A Review on Nano-Scale Precipitation in Steels
Abstract
:1. Introduction
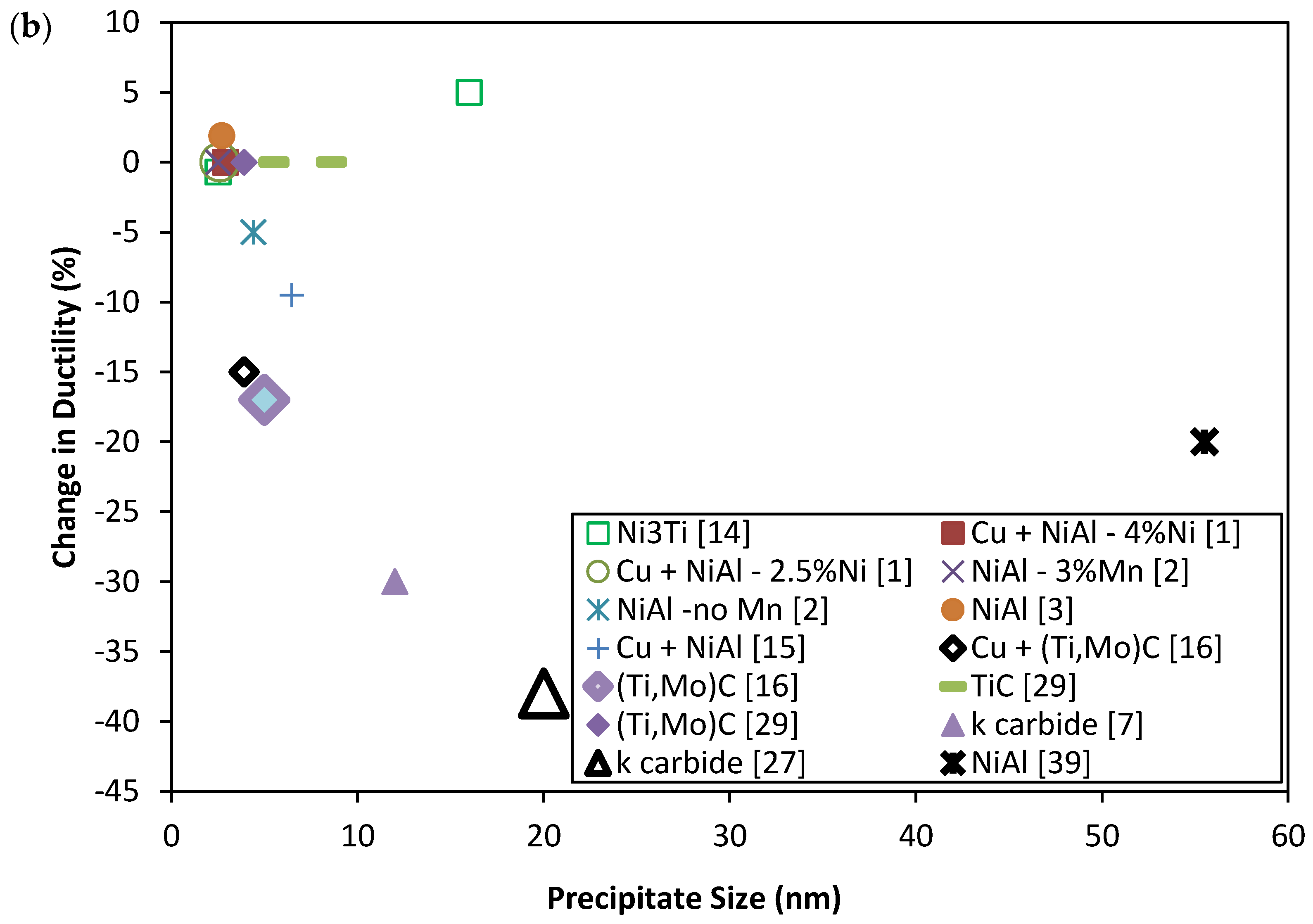
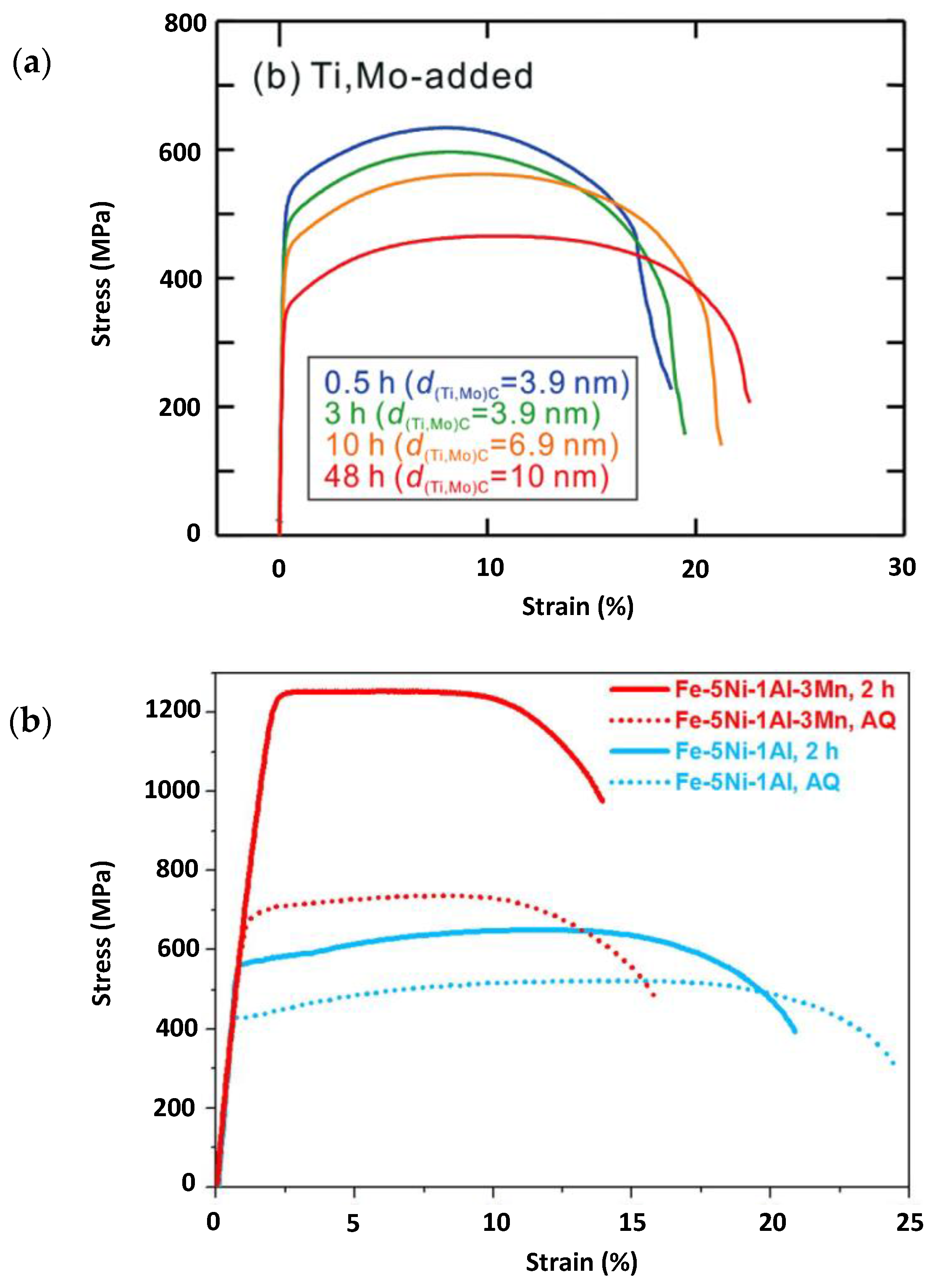
