Enhancing STEM Education by Integrating Research and Teaching in Photochemistry: An Undergraduate Chemistry Laboratory in Spectroscopy and Photochemistry
Abstract
1. Introduction
2. Pedagogical Goals
2.1. Course Approach
2.2. Pedagogical Aims and Learning Outcomes
- (1)
- Perform separation and analysis by chromatography, UV-Vis spectroscopy and photochemistry: students will be expected to identify the number of extracted active ingredients by performing TLC and their chemical identity by recording UV/Vis spectra of the samples.
- (2)
- Perform photo-irradiation studies which will have the aim of testing the extent to which the extracted ingredient can resist UV exposure thus offer photoprotective action. To explore how a sunscreen component resists or degrades upon prolonged UV exposure, students will be asked to expose their sample to UV light for prolonged periods of time to test the efficacy of sunscreen. Student may be able to relate the sun protection factor (SPF) to the chemical composition of a sunscreen lotion.
3. Experimental Design and Overview
3.1. Choice of Sunscreen Lotions
3.2. Choice of Sunscreen Lotions
3.3. Solvent Extraction
3.4. Chromatographic Analysis
3.5. UV Spectroscopy
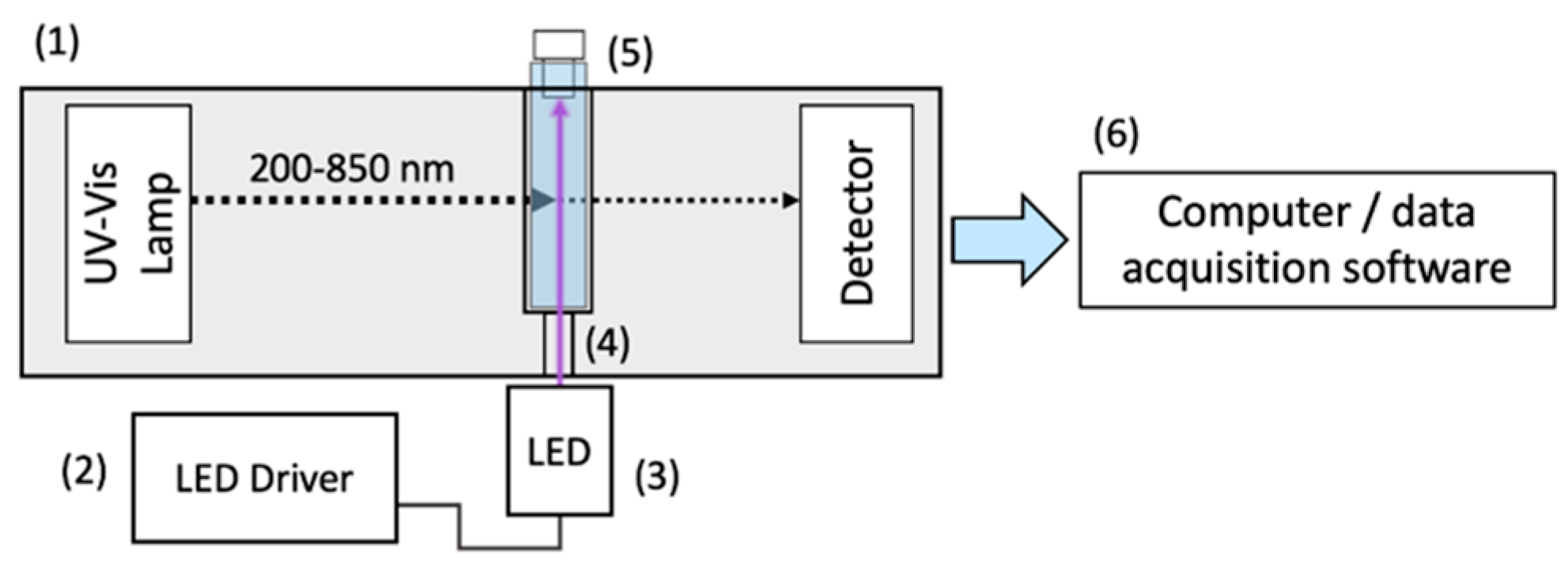
3.6. Photophysics of Sunscreen Lotions
4. Hazards
5. Results and Discussion
5.1. Chromatographic Analysis
5.2. UV/Vis Spectroscopy
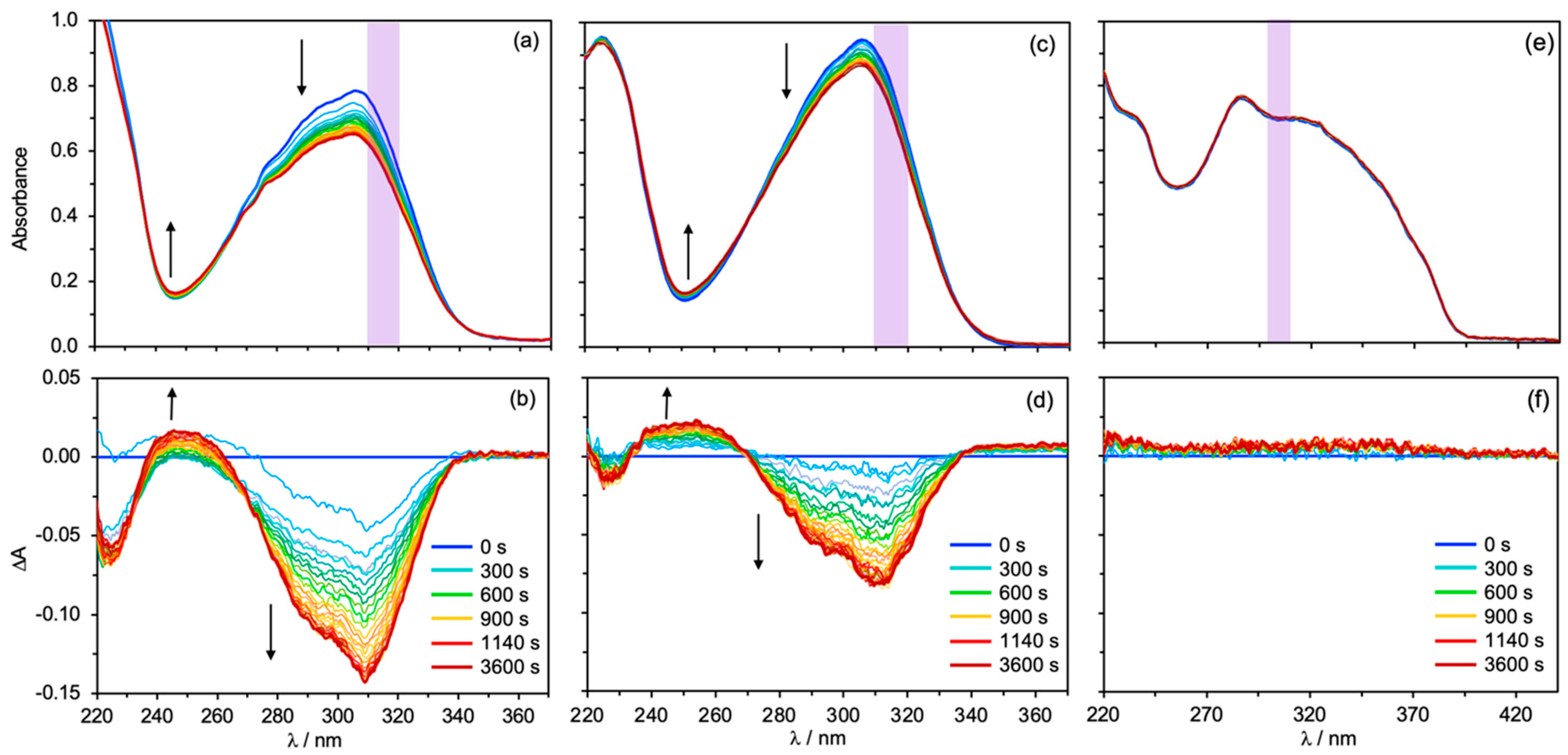
5.3. Steady-State Irradiation and Photostability
- Its absorption spectrum provides UV protection over a broader UV range
- No photochemical reaction is observed
- Its absorbance is not altered by any photophysical process (e.g., photoisomerization)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Credi, A.; Venturi, M. Photochemical conversion of solar energy. ChemSusChem 2008, 1, 26–58. [Google Scholar] [CrossRef] [PubMed]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy conversion in natural and artificial photosynthesis. Chem. Biol. 2010, 17, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Warnan, J.; Reisner, E. Synthetic Organic Design for Solar Fuel Systems. Angew. Chem. Int. Ed. Engl. 2020, 59, 17344–17354. