Surface Hardening of Massive Steel Products in the Low-pressure Glow Discharge Plasma
Abstract
:1. Introduction
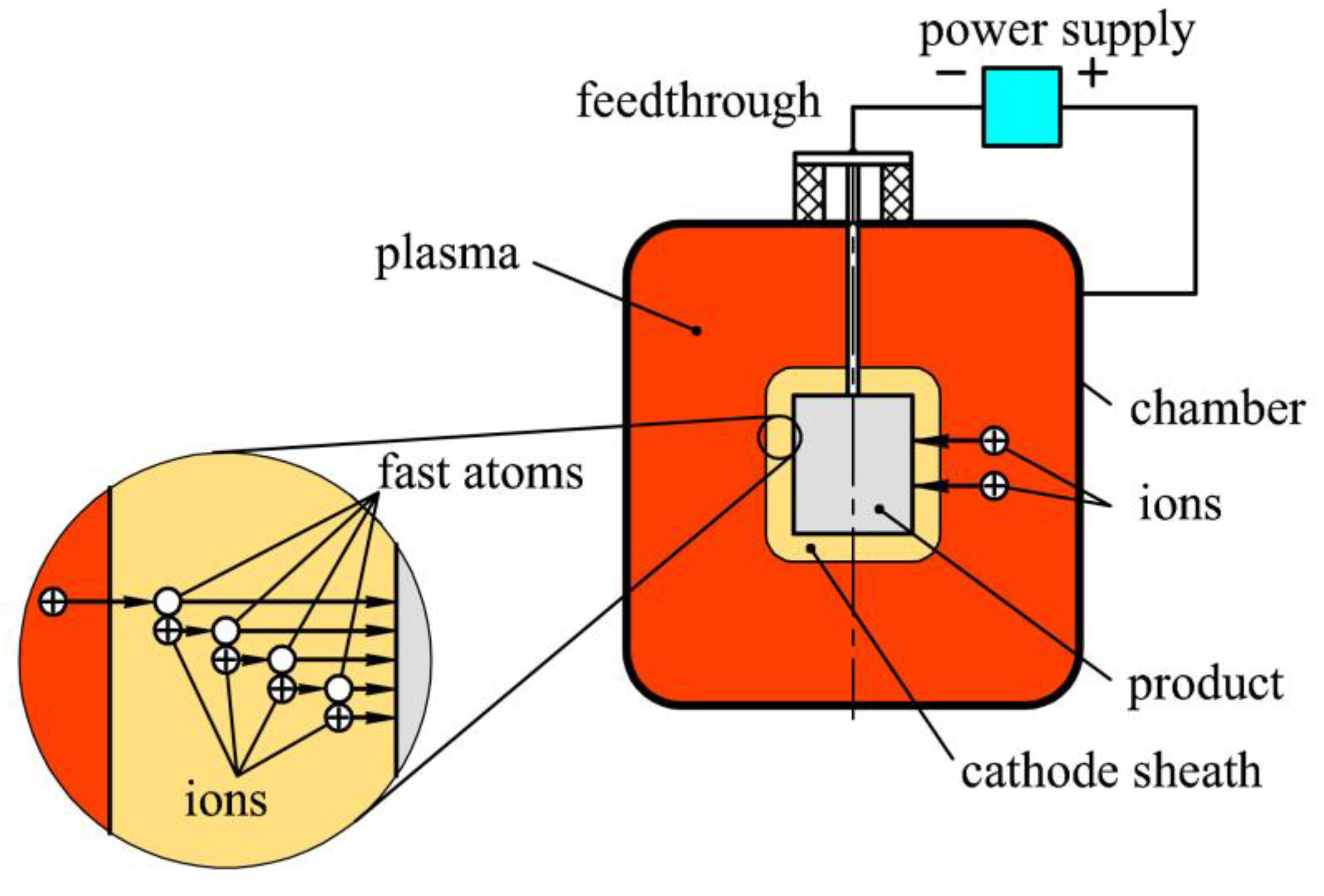
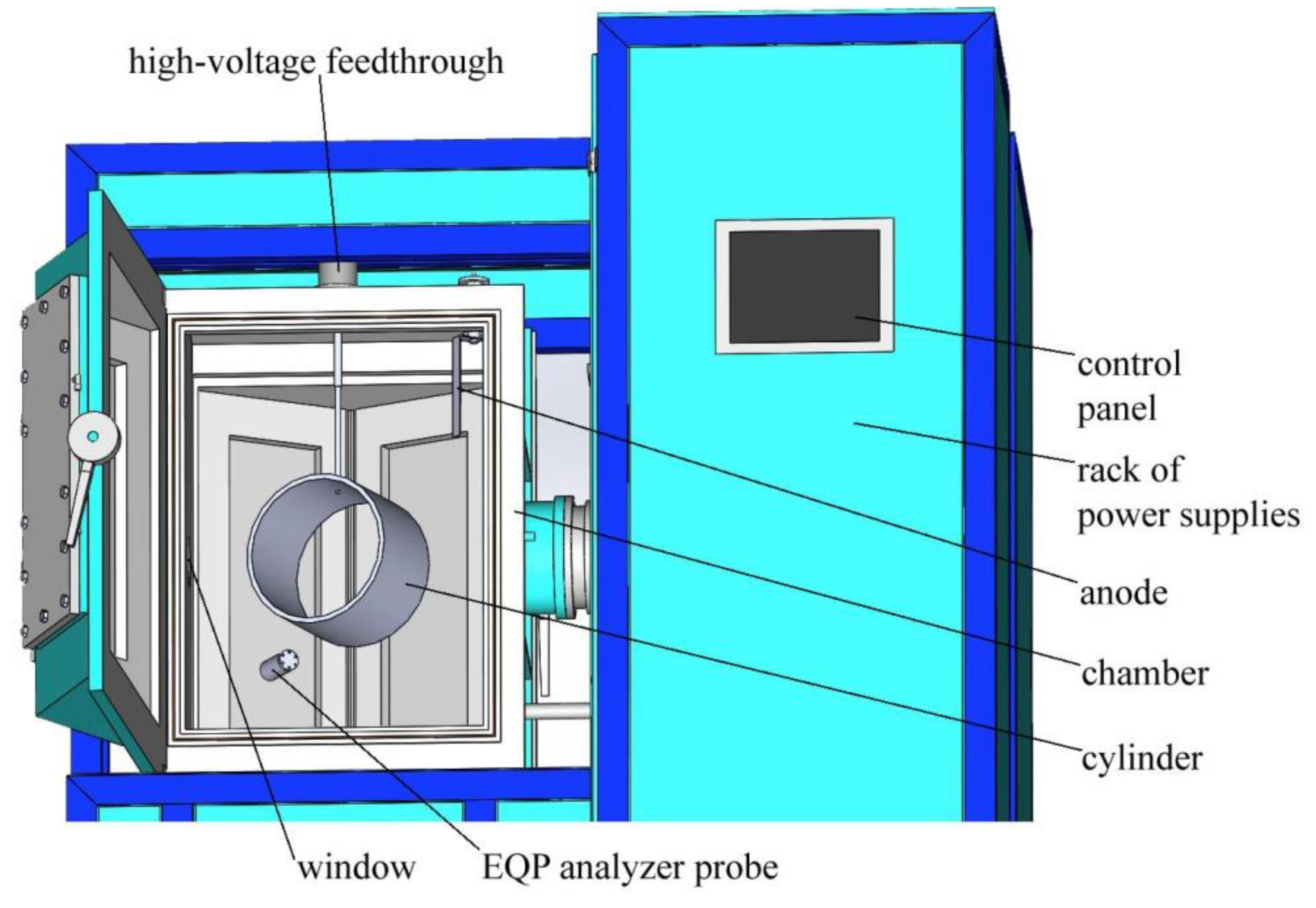
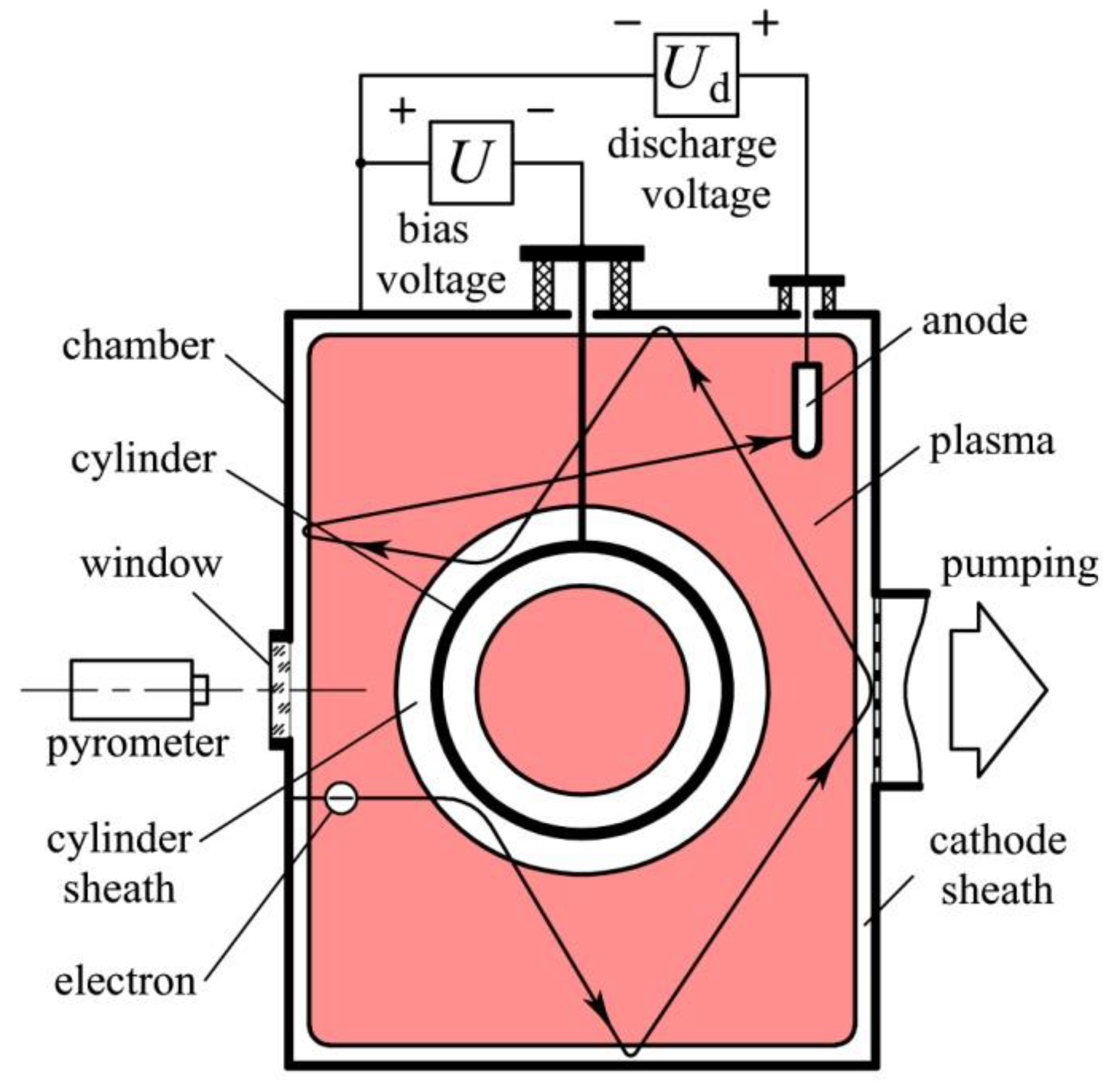
2. Materials and Methods
3. Results
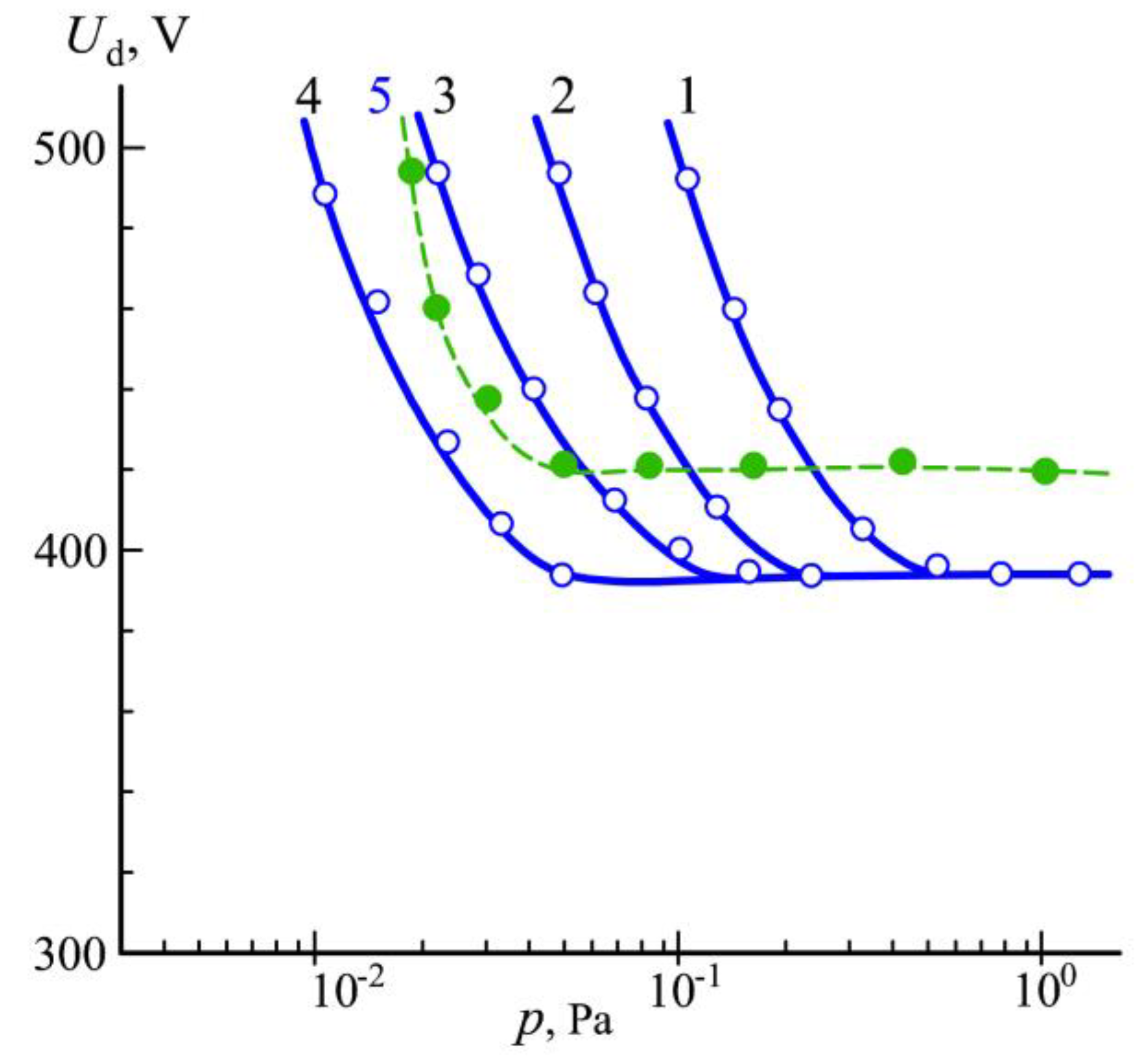
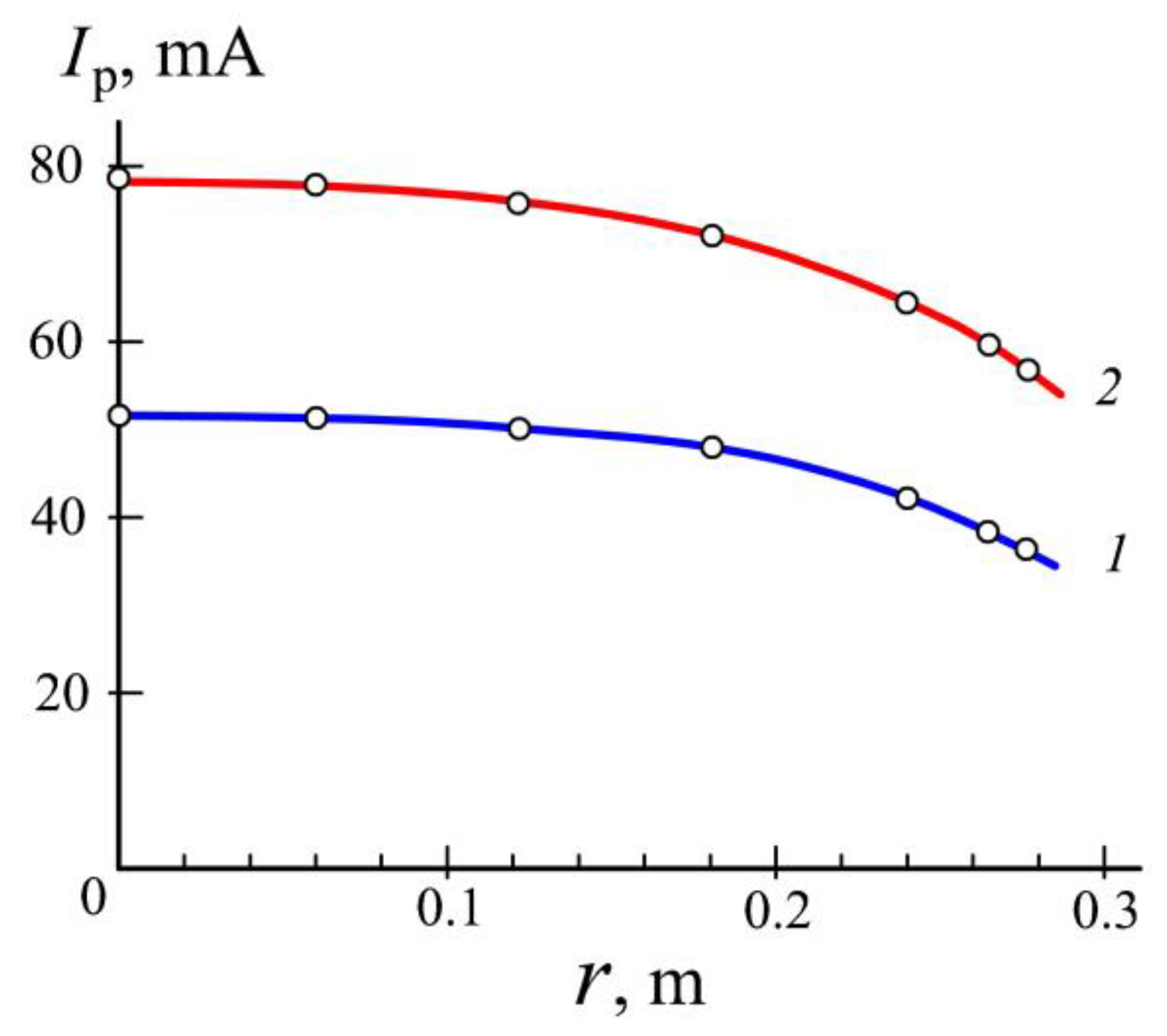
3.1. Generation of Homogeneous Plasma in a Big Chamber Volume
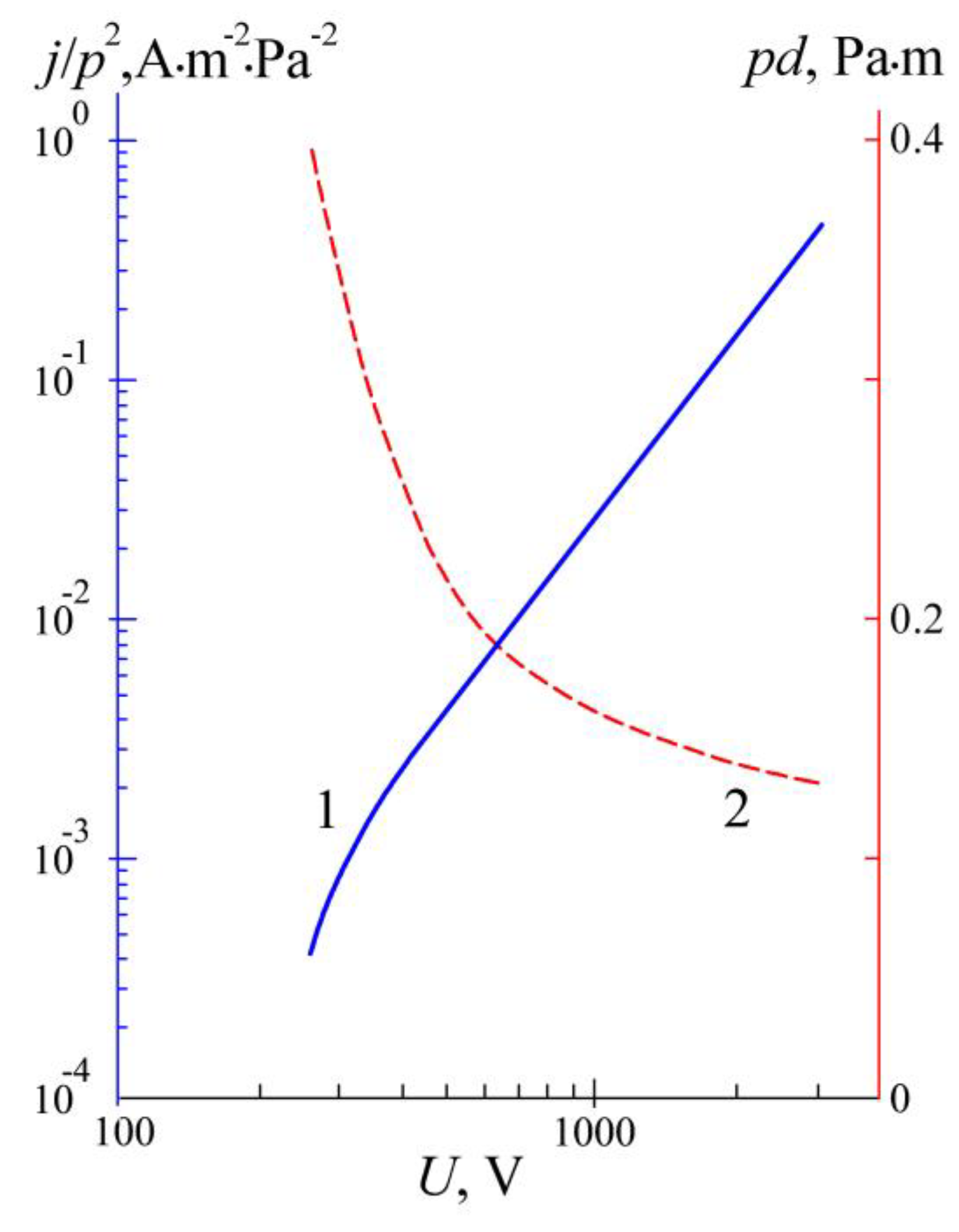
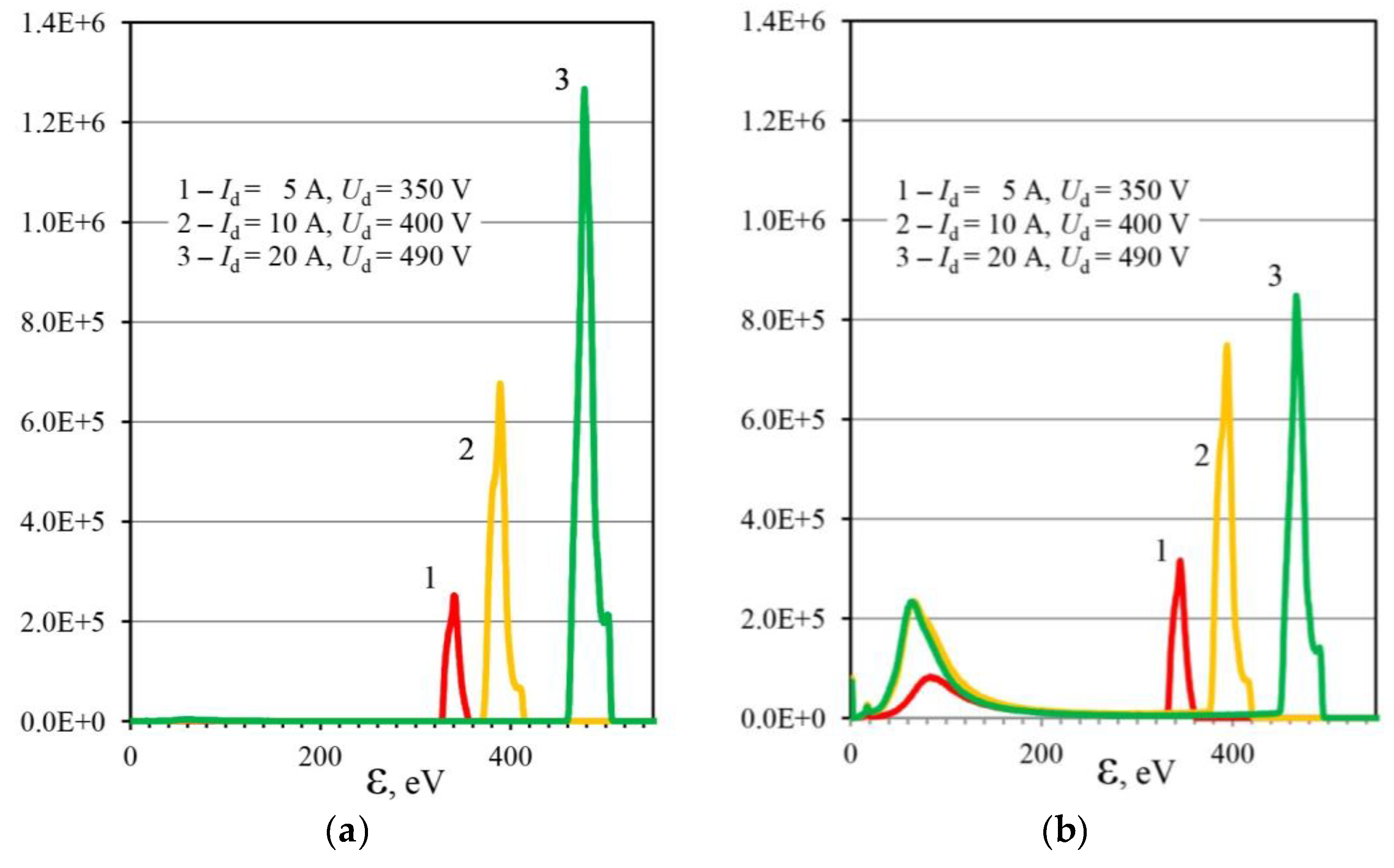
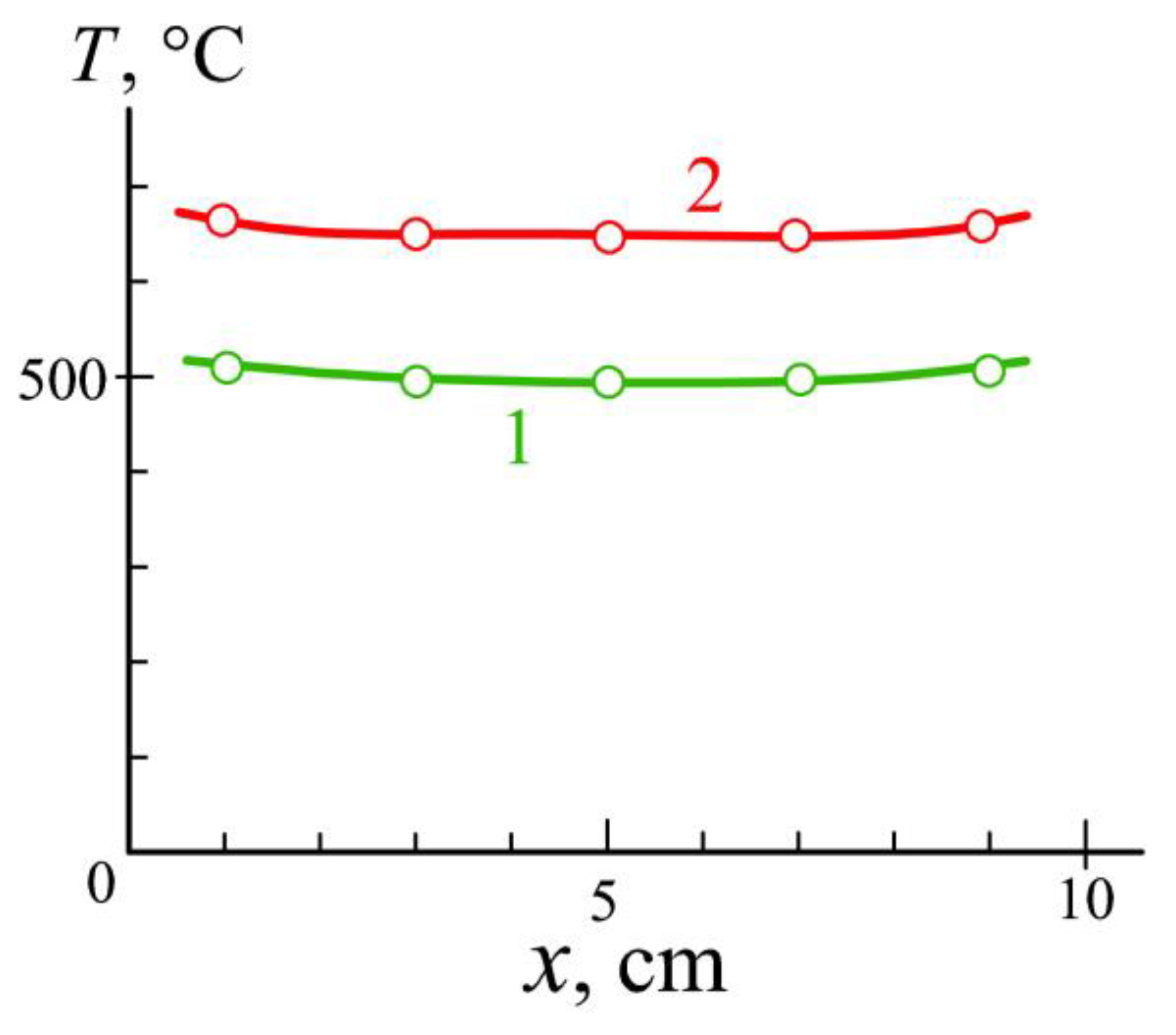
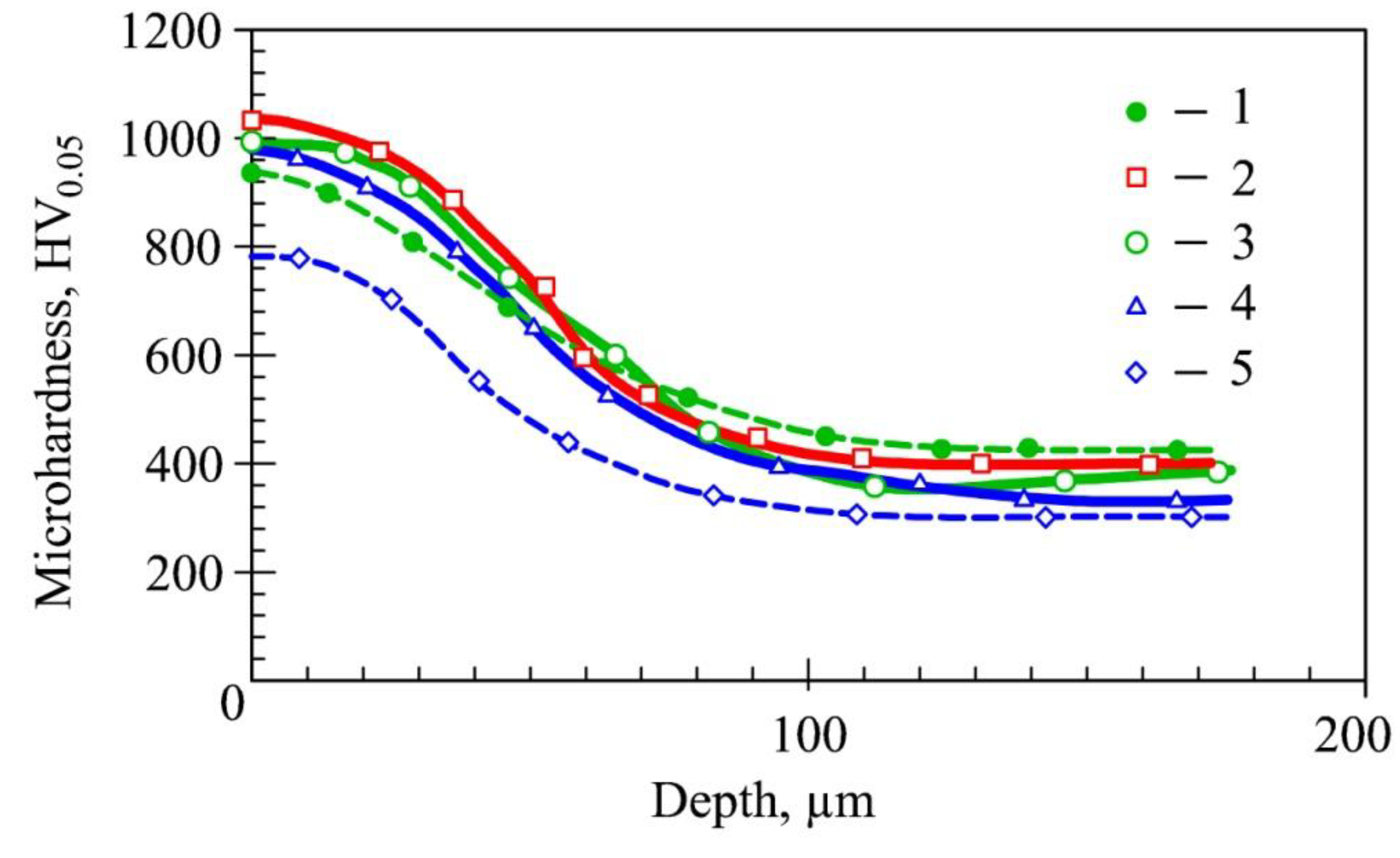
3.2. Plasma Nitriding at a Low Gas Pressure
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuzin, V.; Gurin, V.; Shein, A.; Kochetkova, A.; Mikhailova, M. The influence of duplex vacuum-plasma treatment on the mechanics of complex-profile cutting tool wearing in the production of aircraft engine parts. Mech. Ind. 2018, 19, 704. [Google Scholar] [CrossRef]
- Nayebpashaee, N.; Soltanieh, M.; Kheirandish, S. A Study on Formation and Growth Mechanism of Nitride Layers During Plasma Nitriding Process of Plastic Injection Mold Steel. Mater. Manufact. Proc. 2016, 31, 1192–1200. [Google Scholar] [CrossRef]
- Chong, S.O.; Kim, S.J. Characterization of Cavitation-Erosion Resistance of Plasma Ion Nitrided 316L Stainless Steel Under Shock Wave in Seawater. J. Nanosci. Nanotechnol. 2019, 19, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Alphonsa, J.; Raja, V.; Mukherjee, S. Study of plasma nitriding and nitrocarburizing for higher corrosion resistance and hardness of 2205 duplex stainless steel. Corrosion Sci. 2015, 100, 121–132. