capsules and fibers using food hydrocolloids, so far, these have scarcely been introduced into food engineering courses. Furthermore, current designs of learning science and engineering barely motivate students to develop an interest in learning about EHDP, whereas, at the same time, more human resource talent is needed to promote micro- and nano-manufacturing education in polytechnical universities [13]. Thus, education and high technical attitudes in EHDP are expected to be an interesting vehicle for students and universities to gain more knowledge in science and engineering, contributing to the improvement of their scientific literacy in the near future [14]. In this context, introduction of EHDP at the university level can potentially improve student’s interest, attention, response to issues of science, and motivation towards science, as well as help future professionals make use of it for novel materials and applications.
2. Objectives
The main objective of this educational article was to introduce students in food engineering courses to the EHDP technique, as it is currently used by scientists, researchers, and engineers, and its cutting-edge applications. First, it was aimed to provide students with the ability to prepare and characterize food hydrocolloid solutions, for which it is included an easy-to-follow methodology to prepare and characterize zein solutions with varying protein contents in the university laboratory facilities. Then, students were exposed to the basic principles and management of EHDP. Thus, students were guided to process zein solutions using EHDP and relate their processability and morphology to their properties, determining the most optimal conditions. Furthermore, the experimental steps and information gathered in the present document can provide students and early researchers with the sufficient knowledge to become familiar with the main EHDP parameters. In particular, the proposed procedure described herein has essentially been written for students and faculty members to be followed in two sessions of experimental laboratory practices for a timespan of approximately 4 h in food engineering courses.
3. Materials and Methods
3.1. Materials
Zein from corn (grade Z3625) was purchased from Sigma-Aldrich S.A. (Madrid, Spain) and was used as received without further purification. Ethanol of 96% vol/vol purity was supplied by Panreac S.A. (Barcelona, Spain).
3.2. Preparation of the Zein Solutions
Zein was dissolved at room temperature with magnetic stirring until complete dissolution in an ethanol and water mixture 80% (wt/wt) at different concentrations. Table 1 shows the range of compositions that were prepared for the laboratory practice. Approximately 200 g of each sample was prepared for characterization in duplicate.

Table 1.
Codification and composition of the zein solutions prepared in an ethanol and water mixture 80% (wt/wt) according to the protein weight content (wt%) to provide a total mass of 200 g.
3.3. Characterization of the Zein Solutions
3.3.1. Density
The zein solutions were first characterized in terms of their density using a Hubbard pycnometer (VidraFoc S.A., Barcelona, Spain). Figure 1 includes an image of the pycnometer used and the procedure followed to record the weight on a PB303-s balance (Mettler-Toledo, Barcelona, Spain).
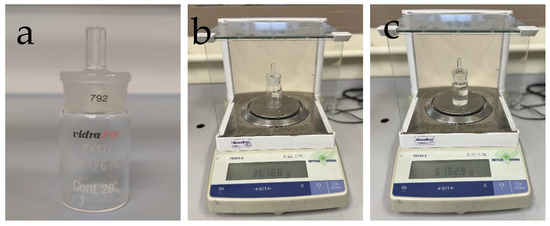
Figure 1.
Pycnometer (a) and procedure used to record its weight when empty (b) and full (c).
The steps followed to determine density are summarized as follow:
- The empty pycnometer was weighed and its mass (mp) noted.
- The pycnometer was filled up with distilled water and its mass (mp+w) weighed, avoiding the formation of bubbles. When closed, the liquid overflowing through the capillary was removed and the pycnometer was dried.
- The pycnometer was cleaned and filled up with the studied sample solution and its mass (mp+s) weighed, considering the previous cautions.
- Equation (1) was applied to determine the density of the sample.
3.3.2. Viscosity
Determination of the viscosity of the zein solutions was carried out in a Brookfield Synchro-Lectric Viscometer, model LVF (Brookfield Engineering Laboratories, Stoughton, MA, USA). This consisted of a rotation viscometer with 4 types of needles, marked from 1 to 4, as shown in Figure 2.

Figure 2.
Rotational viscometer with needles numbered from 1 to 4.
The steps followed to determine viscosity are described below:
- The appropriate needle was selected according to the expected viscosity of the fluid to be studied. The higher the viscosity, the higher the gauge number of the needle.
- The studied solution was poured into a 250-mL beaker up to the needle mark, avoiding the protector to hit the bottom of the beaker.
- The viscometer was turned on with the start selector and a suitable rotation speed was selected to obtain a proper reading: 6, 12, 30, and 60 rpm.
- Once stabilized, the rear selector was pressed and held to fix the reading value.
- Without releasing the selector, the equipment was stopped by pressing the start selector to proceed with the dial reading.
- The viscosity was determined from the speed and needle selected as shown in Table 2.
 Table 2. Determination of the constant (k) according to the speed and needle type.