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Xu, Y.-J. (Eds.) Heterogeneous Photocatalysis, from Fundamentals to Green Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Strunk, J. (Ed.) Heterogeneous Photocatalysis: From Fundamentals to Applications in Energy Conversion and Depollution; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Pelizzetti, E.; Serpone, N. (Eds.) Homogenous and Heterogenous Photocatalysis; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Parrino, F.; Palmisano, G. Highlights on recent developments of heterogeneous and homogeneous photocatalysis. Molecules 2021, 26, 23. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef]
- Jablonski, A. Efficiency of Anti-Stokes Fluorescence in Dyes. Nature 1933, 131, 839–840. [Google Scholar] [CrossRef]
- Baker, L.A.; Marchetti, B.; Karsili, T.N.V.; Stavros, V.G.; Ashfold, M.N.R. Photoprotection: Extending lessons learned from studying natural sunscreens to the design of artificial sunscreen constituents. Chem. Soc. Rev. 2017, 46, 3770–3791. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Baker, L.A.; Greenough, S.E.; Stavros, V.G. A Perspective on the Ultrafast Photochemistry of Solution-Phase Sunscreen Molecules. J. Phys. Chem. Lett. 2016, 7, 4655–4665. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.d.N.; Stavros, V.G. From Fundamental Science to Product: A Bottom-up Approach to Sunscreen Development. Sci. Prog. 2018, 101, 8–31. [Google Scholar] [CrossRef]
- Xie, B.-B.; Tang, X.-F.; Liu, X.-Y.; Chang, X.-P.; Cui, G. Mechanistic photophysics and photochemistry of unnatural bases and sunscreen molecules: Insights from electronic structure calculations. Phys. Chem. Chem. Phys. 2021, 23, 27124–27149. [Google Scholar] [CrossRef] [PubMed]
