Simulating Electronic Absorption Spectra of Atmospherically Relevant Molecules: A Systematic Assignment for Enhancing Undergraduate STEM Education
Abstract
:1. Introduction
2. Pedagogical Goals
2.1. Course Approach
2.2. Pedagogical Aims and Learning Outcomes
- Define and calculate the wavelength, frequency and energy of electromagnetic radiation
- Describe the concepts of excitation and relaxation of an electron
- Define the basic concepts of quantum numbers, shells, subshells, orbitals, electronic charge distribution, and their relative energies
- Write electronic configuration in simple atoms and molecules
- Correlate electronic configuration of a molecule to its shape
- Expose students to practical photochemistry and electronic absorption spectroscopy at an early stage.
- Make students aware of how important chemical concepts translate to the chemistry of the atmosphere.
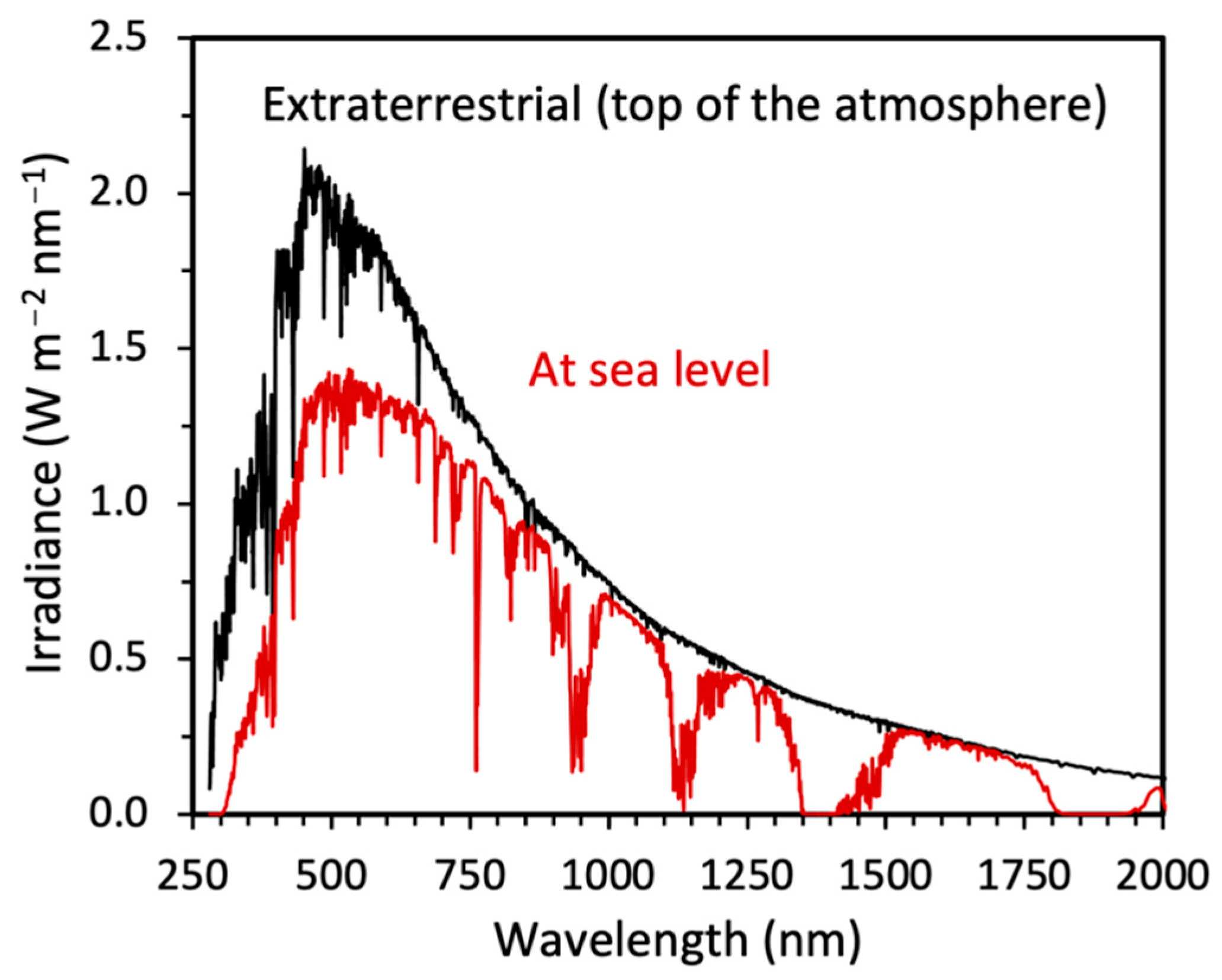
- Be able to relate the lifetimes of long-lived greenhouse (and other) gases in Earth’s troposphere and their overlap with the tropospherically relevant solar irradiance.