Table 2. Determination of the constant (k) according to the speed and needle type.
- Finally, from the dial reading and the constant (k), the viscosity value of each solution was estimated by means of Equation (2).
3.3.3. Conductivity
The solutions were characterized in terms of their conductivity using a portable CON 6/TDS conductivity meter (Thermo Fisher Eutech Instruments Pte Ltd., Singapore), shown in Figure 3. Electrical conductivity meters measure the capacity of ions in an aqueous solution to carry electrical current.
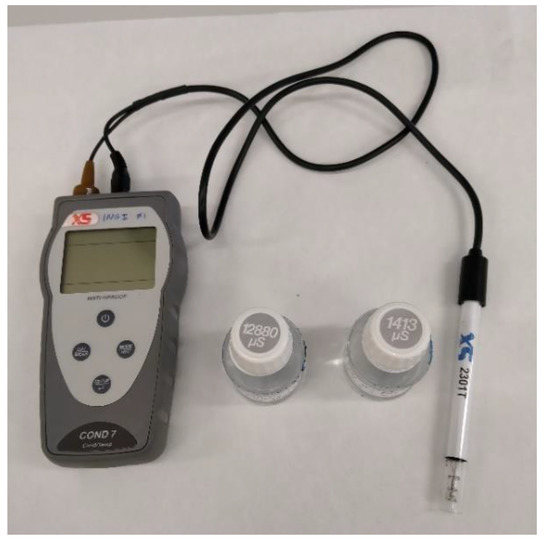
Figure 3.
Conductivity meter with ±1% full-scale accuracy.
The steps followed to carry out the conductivity measurements are shown below:
- The conductivity meter was connected in its “Conductivity” mode.
- The conductivity meter was calibrated with a standard solution of 0.01 M KCl (according to the instructions in the manual, specific conductivity KCl = 1.41 mS/cm at 25 °C) and the “CAL” key was pressed.
- The sensor was placed in a beaker with distilled water. Before measuring, the sensor was gently dried with paper.
- The sensor was introduced in the studied solution, preventing contact with the magnet during stirring.
- The value was read when the measurement stabilized. As the ranges in aqueous solutions are usually small, the basic units of measurements were expressed in milliSiemens/cm (mS/cm) or microSiemens/cm (µS/cm).
- The sensor was washed with distilled water and stored with its protector at the end of the test.
3.3.4. Surface Tension
A K20 force tensiometer (Krüss Scientific, Hamburg, Germany) was used to determine surface tension of the solutions. This is a semi-automatic instrument for measuring the surface and interfacial tension according to the ring, plate, and ring tear-off method, which is based on the force that acts when a measuring probe is wetted. This measurement was carried out using the RI 21 platinum-iridium alloy wire ring and SV20 vessel employing the Du Noüy ring method, as shown in Figure 4.
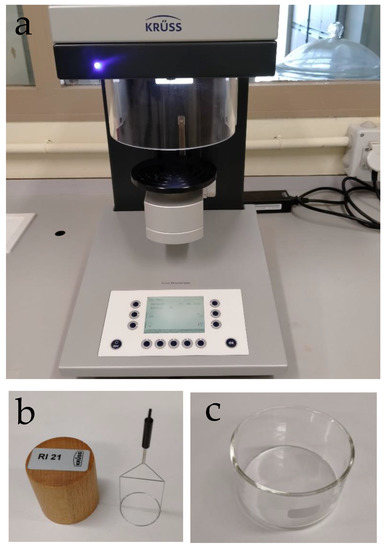
Figure 4.
Experimental setup showing the surface tension equipment (a), RI 21 ring (b), and SV20 vessel (c).
The measurements were performed following these steps:
- The platform was moved down.
- The RI 21 ring was suspended from the hook located in the force sensor.
- The SV20 vessel was placed on the platform with the solution to be studied.
- In the main menu, the “Du Noüg Ring” method was selected.
- The density of the sample to be measured was added in the menu.
- The platform with the sample was moved upwards until the ring was positioned just above the surface of the liquid, waiting until the ring remained stable and the liquid was at rest.
- The “tare” button was pressed to tare the weight of the ring in air, waiting until a weight of “0.0000 g” was displayed in the upper right-hand corner of the display.
- The sample platform finally moved upwards and the ring was immersed in the liquid to a depth of about 1 mm.
- The “start” button was pressed to measure the surface tension of the liquid.
3.4. EHDP of the Zein Solutions
The zein solutions were processed by EHDP using a dual polarization Fluidnatek LE-10 lab tool manufactured by Bioinicia S.L. (Valencia, Spain). The equipment was operated with a single needle injector, horizontally placed toward a fixed metallic collector at room conditions, that is, 25 °C and 40% relative humidity (RH). All of the solutions were processed in the following conditions: 12 kV of voltage, 0.25 mL/h of flow-rate, and 15 cm of tip-to-collector distance. Furthermore, in order to study the influence of the processing conditions, the solution containing 33 wt% zein (solution D) was also processed at 5 kV and 25 kV, 0.1 mL/h and 0.5 mL/h, and 9 cm and 22 cm, fixing the other conditions. Figure 5 shows an illustration of the EHDP device, showing indications of all the different components.