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Xiu, J.; Wang, W.; Zhu, Y.; Hu, B. Wear and corrosion properties of AISI 420 martensitic stainless steel treated by active screen plasma nitriding. Surf. Coat. Technol. 2017, 329, 184–192. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Grigoriev, S.N.; Celis, J.P.; Romanov, R.I.; Oshurko, V.B. Structure and mechanical properties of W-Se-C/diamond-like carbon and W-Se/diamond-like carbon bi-layer coatings prepared by pulsed laser deposition. Thin Solid Films 2012, 520, 6476–6483. [Google Scholar] [CrossRef]
- Alim, M.; Saoula, N.; Tadjine, R.; Hadj-Larbi, F.; Keffous, A.; Kechouane, M. Improvement in nano-hardness and corrosion resistance of low carbon steel by plasma nitriding with negative DC bias voltage. Eur. Phys. J. Appl. Phys. 2016, 75, 30801. [Google Scholar] [CrossRef]
- Kurelo, B.C.S.; de Oliveira, W.; Serbena, F.C.; de Souza, G. Surface mechanics and wear resistance of supermartensitic stainless steel nitrided by plasma immersion ion implantation. Surf. Coat. Technol. 2018, 353, 199–209. [Google Scholar] [CrossRef]
- Kovaci, H.; Baran, O.; Yetim, A.F.; Bozkurt, Y.B.; Kara, L.; Celik, A. The friction and wear performance of DLC coatings deposited on plasma nitrided AISI 4140 steel by magnetron sputtering under air and vacuum conditions. Surf. Coat. Technol. 2018, 349, 969–979. [Google Scholar] [CrossRef]