- Be able to discover the reasons for the scarcity of NO2/NO3 during the day and their higher abundances at night—relating it to the photochemistry.
- Visualize and analyze molecular orbitals
- Assign electronic transitions
- Relate the character of the electronic excitation to the magnitude of the associated oscillator strength (and/or intensity)
- Compare and contrast spectra simulated with the nuclear ensemble method to those predicted by exclusively computing the vertical excitation energies.
- Newton-X [46]
- Our spectral simulation script which will be made available to interested users by contacting the authors.
3. Experimental Overview
- Computation of optimized structures and associated vibrational frequencies. This step is performed using the GaussView6 and Gaussian16 computational packages.
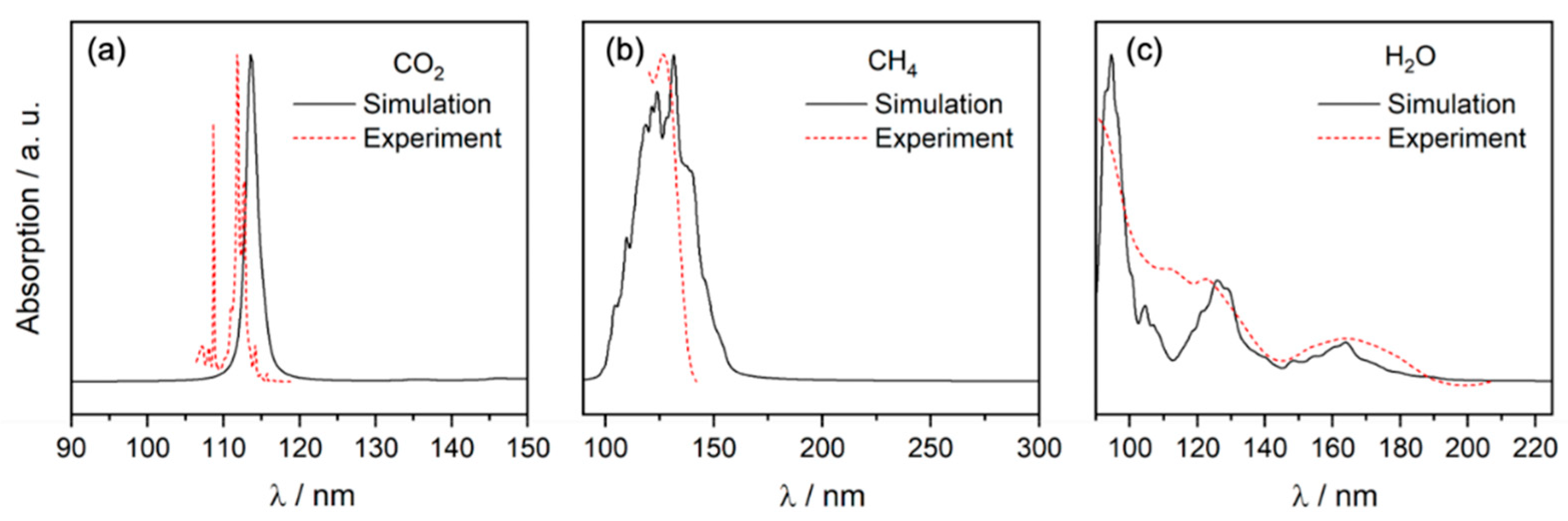
- Generation of initial geometries (Wigner points) and vertical excitation energies to generate the full electronic absorption spectral profile of the selected molecule. This step is performed using our home-written script and is very important because it provides an absorption profile rather than the commonly used (and sometime mis-interpreted) stick spectra.