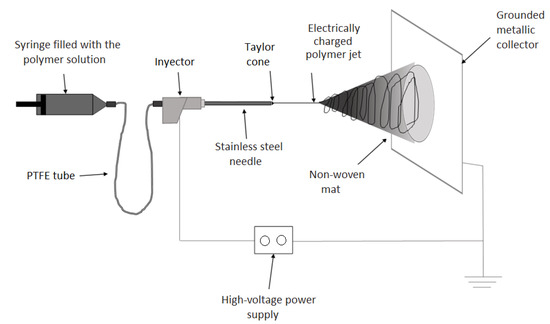
Figure 5.
Electrohydrodynamic processing (EHDP) device with indications of different components.
3.5. Morphology of Zein Materials Processed by EHDP
The morphologies of the top views of the resultant zein materials processed by EHDP were observed using field emission scanning electron microscopy (FESEM) with a JEOL model JSM-5410 (Tokyo, Japan) equipped with a focused ion gun (AURIGA Compact, Zeiss, Oxford Instruments, Abingdon, UK). An accelerating voltage of 10 kV was used during observation. The samples were deposited on aluminum foil and fixed to beveled holders using conductive double-sided adhesive tape and sputtered with a gold–palladium mixture under vacuum prior to observation. The fiber diameter sizes were determined with ImageJ software v 1.41 using a minimum of 50 FESEM micrographs for each sample.
3.6. Student Survey
A student survey was conducted to validate the educational improvement offered by this experimental activity. Online survey data were collected at the end of the academic years 2020–2021 and 2021–2022, with a total of over 100 postgraduate students from food engineering courses experiencing this activity. Table 3 provides the content of the survey, which encompasses ten salient themes about students’ perceptions of the laboratory activities performed. Assessment was performed using a Likert scale of 1–6, where strongly disagree = 1, disagree = 2, somewhat disagree = 3, somewhat agree = 4, agree = 5, and strongly agree = 6. Participation was anonymous and voluntary.

Table 3.
Content of the student survey.
4. Results and Discussion
4.1. Properties of Zein Solutions
The first session dealt with the preparation of the zein solutions by the students, who also performed their characterization to thereafter ascertain, in the second session, the effect of solution properties during EHDP. Zein was easily dissolved in aqueous ethanol, which is an environmentally friendly solvent. Moreover, since this process is carried out at room temperature and takes very few minutes, it can be easily applied to laboratory practices. Figure 6 shows the resultant zein solutions with different protein contents, which were prepared by students in the laboratory. As it can be observed in the image, all solutions showed a yellow-to-brown color, whose tonality increased with the zein content. For most students in the food engineering field, this probably represents the first introduction to polymer solutions, which are characterized by high viscosities and the fact that they are not easy to handle.

Figure 6.
Prepared solutions with different contents of zein, from left to right: 5 wt% (solution A, (a)); 12 wt% (solution B, (b)); 25 wt% (solution C, (c)); 33 wt% (solution D, (d)); 42 wt% (solution E, (e)); 50 wt% (solution F, (f)).
Then, in Figure 7, Figure 8, Figure 9 and Figure 10, the effect of the zein content on the solution properties is shown. These measurements were effectively performed by students, using common laboratory equipment under the right supervision. The understanding of these properties is very relevant for their subsequent processing by EHDP. Moreover, their simple handling and observation helped students to identify the most suitable samples. Figure 7 shows the evolution of the solution density with different zein contents, where it is observed that density increased progressively with the protein content. This increase is due to the high MW of zein, which is approximately 20 kDa for α-zein, the most common form of this protein mixture [15]. The increase in density with the increase in concentration was significantly pronounced from a protein content of 12 wt% to 25 wt%, being then stabilized at 42 wt%. Although this property has nearly no influence on EHDP, it is directly related to other properties of the polymer solution, such as surface tension and viscosity, which are known to highly affect processability [16].
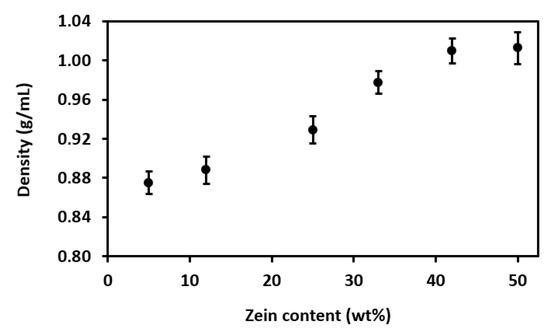
Figure 7.