- Domcke, W.; Yarkony, D.R.; Köppel, H. Conical Intersections: Electronic Structure, Dynamics and Spectroscopy. Adv. Ser. Phys. Chem. 2004, 15, 838. [Google Scholar]
- Domcke, W.; Yarkony, D.R.; Köppel, H. Conical Intersect. Theory Comput. Exp. 2011, 17, 1–754. [Google Scholar]
- Yamazaki, K.; Miyazaki, Y.; Harabuchi, Y.; Taketsugu, T.; Maeda, S.; Inokuchi, Y.; Kinoshita, S.; Sumida, M.; Onitsuka, Y.; Kohguchi, H.; et al. Multistep Intersystem Crossing Pathways in Cinnamate-Based UV-B Sunscreens. J. Phys. Chem. Lett. 2016, 7, 4001–4007. [Google Scholar] [CrossRef]
- Chang, X.-P.; Zhang, T.-S.; Fang, Y.-G.; Cui, G. Quantum Mechanics/Molecular Mechanics Studies on the Photophysical Mechanism of Methyl Salicylate. J. Phys. Chem. A 2021, 125, 1880–1891. [Google Scholar] [CrossRef]
- Abiola, T.T.; Whittock, A.L.; Stavros, V.G. Unravelling the Photoprotective Mechanisms of Nature-Inspired Ultraviolet Filters Using Ultrafast Spectroscopy. Molecules 2020, 25, 3945. [Google Scholar] [CrossRef]
- Dalton, J.; Richings, G.W.; Woolley, J.M.; Abiola, T.T.; Habershon, S.; Stavros, V.G. Experimental and Computational Analysis of Para-Hydroxy Methylcinnamate following Photoexcitation. Molecules 2021, 26, 7621. [Google Scholar] [CrossRef]
- Fang, Y.-G.; Li, C.-X.; Chang, X.-P.; Cui, G. Photophysics of a UV-B Filter 4-Methylbenzylidene Camphor: Intersystem Crossing Plays an Important Role. ChemPhysChem 2018, 19, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, H.H.; Miller, A.L. The fates of electronic excitation energy. J. Chem. Educ. 1966, 43, 469. [Google Scholar] [CrossRef]
- Priestley, E.B.; Haug, A. Phosphorescence Spectrum of Pure Crystalline Naphthalene. J. Chem. Phys. 1968, 49, 622–629. [Google Scholar] [CrossRef]
- Abney, J.R.; Scalettar, B.A. Saving Your Students’ Skin. Undergraduate Experiments that Probe UV Protection by Sunscreens and Sunglasses. J. Chem. Educ. 1998, 75, 757. [Google Scholar] [CrossRef]
- Boesdorfer, S.B. Review of Teaching and Learning STEM: A Practical Guide. J. Chem. Educ. 2016, 93, 1682–1683. [Google Scholar] [CrossRef]