- Analysis and interpretation of the simulated spectra, with comparison to the known experimental spectra.
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharifi, A.; Simangan, D.; Kaneko, S. Three decades of research on climate change and peace: A bibliometrics analysis. Sustain. Sci. 2021, 16, 1079–1095. [Google Scholar] [CrossRef]
- Hall, C.M.; Saarinen, J. 20 years of Nordic climate change crisis and tourism research: A review and future research agenda. Scand. J. Hosp. Tour. 2021, 21, 102–110. [Google Scholar] [CrossRef]
- Ortiz, A.M.D.; Outhwaite, C.L.; Dalin, C.; Newbold, T. A review of the interactions between biodiversity, agriculture, climate change, and international trade: Research and policy priorities. One Earth 2021, 4, 88–101. [Google Scholar] [CrossRef]
- Mach, K.J.; Kraan, C.M. Science–policy dimensions of research on climate change and conflict. J. Peace Res. 2020, 58, 168–176. [Google Scholar] [CrossRef]
- D’Eon, J.C.; Stirchak, L.T.; Brown, A.-S.; Saifuddin, Y. Project-Based Learning Experience That Uses Portable Air Sensors to Characterize Indoor and Outdoor Air Quality. J. Chem. Educ. 2021, 98, 445–453. [Google Scholar] [CrossRef]
- D’Eon, J.C.; Faust, J.A.; Browning, C.S.; Quinlan, K.B. Exploring the Phases of Carbon Dioxide and the Greenhouse Effect in an Introductory Chemistry Laboratory. J. Chem. Educ. 2019, 96, 329–334. [Google Scholar] [CrossRef]
- Wagner, E.P.; Murray, B.E. An Undergraduate Experiment to Investigate Air Pollutants Created from Lightning. J. Chem. Educ. 2021, 98, 1361–1370. [Google Scholar] [CrossRef]
- Roberts, J.E.; Zeng, G.; Maron, M.K.; Mach, M.; Dwebi, I.; Liu, Y. Measuring Heterogeneous Reaction Rates with ATR-FTIR Spectroscopy to Evaluate Chemical Fates in an Atmospheric Environment: A Physical Chemistry and Environmental Chemistry Laboratory Experiment. J. Chem. Educ. 2016, 93, 733–737. [Google Scholar] [CrossRef]
- Ballard, J.; Mooring, S.R. Cleaning Our World through Green Chemistry: Introducing High School Students to the Principles of Green Chemistry Using a Case-Based Learning Module. J. Chem. Educ. 2021, 98, 1290–1295. [Google Scholar] [CrossRef]
- Bopegedera, A.M.R.P.; Coughenour, C.L. An Interdisciplinary, Project-Based Inquiry into the Chemistry and Geology of Alkaline Surface Lake Waters in the General Chemistry Laboratory. J. Chem. Educ. 2021, 98, 1352–1360. [Google Scholar] [CrossRef]
- Darr, J.P. Combining Experiment and Theory to Probe Salt Aerosol Deliquescence. J. Chem. Educ. 2013, 90, 1392–1395. [Google Scholar] [CrossRef]
- Fillman, K.L.; Palkendo, J.A. Collection, Extraction, and Analysis of Lead in Atmospheric Particles. J. Chem. Educ. 2014, 91, 590–592. [Google Scholar] [CrossRef]
- Crosby, C.M.; Maldonado, R.A.; Hong, A.; Caylor, R.L.; Kuhn, K.L.; Wise, M.E. Investigating NOx Concentrations on an Urban University Campus Using Passive Air Samplers and UV–Vis Spectroscopy. J. Chem. Educ. 2018, 95, 2023–2027. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView Version 6; Semichem Inc.: Shawnee, KS, USA, 2019. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kong, J.; White, C.A.; Krylov, A.I.; Sherrill, D.; Adamson, R.D.; Furlani, T.R.; Lee, M.S.; Lee, A.M.; Gwaltney, S.R.; Pople, J.; et al. Q-Chem 2.0: A high-performance ab initio electronic structure program package. J. Comput. Chem. 2000, 21, 1532–1548. [Google Scholar] [CrossRef]