Effect of zein content on solution density.
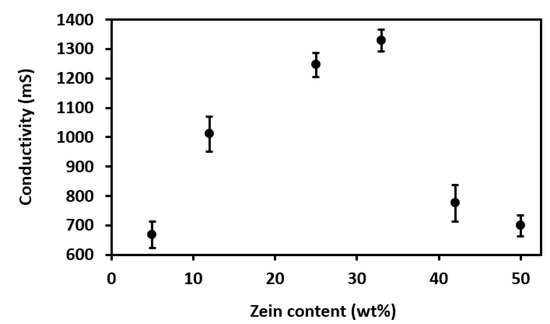
Figure 8.
Effect of zein content on solution conductivity.
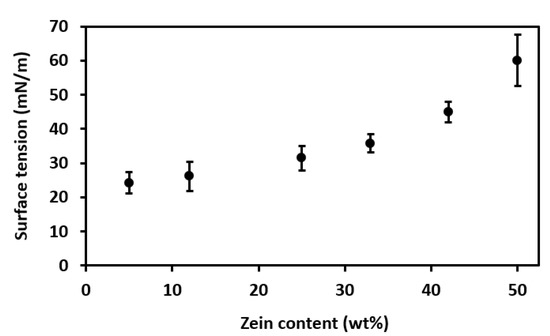
Figure 9.
Effect of zein content on solution surface tension.
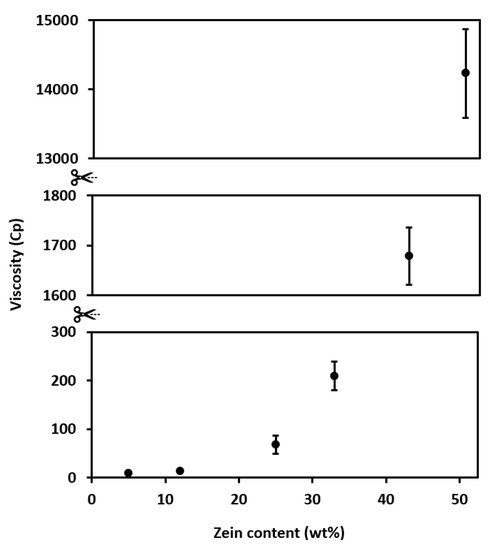
Figure 10.
Effect of zein content on solution viscosity.
Figure 8 shows the evolution of solution conductivity with different zein contents. A progressive increase in conductivity was seen with zein contents up to 33 wt%. Then, for higher protein contents, that is, 42 wt% and 50 wt%, the conductivity values sharply decreased. This suggests that, up to a certain protein concentration, conductivity increases due to the enhancement of the available surface charges of amino groups. However, zein is rich in nonpolar amino acids and deficient in charged amino acids and the solutions with the highest contents tended to present lower conductivity values. The latter effect can be ascribed to the fact that, at higher concentrations, the availability of surface charges is reduced as a result of molecular agglomeration, as well as the use of lower amounts of solvent [12]. In any case, as shown herein, conductivity is another important parameter that can be easily studied in food engineering courses to foresee the outcome of EHDP. In this regard, extreme conductivities, either low or high, can hinder solution processability in EHDP or cause severe morphological changes in the resultant structures. For instance, extremely high values of solution viscosity and electrical conductivity, obtained by increasing the zein concentration, have resulted in the production of fibers with larger diameters, in the micrometer range, impairing processability by EHDP [17]. Therefore, this experience was easily conducted by the students and the influence of the protein content on solution conductivity can be related to the availability of surface charges in the macromolecule.
In Figure 9, one can observe that solution surface tension increased with increasing zein contents for all the prepared solutions. This increase was particularly noticeable at the highest protein contents, that is, 42 wt% and, more importantly, 50 wt%. Surface tension values can be related to both the solvent type and its content, as well as the viscosity of the resultant solutions, being that the latter is mainly influenced by the MW of the protein. At this point, students should pay particular attention to achieving polymer solutions with sufficient but not excessive surface tension. This requirement is due to the fact that having a low or low-to-medium surface tension is generally desirable for the formation of Taylor’s cone during EHDP because the applied voltage must be able to overcome it [18]. However, excessively low values of surface tension can be detrimental for EHDP because a minimal or threshold value is also habitually needed to stabilize the process.
Finally, Figure 10 shows the variation of solution viscosity with different zein contents. As can be observed, in all cases, solution viscosity increased exponentially with increasing zein content. It is well-known that both polymer concentration and polymer’s MW greatly influence the solution viscosity, and the latter property is paramount for optimizing EHDP. In this regard, fiber-based morphologies can only be stabilized using solutions in medium and medium-to-high viscosity ranges. On the contrary, below a critical process viscosity, EHDP results in beads or beaded fibers, whereas the process is impaired when the solution is too viscous due to clogging issues [18]. For instance, values of viscosity > 100 mPa·s are needed to obtain bead-free and uniform fibers of zein [19]. As a result, students should pay particular attention to preparing polymer solutions with the right viscosity by choosing the adequate polymer, when possible, and the right concentration to avoid encountering processability issues and also to attain the desired morphology during EHDP.