- York, S.; Lavi, R.; Dori, Y.J.; Orgill, M. Applications of Systems Thinking in STEM Education. J. Chem. Educ. 2019, 96, 2742–2751. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M.; Román Suero, S. Teaching How to Research: A Case Study on Chemical and Industrial Engineering Degrees. Educ. Sci. 2022, 12, 673. [Google Scholar] [CrossRef]
- Galloway, K.R.; Bretz, S.L.; Novak, M. Paper Chromatography and UV–Vis Spectroscopy to Characterize Anthocyanins and Investigate Antioxidant Properties in the Organic Teaching Laboratory. J. Chem. Educ. 2015, 92, 183–188. [Google Scholar] [CrossRef]
- Hamad, M.L. Measuring Tablet Dissolution: An Experiment for Teaching Quantitative Ultraviolet Absorption Spectroscopy. J. Chem. Educ. 2013, 90, 1662–1664. [Google Scholar] [CrossRef]
- Ren, J.; Lin, T.; Sprague, L.W.; Peng, I.; Wang, L.-Q. Exploring Chemical Equilibrium for Alcohol-Based Cobalt Complexation through Visualization of Color Change and UV–vis Spectroscopy. J. Chem. Educ. 2020, 97, 509–516. [Google Scholar] [CrossRef]
- Macomber, R.S. A Unifying Approach to Absorption Spectroscopy at the Undergraduate Level. J. Chem. Educ. 1997, 74, 65. [Google Scholar] [CrossRef]
- Hansbury, E.; Ott, D.G.; Perrings, J.D. Thin layer chromatography apparatus. J. Chem. Educ. 1963, 40, 31. [Google Scholar] [CrossRef]
- Lincoln, S.; Pohl, R.A.; Haworth, D.T. A thin-layer chromatography experiment for general chemistry. J. Chem. Educ. 1970, 47, 401. [Google Scholar] [CrossRef]
- Lott, P.F.; Hurtubise, R.J. Instrumentation for thin-layer chromatography. J. Chem. Educ. 1971, 48, A437. [Google Scholar] [CrossRef]
- Neman, R.L. The thin-layer chromatography of drugs. A laboratory experiment. J. Chem. Educ. 1972, 49, 834. [Google Scholar] [CrossRef]
- Dahlberg, D.B.; Ferguson, E. Thin-layer chromatography (Zellmer, D.L.; Gump, B.H.). J. Chem. Educ. 1988, 65, A116. [Google Scholar] [CrossRef]