- Devi, M.; Mohanty, O. Plasma-nitriding of tool steel for combined percussive impact and rolling fatigue wear applications. Surf. Coat. Technol. 1998, 107, 55–64. [Google Scholar] [CrossRef]
- Kaplun, P.V.; Gonchar, V.A. Low-cycle fatigue of steels after ion nitriding in hydrogen-free atmospheres. Mater. Sci. 2016, 52, 402–406. [Google Scholar] [CrossRef]
- Bobadilla, M.; Tschiptschin, A. On the nitrogen diffusion in duplex stainless steel. Mater. Res. 2015, 18, 390–394. [Google Scholar] [CrossRef]
- Vales, S.; Brito, P.; Pineda, F.; Ochoa, E.; Droppa, R.; Garcia, J.; Morales, M.; Alvarez, F.; Pinto, H. Influence of substrate pre-treatments by Xe+ ion bombardment and plasma nitriding on the behavior of TiN coatings deposited by plasma reactive sputtering on 100Cr6 steel. Mater. Chem. Phys. 2016, 177, 156–163. [Google Scholar] [CrossRef]
- Hamann, S.; Burlacov, I.; Spies, H.J.; Biermann, H.; Ropcke, J. Spectroscopic investigations of plasma nitriding processes: A comparative study using steel and carbon as active screen materials. J. Appl. Phys. 2017, 121, 153301. [Google Scholar] [CrossRef]
- Stechyshyn, M.S.; Martynyuk, A.V.; Bilyk, Y.M.; Oleksandrenko, V.P.; Stechyshyna, N.M. Influence of the Ionic Nitriding of Steels in Glow Discharge on the Structure and Properties of the Coatings. Mater. Sci. 2017, 53, 343–350. [Google Scholar] [CrossRef]