4.2. Characterization of Zein Materials Processed by EHDP
The second session was devoted to processing the previously prepared zein solutions using EHDP. This task was performed by the supervisor who had previous experience in EHDP, but all phenomena was observed and explained to the students. In addition, during this experimental activity, the previously measured properties of each solution were elucidated and related to the different processability aspects during EHDP. At the beginning of the process, for all the zein solutions, the change in the polymer solution from a spherical drop to Taylor’s cone as a result of the applied voltage was observed. This is illustrated in Figure 11 and it was naked-eye observed by students. Thereafter, students could also see the formation of beads or fibers after a few minutes on the collector. The electrosprayed or electrospun zein materials were easily seen as a white coating or mat deposited onto the aluminum foil. At this point, it was important to remark that the zein material changed its color from yellow, in the solution, to white, in mat form. This color change is due to the ultrathin size of the fibers or particles, which increases scattering of visible light.
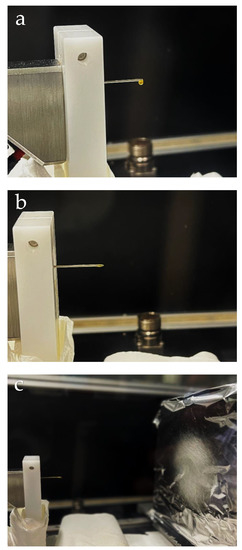
Figure 11.
Illustration of the electrohydrodynamic processing (EHDP) of zein showing: (a) detail of solution drop on the needle tip; (b) formation of Taylor’s cone after application of electrical field; (c) electrospun mat of fibers being deposited onto the collector.
All the zein solutions were processed by EHDP, fixing the following conditions: 12 kV, 0.25 mL/h, and 15 cm. These conditions were kept constant as they have been previously optimized for EHDP of zein [10] and, thus, were expected to be useful for the students to understand the effect of the solution properties on the process. In this regard, both the solutions with the lowest and highest zein contents were unable to be processed by EHDP. In the case of the 5 wt% zein (solution A), the process resulted in the formation of microdroplets due to the low surface tension and insufficient viscosity. Therefore, students understood that the minimum content of zein in the solution was not reached. Conversely, for a zein content of 50 wt% (solution F), the solution presented excessive viscosity, blocking both the PTFE tube and needle tip. So, students realized that only solutions within the adequate viscosity range can be adequately processed by EHDP. The other solutions, namely B, C, D, and E, were processed by EHDP and the zein materials were successfully collected on aluminum foil and, thereafter, observed by FESEM. Figure 12 shows the FESEM micrographs, taken right after deposition, showing the morphology of the attained zein materials, where students could correlate the different morphologies attained with the solution properties measured during the first session. Figure 12a, corresponding to the zein material obtained from solution B, revealed the formation of beads or capsules in the 100–300 nm range. This morphology results from the use of a zein solution with low conductivity, low surface tension and, more importantly, low viscosity. This version of EHDP is habitually termed “electrospraying” since it yields bead-like particles by means of high electrical fields. It is important to remark that these nanobeads can be useful for encapsulation or food fortification purposes, which are of great interest in the field of food engineering [20]. Furthermore, the use of zein solutions with higher values of viscosity resulted in fibers instead of beads and this version of EHDP is called “electrospinning”. Therefore, zein contents of 25 wt% (solution C) and 33 wt% (solution D) produced thin fibers with diameters in the submicron range. These zein electrospun mats can be seen in Figure 12b,c, respectively, showing mean diameters of 180 nm and 330 nm. In this regard, the use of ultrathin fiber mats can be very promising as barrier coatings [21] or interlayers [22] in food packaging. In contrast, the zein solution E, which was still processable by EHDP but showed very high viscosity and low conductivity, yielded thick fibers of above 1 μm (see Figure 12d). This morphology can be detrimental for the previously described applications in food engineering because electrospun mats composed of large fibers tend to show higher porosity and thus lower physical performance.
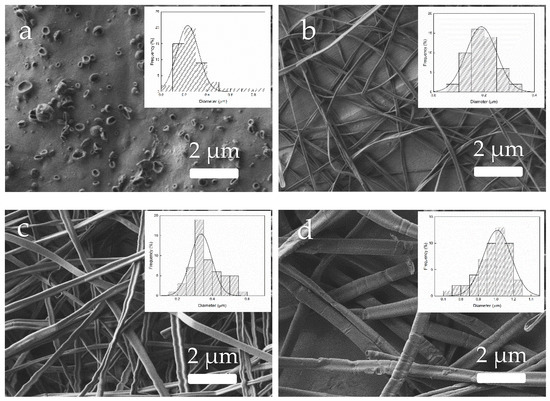
Figure 12.