- Silver, J. Let Us Teach Proper Thin Layer Chromatography Technique! J. Chem. Educ. 2020, 97, 4217–4219. [Google Scholar] [CrossRef]
- Ketchum, A.R.; Wolf, A.K.; Meyerhoff, M.E. Synthesis, Spectroscopy, and Stability of the Nitric Oxide Donor S-nitroso-N-acetylpenicillamine: An Undergraduate Laboratory Experiment. Sci. Acad. Publ. 2017, 5, 79–85. [Google Scholar]
- Barrett, S.M.; Szalay, P.S.; Zook-Gerdau, L.A.; Schurter, E.J. Exploring Emission and Absorption Spectroscopy in the First-Year General Chemistry Laboratory. J. Chem. Educ. 2020, 97, 4097–4102. [Google Scholar] [CrossRef]
- Kimbrough, D.R. The Photochemistry of Sunscreens. J. Chem. Educ. 1997, 74, 51. [Google Scholar] [CrossRef]
- Moeur, H.P.; Zanella, A.; Poon, T. An Introduction to UV-Vis Spectroscopy Using Sunscreens. J. Chem. Educ. 2006, 83, 769. [Google Scholar] [CrossRef]
- Tran, J.B.; McCoy, J.C.; Bailey, L.M.; McDaniel, B.P.; Simon, R.L.; Marchetti, B.; Karsili, T.N.V. Affordable Setup for Studying Photochemistry in Action in Undergraduate Teaching Laboratories: Principles and Applications. J. Chem. Educ. 2020, 97, 2203–2211. [Google Scholar] [CrossRef]
- Vygotsky, S.L. Mind in Society: The Development of Higher Psychological Processes; Harvard University Press: Cambridge, MA, USA, 1978. [Google Scholar]
- Abdul-Rasheed, O.F.; Farid, Y.Y. Development of a new high performance liquid chromatography method for measurement of coenzyme Q10 in healthy blood plasma. Saudi Med. J. 2009, 30, 1138–1143. [Google Scholar]
- Moschetti, A.; Vine, L.N.; Lethcoe, K.; Dagda, R.K.; Ellison, P.; Ryan, R.O. Assembly and Characterization of Biocompatible Coenzyme Q(10)-Enriched Lipid Nanoparticles. Lipids 2020, 55, 141–149. [Google Scholar] [CrossRef]
- Speirs, M.P.; Zhao, L. Determination of UV Filters in Sunscreens Using Agilent Captiva EMR—Lipid Cleanup by HPLC. Available online: https://www.biocompare.com/Media/37/Document/application-uv-filter-sunscreen-captiva-emr-Lipid-5994-1611en-agilent.pdf (accessed on 1 January 2020).