- Foerster, C.E.; Souza, J.F.P.; Silva, C.A.; Ueda, M.; Kuromoto, N.K.; Serbena, F.C.; Silva, S.L.R.; Lepienski, C.M. Effect of cathodic hydrogenation on the mechanical properties of AISI 304 stainless steel nitrided by ion implantation, glow discharge and plasma immersion ion implantation. Nucl. Instrum. Meth. Phys. Res. B 2007, 257, 727–731. [Google Scholar] [CrossRef]
- Güntherschulze, A. Der Kathodenfall der Glimmentladung in Abhängigkeit von der Stromdichte bei Spannungen bis 3000 Volt. Z. Phys. 1930, 59, 433–445. [Google Scholar] [CrossRef]
- Leyland, A.; Fancey, K.S.; James, A.S.; Matthews, A. Enhanced plasma nitriding at low pressures: A comparative study of DC and RF techniques. Surf. Coat. Technol. 1990, 41, 295–304. [Google Scholar] [CrossRef]
- Metel, A.S.; Grigoriev, S.N.; Melnik, Y.A.; Bolbukov, V.P. Broad beam sources of fast molecules with segmented cold cathodes and emissive grids. Instrum. Exp. Tech. 2012, 55, 122–130. [Google Scholar] [CrossRef]
- Avelar-Batista, J.C.; Spain, E.; Housden, J.; Matthews, A.; Fuentes, G.G. Plasma nitriding of Ti6Al4V alloy and A1S1M2 steel substrates using DC glow discharge under a triode configuration. Surf. Coat. Technol. 2005, 200, 1954–1961. [Google Scholar] [CrossRef]
- Koval, N.N.; Ryabchikov, A.I.; Sivin, D.O.; Lopatin, I.V.; Krysina, O.V.; Akhmadeev, Y.H.; Ignatov, D.Y. Low-energy high-current plasma immersion implantation of nitrogen ions in plasma of non-self-sustained arc discharge with thermionic and hollow cathodes. Surf. Coat. Technol. 2018, 340, 152–158. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Melnik, Y.A.; Metel, A.S.; Panin, V.V. Broad beam source of fast atoms produced as a result of charge exchange collisions of ions accelerated between two plasmas. Instrum. Exp. Tech. 2009, 52, 602–608. [Google Scholar] [CrossRef]