Morphology of the zein materials processed by electrohydrodynamic processing (EHDP) obtained from solutions: (a) 12 wt% (solution B); (b) 25 wt% (solution C); (c) 33 wt% (solution D); (d) 42 wt% (solution E). The processing conditions were fixed at 12 kV, 0.25 mL/h, and 15 cm. Images taken with a magnification of 5000× and scale markers of 2 μm. Insets show particle or fiber diameter distributions.
After studying the effect on EHDP of zein concentration in the solutions, which was related to the solution properties, the last part of this session was devoted to modifying and analyzing the process parameters. It was explained to students that three main parameters should be controlled during EHDP, namely applied voltage, flow-rate, and tip-to-collector distance. During this task, solution D was selected since it provided the optimal processability. Thus, for this zein solution, the process parameters were modified to ascertain their effect on EHDP. In particular, this was carried out by selecting two new processing conditions and then observing the resultant zein materials attained by EHDP. Regarding applied voltage, Figure 13 shows the morphology of electrospun zein fibers obtained at 5 kV (Figure 13a) and 25 kV (Figure 13b). Students observed that the use of higher electrical fields tends to promote the formation of fibers but the mean fiber diameter also increased from 340 nm to 680 nm, also showing a broader size distribution [23]. Then, although high voltages would be recommended, the use of higher values than the optimal one would not be beneficial. This is due to the increase in the diameter size distribution of the fibers that results from the process instability faced at excessively high voltages. Therefore, students became aware of the fact that the right range of applied voltage should be explored, where the polymer solution is stable during EHDP.
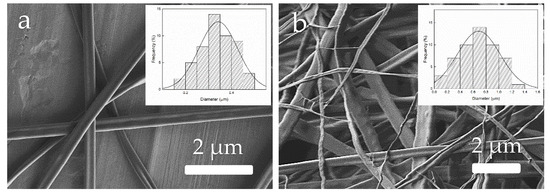
Figure 13.
Morphology of the zein materials obtained from solution D processed by electrohydrodynamic processing (EHDP) as a function of the voltage: (a) 5 kV; (b) 25 kV. Images taken with a magnification of 5000× and scale markers of 2 μm. Insets show fiber diameter distributions.
In Figure 14, the effect of modifying the flow-rate is illustrated. During the experimental activity, as expected, students observed that the production of fibers was substantially improved with the flow-rate. However, as observed in the FESEM micrographs, low flow-rates of 0.1 mL/h were more beneficial to attain ultrathin fibers, with a mean diameter of approximately 300 nm (Figure 14a). Conversely, high flow-rates of 0.5 mL/h resulted in thick fibers, with fiber diameters in the 1–2 μm range (Figure 14b). As mentioned above, electrospun fibers in the submicron range are habitually preferable from the point of view of application in food engineering. Therefore, students were advised to find the optimal flow-rate during EHDP to attain ultrathin fibers at the highest production. It is important to remark that this property will be of utmost importance during the scale-up of the process and, in this regard, it should be considered that other spinneret configurations are available to increase material throughput by using multi-needle, needle-less, or multi-pin configurations [24].
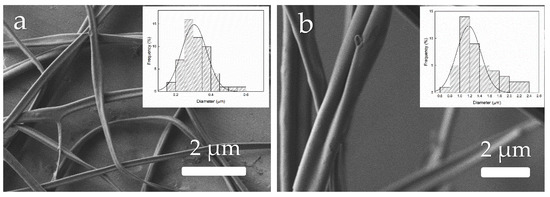
Figure 14.
Morphology of the zein materials obtained from solution D processed by electrohydrodynamic processing (EHDP) as a function of the flow-rate: (a) 0.1 mL/h; (b) 0.5 mL/h. Images taken with a magnification of 5000× and scale markers of 2 μm. Insets show fiber diameter distributions.
Finally, Figure 15 reveals the effect of the distance between tip and collector on the morphology of the zein materials processed by EHDP. Students could easily move the collector and measure the distance to the needle tip. Thereafter, it was observed that fiber sizes reduced after increasing the tip-to-collector distance. In particular, a distance of 9 cm (Figure 15a) yielded thick fibers, with a mean diameter above 1 μm, while ultrathin fibers of nearly 230 nm were attained at a distance of 22 cm (Figure 15b). Students could then observe that, among the three tested process parameters during the experimental activity of the second session, the tip-to-collector distance has the strongest influence on fiber size [25]. Thus, students learned that this process parameter can be effectively applied to successfully produce ultrathin fibers. In this regard, however, it also worth noting that longer distances can impair the process because the fibers encounter difficulties in reaching the collector.