- Baker, L.A.; Horbury, M.D.; Greenough, S.E.; Ashfold, M.N.R.; Stavros, V.G. Broadband ultrafast photoprotection by oxybenzone across the UVB and UVC spectral regions. Photochem. Photobiol. Sci. 2015, 14, 1814–1820. [Google Scholar] [CrossRef]
- Kosenkov, D.; Shaw, J.; Zuczek, J.; Kholod, Y. Transient-Absorption Spectroscopy of Cis–Trans Isomerization of N,N-Dimethyl-4,4′-azodianiline with 3D-Printed Temperature-Controlled Sample Holder. J. Chem. Educ. 2016, 93, 1299–1304. [Google Scholar] [CrossRef]
- Contreras-Cruz, D.A.; Cantú-Reyes, M.; García-Sánchez, J.M.; Peña-Ortíz, D.; Sánchez-Carmona, M.A.; Miranda, L.D. Shedding Blue Light on the Undergraduate Laboratory: An Easy-to-Assemble LED Photoreactor for Aromatization of a 1,4-Dihydropyridine. J. Chem. Educ. 2019, 96, 2015–2020. [Google Scholar] [CrossRef]
- Walden, S.L.; Frisch, H.; Unterreiner, B.V.; Unterreiner, A.-N.; Barner-Kowollik, C. Revealing the Wavelength Dependence of Photochemical Reactions: Cutting-Edge Research in the Teaching Lab. J. Chem. Educ. 2020, 97, 543–548. [Google Scholar] [CrossRef]
- Volpe, K.; Podlesny, E.E. Modernization of a Photochemical Reaction for the Undergraduate Laboratory: Continuous Flow Photopinacol Coupling. J. Chem. Educ. 2020, 97, 586–591. [Google Scholar] [CrossRef]