- Gavrilov, N.V.; Mamaev, A.S.; Chukin, A.V. Nitriding of Stainless Steel in Electron-Beam Plasma in the Pulsed and DC Generation Modes. J. Surf. Invest. 2017, 11, 1167–1172. [Google Scholar] [CrossRef]
- Burdovitsin, V.A.; Golosov, D.A.; Oks, E.M.; Tyunkov, A.V.; Yushkov, Y.G.; Zolotukhin, D.B.; Zavadsky, S.M. Electron beam nitriding of titanium in a medium vacuum. Surf. Coat. Technol. 2019, 358, 726–731. [Google Scholar] [CrossRef]
- Tahchieva, A.B.; Llorca-Isern, N.; Cabrera, J.M. Duplex and Superduplex Stainless Steels: Microstructure and Property Evolution by Surface Modification Processes. Metals 2019, 9, 347. [Google Scholar] [CrossRef]
- De Oliveira, W.; Kurelo, B.; Ditzel, D.; Serbena, F.; Foerster, C.; de Souza, G. On the S-phase formation and the balanced plasma nitriding of austenitic-ferritic super duplex stainless steel. Appl. Surf. Sci. 2018, 434, 1161–1174. [Google Scholar] [CrossRef]
- Zdravecka, E.; Slota, J.; Solfronk, P.; Kolnerova, M. Evaluation of the effect of different plasma-nitriding parameters on the properties of low-alloy steel. J. Mater. Eng. Perf. 2017, 26, 3588–3596. [Google Scholar] [CrossRef]
- Li, S.L.; Ma, C.Y.; Zhang, Q.Y.; Ren, C.S.; Lu, W.Q. Ion nitriding of pure iron using high-density plasma beam generated by a tubular plasma source. Surf. Coat. Technol. 2017, 309, 47–53. [Google Scholar] [CrossRef]