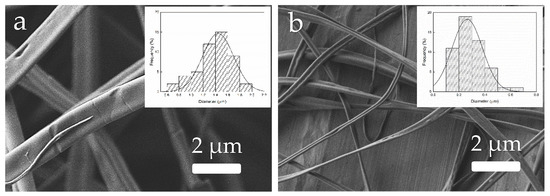
Figure 15.
Morphology of the zein materials obtained from solution D processed by electrohydrodynamic processing (EHDP) as a function of the tip-to-collector distance: (a) 9 cm; (b) 22 cm. Images taken with a magnification of 5000× and scale markers of 2 μm. Insets show fiber diameter distributions.
4.3. Student Feedback
Finally, the experimental activities carried out during the course were evaluated by the students to ascertain their effect on the scientific education and literacy in the EHDP technology. The results for the surveys completed after the laboratory practice are presented in Table 4. The high rating given for Statements #5, #6, #9, and #10 indicate that the students saw a significant value in being exposed to the activities. These benefits point out the fact that students received the sufficient knowledge to familiarize themselves with food hydrocolloid solutions and the EHDP technology. Furthermore, according to Statements #2 and #3, dealing with their knowledge and confidence in the content area, students reported foreseeing a very positive reinforcement in their scientific education. In addition, according to Statement #4, the preparation and processing of zein solutions by EHDP seemed to serve as an appropriate methodology to introduce this novel technology at the university level. However, students still did not fully connect the activities to their academic area (Statement #1), food engineering, which indicates that this technology is still properly not yet integrated in their field of knowledge. Finally, some of them still perceived that more than 4 h would be needed to perform these activities (Statement #8), suggesting that the laboratory practice can be extended.

Table 4.
Average survey scores.
5. Conclusions
In this educational article, the fundamentals for obtaining zein materials using EHDP have been presented. The steps to be followed to obtain the solutions with different zein contents, the characterization of such contents, and the way they can be processed by EHDP have been described in a simple and effective manner to be successfully applied in food engineering courses. Once the different zein solutions were processed, the morphologies obtained were observed using FESEM and related to the solution properties analyzed and the different process conditions explored. This whole procedure was presented in a sequential way, to be performed in two sessions, and it was described in sufficient detail to be applied in courses dealing with food emulsion. Therefore, this document can serve to introduce the EHDP technique to students in food engineering and related areas and contribute to the training of new professionals in this area. In particular, the educational learning outcomes expected include: (1) The students should be able to prepare, characterize, and then select the most optimal solutions in terms of their conductivity, surface tension, and viscosity to be thereafter processed by EHDP; (2) The students should be ready to articulate the operational principle and optimize the main parameters of EHDP, including the applied voltage, flow-rate, and tip-to-collect distance; (3) The students should have gained familiarity with food hydrocolloid solutions and the basic equipment of EHDP, as well as the potential applications of the resultant materials in the field of food engineering. To conclude, the students validated the proposed methodology as a potential scientific literacy in food hydrocolloids and EHDP and, therefore, one can foresee a great application in food engineering courses.
Author Contributions
Conceptualization, S.T.-G.; methodology, A.Y. and S.T.-G.; writing—original draft preparation, S.T.-G.; writing—review and editing, C.G.-M. and S.T.-G.; supervision, C.G.-M.; visualization S.T.-G. All authors have read and agreed to the published version of the manuscript.
Funding
S.T.-G. acknowledges the Spanish Ministry of Science and Innovation (MICI) for the funding received during his Ramón y Cajal contract (RYC2019-027784-I).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that the questionnaire questions were not personal and did not involve sensitive information.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and also available on request.