- Pattanaargson, S.; Munhapol, T.; Hirunsupachot, P.; Luangthongaram, P. Photoisomerization of octyl methoxycinnamate. J. Photochem. Photobiol. A Chem. 2004, 161, 269–274. [Google Scholar] [CrossRef]
- Hanson, K.M.; Narayanan, S.; Nichols, V.M.; Bardeen, C.J. Photochemical degradation of the UV filter octyl methoxycinnamate in solution and in aggregates. Photochem. Photobiol. Sci. 2015, 14, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
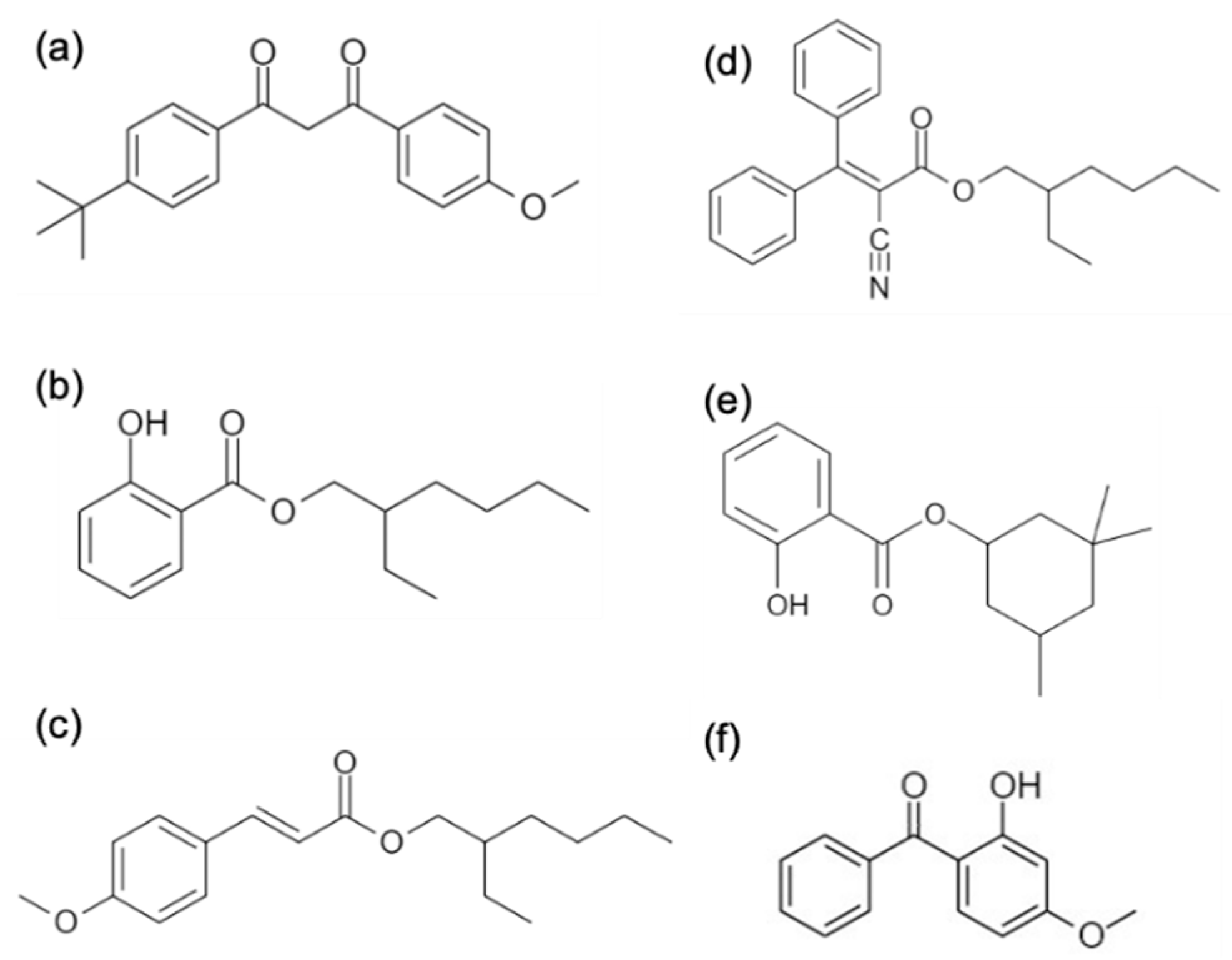
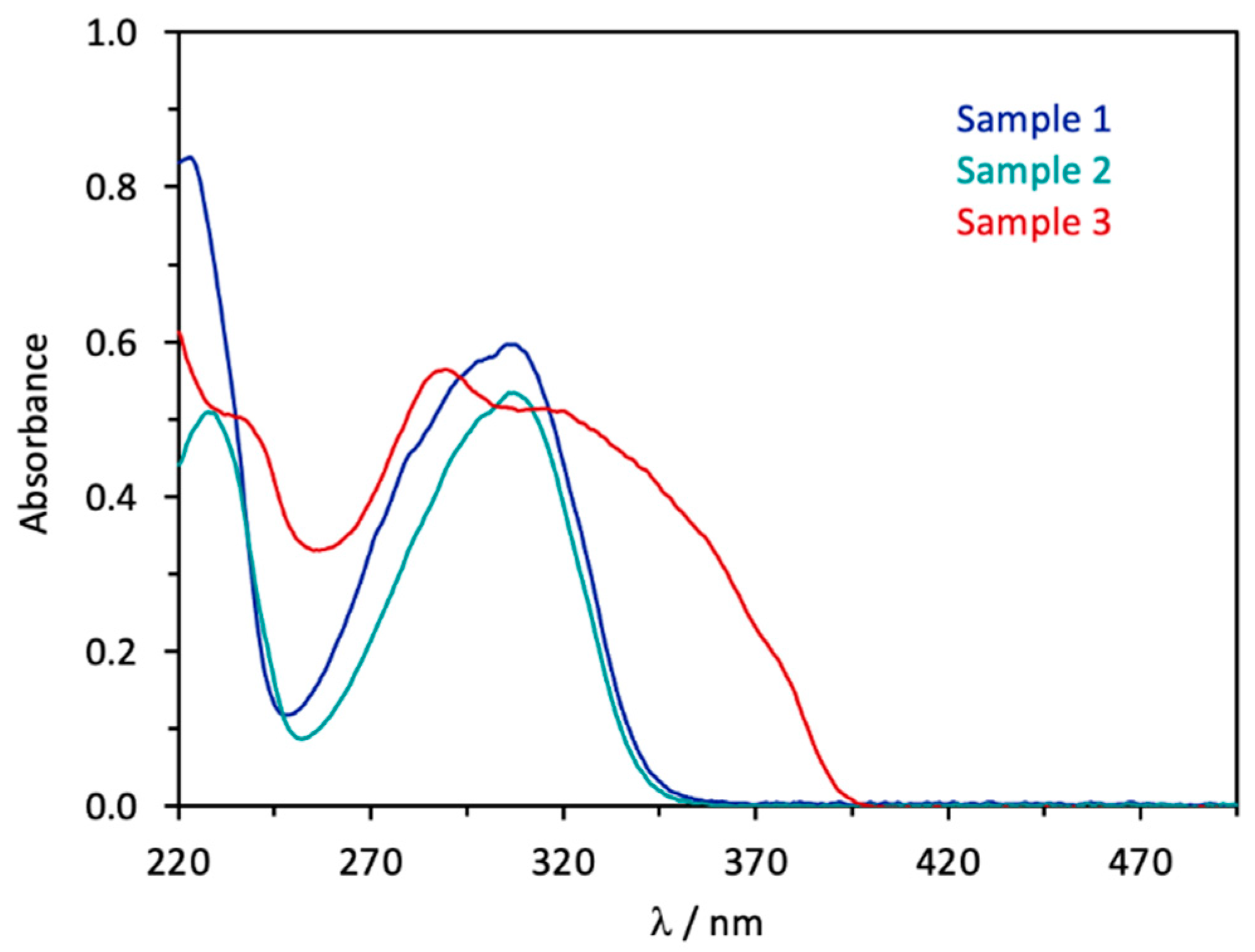

| Samples | Sample 1 * (Panama Jack) | Sample 2 ** (Coppertone Babies) | Sample 3 (Banana Boat) | |
|---|---|---|---|---|
| Information | ||||
| SPF | 4 | 30 | 100 | |
| # of UV filters | 1 | 2 | 3 | |
| Avobenzone | ✓ (2.5%) | |||
| Octisalate (%) | ✓ (5.0%) | |||
| Octinoxate (%) | ✓ (2%) | ✓ (7.5%) | ||
| Octocrylene (%) | ✓ (8.0%) | |||
| Oxybenzone (%) | ✓ (3.5%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelz-Sullivan, E.J.; Racca, J.M.; McCoy, J.C.; Charif, D.L.; Islam, L.; Zhou, X.-D.; Marchetti, B.; Karsili, T.N.V. Enhancing STEM Education by Integrating Research and Teaching in Photochemistry: An Undergraduate Chemistry Laboratory in Spectroscopy and Photochemistry. Educ. Sci. 2022, 12, 729. https://doi.org/10.3390/educsci12100729