- Kurelo, B.; de Souza, G.; Rutz da Silva, S.; Serbena, F.; Foerster, C.; Alves, C. Plasma nitriding of HP13Cr supermartensitic stainless steel. Appl. Surf. Sci. 2015, 349, 403–414. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Low-temperature nitriding of AISI 300 and 200 series austenitic stainless steels. Vacuum 2016, 127, 51–60. [Google Scholar] [CrossRef]
- Kaplun, P.V.; Soroka, E.B.; Snozik, A.V. The impact of hydrogen-free ion nitriding on physicomechanical and performance characteristics of hard alloys T5K10 and T15K6. J. Superhard Mater. 2018, 40, 384–391. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Ye, Q. Effect of Hybrid Surface Treatment Composed of Plasma Nitriding and W-Cr-Ti-Al-N Coating on Tribological Behavior of AISI 316L Steel. Tribol. Online 2018, 13, 316–319. [Google Scholar] [CrossRef]
- Kovaci, H.; Baran, O.; Bayrak, O.; Yetim, A.F.; Celik, A. Influence of plasma nitriding treatment on the adhesion of DLC films deposited on AISI 4140 steel by PVD magnetron sputtering. J. Adhes. Sci. Technol. 2017, 31, 2015–2027. [Google Scholar] [CrossRef]
- Conrad, J.R.; Dodd, R.A.; Worzala, F.J.; Qiu, X. Plasma source ion implantation: a new, cost-effective, non-line-of-sight technique for ion implantation of materials. Surf. Coat. Technol. 1988, 36, 927–937. [Google Scholar] [CrossRef]
- Emmert, G.A.; Henry, M.A. Numerical simulation of plasma sheath expansion, with applications to plasma-source ion implantation. J. Appl. Phys. 1992, 71, 113–117. [Google Scholar] [CrossRef]