Acknowledgments
The authors are grateful to the microscopy services at UPV for their help in collecting the images and the students for participating in the research survey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Dhahebi, A.M.; Ling, J.; Krishnan, S.G.; Yousefzadeh, M.; Elumalai, N.K.; Saheed, M.S.M.; Ramakrishna, S.; Jose, R. Electrospinning research and products: The road and the way forward. Appl. Phys. Rev. 2022, 9, 011319. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Coppola, S.; Nasti, G.; Vespini, V.; Mecozzi, L.; Castaldo, R.; Gentile, G.; Ventre, M.; Netti, P.A.; Ferraro, P. Quick liquid packaging: Encasing water silhouettes by three-dimensional polymer membranes. Sci. Adv. 2019, 5, 5189. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Yousif, M.; Mehdi, M.; Ali, H.F.; Hussain, T.; Hashemikia, S.; Van Langenhove, L.; Kim, I.S. Electroless Deposition: A Superficial Route to Synthesis of Highly Conductive Electrospun Nylon 6 Nanofibers. Fibers Polym. 2022, 23, 680–689. [Google Scholar] [CrossRef]
- Hussain, N.; Yousif, M.; Ali, A.; Mehdi, M.; Ullah, S.; Ullah, A.; Mahar, F.K.; Kim, I.S. A facile approach to synthesize highly conductive electrospun aramid nanofibers via electroless deposition. Mater. Chem. Phys. 2020, 255, 123614. [Google Scholar] [CrossRef]
- Hussain, N.; Mehdi, M.; Yousif, M.; Ali, A.; Ullah, S.; Hussain Siyal, S.; Hussain, T.; Kim, I.S. Synthesis of Highly Conductive Electrospun Recycled Polyethylene Terephthalate Nanofibers Using the Electroless Deposition Method. Nanomaterials 2021, 11, 531. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Gennari, O.; Battista, L.; Silva, B.; Grilli, S.; Miccio, L.; Vespini, V.; Coppola, S.; Orlando, P.; Aprin, L.; Slangen, P.; et al. Investigation on cone jetting regimes of liquid droplets subjected to pyroelectric fields induced by laser blasts. Appl. Phys. Lett. 2015, 106, 054103. [Google Scholar] [CrossRef]
- Lawton, J.W. Zein: A History of Processing and Use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimenez, E.; Lagaron, J.M. Characterization of the morphology and thermal properties of Zein Prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 2008, 22, 601–614. [Google Scholar] [CrossRef]
- Khatri, M.; Khatri, Z.; El-Ghazali, S.; Hussain, N.; Qureshi, U.A.; Kobayashi, S.; Ahmed, F.; Kim, I.S. Zein nanofibers via deep eutectic solvent electrospinning: Tunable morphology with super hydrophilic properties. Sci. Rep. 2020, 10, 15307. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Isobe, S.; Maekawa, T. Influence of preparation conditions on the physical properties of zein films. J. Am. Oil Chem. Soc. 2002, 79, 345–349. [Google Scholar] [CrossRef]
- Samuel, J.; Spackman, C.; Ruff, L.; Crucetti, J.J.; Chiappone, S.; Schadler, L. A Research University and Community College Collaboration Model to Promote Micro-manufacturing Education: Preliminary Findings. Procedia Manuf. 2016, 5, 1168–1182. [Google Scholar] [CrossRef][Green Version]
- Nuryantini, A.Y.; Rajak, A.; Ekaputra, M.P.; Rahma, A.; Munir, M.M.; Khairurrijal. Introducing Electrospinning Technique for Producing Nanofibers to Improve Scientific Literacy of Senior High School Students. In Proceedings of the International Conference on Advances in Education Technology (ICAET-14), Bandung, Indonesia, 16 October 2014; pp. 129–132.
- Xinrui, Z. Zein as a structural protein in gluten-free systems: An overview. Food Sci. Hum. Wellness 2021, 10, 270–277. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of active antimicrobial fiber based chitosan polysaccharide nanostructures using electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Incorporation of zein nanofibers produced by needle-less electrospinning within the casted gelatin film for improvement of its physical properties. Food Bioprod. Process. 2020, 122, 193–204. [Google Scholar] [CrossRef]
- Silva, P.M.; Torres-Giner, S.; Vicente, A.A.; Cerqueira, M.A. Electrohydrodynamic processing for the production of zein-based microstructures and nanostructures. Curr. Opin. Colloid Interface Sci. 2021, 56, 101504. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Easteal, A.J.; Nikolaidis, M.G.; Quek, S.Y. Influence of solution and processing parameters towards the fabrication of electrospun zein fibers with sub-micron diameter. J. Food Eng. 2012, 109, 645–651. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of Aloe vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer structures based on annealed electrospun biopolymer coatings of interest in water and aroma barrier fiber-based food packaging applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Lagaron, J.M. Zein-based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. II. Mechanical properties, gas barrier, and sustained release capacity of biocide thymol in multilayer polylactide films. J. Appl. Polym. Sci. 2014, 131, 9270–9276. [Google Scholar] [CrossRef]
- Correia, D.M.; Gonçalves, R.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S. Electrosprayed poly(vinylidene fluoride) microparticles for tissue engineering applications. RSC Adv. 2014, 4, 33013–33021. [Google Scholar] [CrossRef]
- Silva, P.M.; Torres-Giner, S.; Vicente, A.A.; Cerqueira, M.A. Management of Operational Parameters and Novel Spinneret Configurations for the Electrohydrodynamic Processing of Functional Polymers. Macromol. Mater. Eng. 2022, 307, 2100858. [Google Scholar] [CrossRef]
- Jain, E.; Scott, K.M.; Zustiak, S.P.; Sell, S.A. Fabrication of Polyethylene Glycol-Based Hydrogel Microspheres Through Electrospraying. Macromol. Mater. Eng. 2015, 300, 823–835. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).