- Martini, S.R.; Hartzell, C.J. Integrating Computational Chemistry into a Course in Classical Thermodynamics. J. Chem. Educ. 2015, 92, 1201–1203. [Google Scholar] [CrossRef]
- Martin, N.H. Integration of Computational Chemistry into the Chemistry Curriculum. J. Chem. Educ. 1998, 75, 241. [Google Scholar] [CrossRef]
- Marzzacco, C.J.; Baum, J.C. Computational Chemistry Studies on the Carbene Hydroxymethylene. J. Chem. Educ. 2011, 88, 1667–1671. [Google Scholar] [CrossRef]
- Howe, J.J.; Lever, L.S.; Whisnant, D.M. Collaborative Physical Chemistry Projects Involving Computational Chemistry. J. Chem. Educ. 2000, 77, 199. [Google Scholar] [CrossRef]
- Pearson, J.K. Introducing the Practical Aspects of Computational Chemistry to Undergraduate Chemistry Students. J. Chem. Educ. 2007, 84, 1323. [Google Scholar] [CrossRef]
- Lipkowitz, K.B.; Jalaie, M.; Robertson, D.; Barth, A. Interdisciplinary Learning with Computational Chemistry: A Collaboration between Chemistry and Geology. J. Chem. Educ. 1999, 76, 684. [Google Scholar] [CrossRef]
- Esselman, B.J.; Hill, N.J. Integration of Computational Chemistry into the Undergraduate Organic Chemistry Laboratory Curriculum. J. Chem. Educ. 2016, 93, 932–936. [Google Scholar] [CrossRef]
- Snyder, H.D.; Kucukkal, T.G. Computational Chemistry Activities with Avogadro and ORCA. J. Chem. Educ. 2021, 98, 1335–1341. [Google Scholar] [CrossRef]
- Metz, I.K.; Bennett, J.W.; Mason, S.E. Examining the Aufbau Principle and Ionization Energies: A Computational Chemistry Exercise for the Introductory Level. J. Chem. Educ. 2021, 98, 4017–4025. [Google Scholar] [CrossRef]
- Kobayashi, R.; Goumans, T.P.; Carstensen, N.O.; Soini, T.M.; Marzari, N.; Timrov, I.; Poncé, S.; Linscott, E.B.; Sewell, C.J.; Talirz, L.; et al. Virtual Computational Chemistry Teaching Laboratories—Hands-On at a Distance. J. Chem. Educ. 2021, 98, 3163–3171. [Google Scholar] [CrossRef]
- Ji, X.; Liu, X.; Li, M.; Shao, S.; Chang, J.; Du, J.; Ma, X.; Feng, X.; Zhu, L.; Yu, X.; et al. Study of the Redox Potentials of Benzoquinone and Its Derivatives by Combining Electrochemistry and Computational Chemistry. J. Chem. Educ. 2021, 98, 3019–3025. [Google Scholar] [CrossRef]
- Heimann, J.E.; Williams, T.H.; Bennett, J.W.; Rosenzweig, Z. Baltimore SCIART: A Fully Virtual Undergraduate Research Experience at the Interface of Computational Chemistry and Art. J. Chem. Educ. 2021, 98, 3172–3179. [Google Scholar] [CrossRef]
- Chhantyal-Pun, R.; Khan, M.A.H.; Martin, R.; Zachhuber, N.; Buras, Z.J.; Percival, C.J.; Shallcross, D.E.; Orr-Ewing, A.J. Direct Kinetic and Atmospheric Modeling Studies of Criegee Intermediate Reactions with Acetone. ACS Earth Space Chem. 2019, 3, 2363–2371. [Google Scholar] [CrossRef]
- McCoy, J.C.; Marchetti, B.; Thodika, M.; Karsili, T.N.V. A Simple and Efficient Method for Simulating the Electronic Absorption Spectra of Criegee Intermediates: Benchmarking on CH2OO and CH3CHOO. J. Phys. Chem. A 2021, 125, 4089–4097. [Google Scholar] [CrossRef]
- McGillen, M.R.; Curchod, B.F.; Chhantyal-Pun, R.; Beames, J.M.; Watson, N.P.; Khan, M.A.H.; McMahon, L.; Shallcross, D.E.; Orr-Ewing, A.J. Criegee Intermediate–Alcohol Reactions, A Potential Source of Functionalized Hydroperoxides in the Atmosphere. ACS Earth Space Chem. 2017, 1, 664–672. [Google Scholar] [CrossRef]