- Mändl, S.; Gerlach, J.W.; Rauschenbach, B. Nitride formation in transition metals during high fluence–high temperature implantation. Surf. Coat. Technol. 2005, 200, 584–588. [Google Scholar] [CrossRef]
- Metel, A.S. Ionization effect in cathode layers on glow-discharge characteristics with oscillating electrons. 1. Discharge with the hollow cathode. Zh. Techn. Fiz. 1985, 55, 1928–1934. [Google Scholar]
- Metel, A.; Bolbukov, V.; Volosova, M.; Grigoriev, S.; Melnik, Y. Source of metal atoms and fast gas molecules for coating deposition on complex shaped dielectric products. Surf. Coat. Technol. 2013, 225, 34–39. [Google Scholar] [CrossRef]
- Metel, A.; Bolbukov, V.; Volosova, M.; Grigoriev, S.; Melnik, Y. Equipment for deposition of thin metallic films bombarded by fast argon atoms. Instrum. Exp. Tech. 2014, 57, 345–351. [Google Scholar] [CrossRef]
- Dalgarno, A. Range and energy loss. In Atomic and Molecular Processes; Bates, D.R., Ed.; Academic Press: New York, NY, USA, 1962; pp. 622–642. [Google Scholar]
- Grigoriev, S.N.; Melnik, Y.A.; Metel, A.S.; Panin, V.V.; Prudnikov, V.V. A compact vapor source of conductive target material sputtered by 3-keV ions at 0.05-Pa pressure. Instrum. Exp. Tech. 2009, 52, 731–737. [Google Scholar] [CrossRef]
- Phelps, A.V. Cross sections and swarm coefficients for nitrogen ions and neutrals in N2 and argon ions and neutrals in Ar for energies from 0.1 eV to 10 keV. J. Phys. Chem. Ref. Data 1991, 20, 557–573. [Google Scholar] [CrossRef]