Stelz-Sullivan EJ, Racca JM, McCoy JC, Charif DL, Islam L, Zhou X-D, Marchetti B, Karsili TNV. Enhancing STEM Education by Integrating Research and Teaching in Photochemistry: An Undergraduate Chemistry Laboratory in Spectroscopy and Photochemistry. Education Sciences. 2022; 12(10):729. https://doi.org/10.3390/educsci12100729
Chicago/Turabian StyleStelz-Sullivan, Eleanor J., Jared M. Racca, Julia C. McCoy, Dana L. Charif, Lajmi Islam, Xiao-Dong Zhou, Barbara Marchetti, and Tolga N. V. Karsili. 2022. "Enhancing STEM Education by Integrating Research and Teaching in Photochemistry: An Undergraduate Chemistry Laboratory in Spectroscopy and Photochemistry" Education Sciences 12, no. 10: 729. https://doi.org/10.3390/educsci12100729
APA StyleStelz-Sullivan, E. J., Racca, J. M., McCoy, J. C., Charif, D. L., Islam, L., Zhou, X.-D., Marchetti, B., & Karsili, T. N. V. (2022). Enhancing STEM Education by Integrating Research and Teaching in Photochemistry: An Undergraduate Chemistry Laboratory in Spectroscopy and Photochemistry. Education Sciences, 12(10), 729. https://doi.org/10.3390/educsci12100729