- Crespo-Otero, R.; Barbatti, M. Spectrum simulation and decomposition with nuclear ensemble: Formal derivation and application to benzene, furan and 2-phenylfuran. Theor. Chem. Acc. 2012, 131, 1237. [Google Scholar] [CrossRef]
- Yu, X.; Hou, H.; Wang, B. Atmospheric Chemistry of Perfluoro-3-methyl-2-butanone [CF3C(O)CF(CF3)2]: Photodissociation and Reaction with OH Radicals. J. Phys. Chem. A 2018, 122, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Francés-Monerris, A.; Carmona-García, J.; Acuña, A.U.; Dávalos, J.Z.; Cuevas, C.A.; Kinnison, D.E.; Francisco, J.S.; Saiz-Lopez, A.; Roca-Sanjuán, D. Photodissociation Mechanisms of Major Mercury(II) Species in the Atmospheric Chemical Cycle of Mercury. Angew. Chemie Int. Ed. 2020, 59, 7605–7610. [Google Scholar] [CrossRef] [PubMed]
- Prlj, A.; Ibele, L.M.; Marsili, E.; Curchod, B.F.E. On the Theoretical Determination of Photolysis Properties for Atmospheric Volatile Organic Compounds. J. Phys. Chem. Lett. 2020, 11, 5418–5425. [Google Scholar] [CrossRef]
- Röder, A.; de Oliveira, N.; Grollau, F.; Mestdagh, J.-M.; Gaveau, M.-A.; Briant, M. Vacuum-Ultraviolet Absorption Spectrum of 3-Methoxyacrylonitrile. J. Phys. Chem. A 2020, 124, 9470–9477. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.P.; Ventura, E.; Monte, S.A.D.; Barbatti, M. UV-photoexcitation and ultrafast dynamics of HCFC-132b (CF2ClCH2Cl). J. Comput. Chem. 2016, 37, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Saiz-Lopez, A.; Sitkiewicz, S.P.; Roca-Sanjuán, D.; Oliva-Enrich, J.M.; Dávalos, J.Z.; Notario, R.; Jiskra, M.; Xu, Y.; Wang, F.; Thackray, C.P.; et al. Photoreduction of gaseous oxidized mercury changes global atmospheric mercury speciation, transport and deposition. Nat. Commun. 2018, 9, 4796. [Google Scholar] [CrossRef] [Green Version]
- Keller-Rudek, H.; Moortgat, G.K.; Sander, R.; Sörensen, R. The MPI-Mainz UV/VIS Spectral Atlas of Gaseous Molecules of Atmospheric Interest. Earth Syst. Sci. Data 2013, 5, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.L.; Peeters, J.; Vereecken, L. Theoretical study of the gas-phase ozonolysis of β-pinene (C10H16). Phys. Chem. Chem. Phys. 2009, 11, 5643–5656. [Google Scholar] [CrossRef]
- Vansco, M.; Zuraski, K.; Winiberg, F.; Au, K.; Trongsiriwat, N.; Walsh, P.; Osborn, D.; Percival, C.; Klippenstein, S.; Taatjes, C.; et al. Functionalized Hydroperoxide Formation from the Reaction of Methacrolein-Oxide, an Isoprene-Derived Criegee Intermediate, with Formic Acid: Experiment and Theory. Molecules 2021, 26, 3058. [Google Scholar] [CrossRef]
- Kuwata, K.T.; Kujala, B.J.; Morrow, Z.W.; Tonc, E. Quantum chemical and RRKM/master equation studies of cyclopropene ozonolysis. Comput. Theor. Chem. 2011, 965, 305–312. [Google Scholar] [CrossRef]
- Vansco, M.F.; Marchetti, B.; Lester, M.I. Electronic spectroscopy of methyl vinyl ketone oxide: A four-carbon unsaturated Criegee intermediate from isoprene ozonolysis. J. Chem. Phys. 2018, 149, 244309. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, P.; Cox, R.; Crowley, J.; Destriau, M.; Hayman, G.; Jenkin, M.; Moortgat, G.; Zabel, F. Organic peroxy radicals: Kinetics, spectroscopy and tropospheric chemistry. Atmospheric Environ. Part A Gen. Top. 1992, 26, 1805–1961. [Google Scholar] [CrossRef]