- Phelps, A.V.; Greene, C.H.; Burke, J.P. Collision cross sections for argon atoms with argon atoms for energies from 0.01 eV to 10 keV. J. Phys. B At. Mol. Opt. Phys. 2000, 33, 2965–2981. [Google Scholar] [CrossRef]
- Metel, A.S.; Grigoriev, S.N.; Melnik, Y.A.; Prudnikov, V.V. Glow discharge with electrostatic confinement of electrons in a chamber bombarded by fast electrons. Plasma Phys. Rep. 2011, 37, 628–637. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, S.; Metel, A.; Volosova, M.; Melnik, Y.; Ney, H.A.; Mustafaev, E. Surface Hardening of Massive Steel Products in the Low-pressure Glow Discharge Plasma. Technologies 2019, 7, 62. https://doi.org/10.3390/technologies7030062
Grigoriev S, Metel A, Volosova M, Melnik Y, Ney HA, Mustafaev E. Surface Hardening of Massive Steel Products in the Low-pressure Glow Discharge Plasma. Technologies. 2019; 7(3):62. https://doi.org/10.3390/technologies7030062
Chicago/Turabian StyleGrigoriev, Sergey, Alexander Metel, Marina Volosova, Yury Melnik, Htet A. Ney, and Enver Mustafaev. 2019. "Surface Hardening of Massive Steel Products in the Low-pressure Glow Discharge Plasma" Technologies 7, no. 3: 62. https://doi.org/10.3390/technologies7030062
APA StyleGrigoriev, S., Metel, A., Volosova, M., Melnik, Y., Ney, H. A., & Mustafaev, E. (2019). Surface Hardening of Massive Steel Products in the Low-pressure Glow Discharge Plasma. Technologies, 7(3), 62. https://doi.org/10.3390/technologies7030062