- Barbatti, M.; Ruckenbauer, M.; Plasser, F.; Pittner, J.; Granucci, G.; Persico, M.; Lischka, H. Newton-X: A surface-hopping program for nonadiabatic molecular dynamics. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 4, 26–33. [Google Scholar] [CrossRef]
- Bode, B.; Gordon, M.S. Macmolplt: A graphical user interface for GAMESS. J. Mol. Graph. Model. 1998, 16, 133–138. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Parrish, R.M.; Burns, L.A.; Smith, D.G.A.; Simmonett, A.C.; DePrince, A.E.; Hohenstein, E.G.; Bozkaya, U.; Sokolov, A.; Di Remigio, R.; Richard, R.; et al. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput. 2017, 13, 3185–3197. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Barbatti, M.; Granucci, G.; Persico, M.; Ruckenbauer, M.; Vazdar, M.; Eckert-Maksić, M.; Lischka, H. The on-the-fly surface-hopping program system Newton-X: Application to ab initio simulation of the nonadiabatic photodynamics of benchmark systems. J. Photochem. Photobiol. A Chem. 2007, 190, 228–240. [Google Scholar] [CrossRef]
- Gueymard, C.A. The sun’s total and spectral irradiance for solar energy applications and solar radiation models. Sol. Energy 2004, 76, 423–453. [Google Scholar] [CrossRef]
- Gueymard, C.; Myers, D.; Emery, K. Proposed reference irradiance spectra for solar energy systems testing. Sol. Energy 2002, 73, 443–467. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Hodnebrog, Ø.; Myhre, G.; Samset, B.H.; Alterskjær, K.; Andrews, T.; Boucher, O.; Faluvegi, G.; Fläschner, D.; Forster, P.M.; Kasoar, M.; et al. Water vapour adjustments and responses differ between climate drivers. Atmos. Chem. Phys. 2019, 19, 12887–12899. [Google Scholar] [CrossRef] [Green Version]
- Kenagy, H.S.; Sparks, T.L.; Ebben, C.J.; Wooldrige, P.J.; Lopez-Hilfiker, F.D.; Lee, B.H.; Thornton, J.A.; McDuffie, E.E.; Fibiger, D.L.; Brown, S.S.; et al. NOx Lifetime and NOy Partitioning During WINTER. J. Geophys. Res. Atmos. 2018, 123, 9813–9827. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelz-Sullivan, E.J.; Marchetti, B.; Karsili, T. Simulating Electronic Absorption Spectra of Atmospherically Relevant Molecules: A Systematic Assignment for Enhancing Undergraduate STEM Education. Educ. Sci. 2022, 12, 252. https://doi.org/10.3390/educsci12040252
Stelz-Sullivan EJ, Marchetti B, Karsili T. Simulating Electronic Absorption Spectra of Atmospherically Relevant Molecules: A Systematic Assignment for Enhancing Undergraduate STEM Education. Education Sciences. 2022; 12(4):252. https://doi.org/10.3390/educsci12040252
Chicago/Turabian StyleStelz-Sullivan, Eleanor J., Barbara Marchetti, and Tolga Karsili. 2022. "Simulating Electronic Absorption Spectra of Atmospherically Relevant Molecules: A Systematic Assignment for Enhancing Undergraduate STEM Education" Education Sciences 12, no. 4: 252. https://doi.org/10.3390/educsci12040252
APA StyleStelz-Sullivan, E. J., Marchetti, B., & Karsili, T. (2022). Simulating Electronic Absorption Spectra of Atmospherically Relevant Molecules: A Systematic Assignment for Enhancing Undergraduate STEM Education. Education Sciences, 12(4), 252. https://doi.org/10.3390/educsci12040252