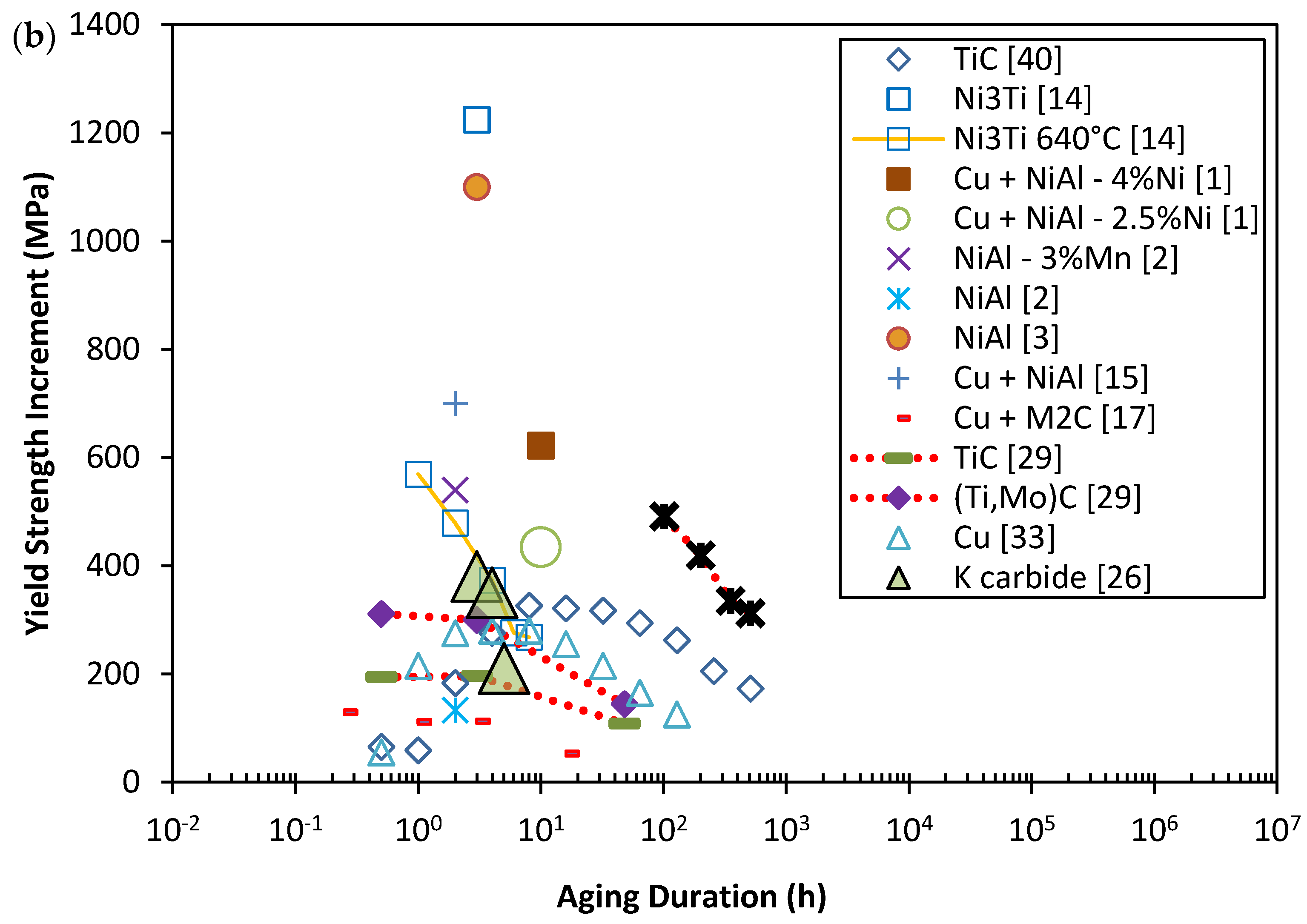
2. Mechanical Properties of Precipitate Strengthened Steels
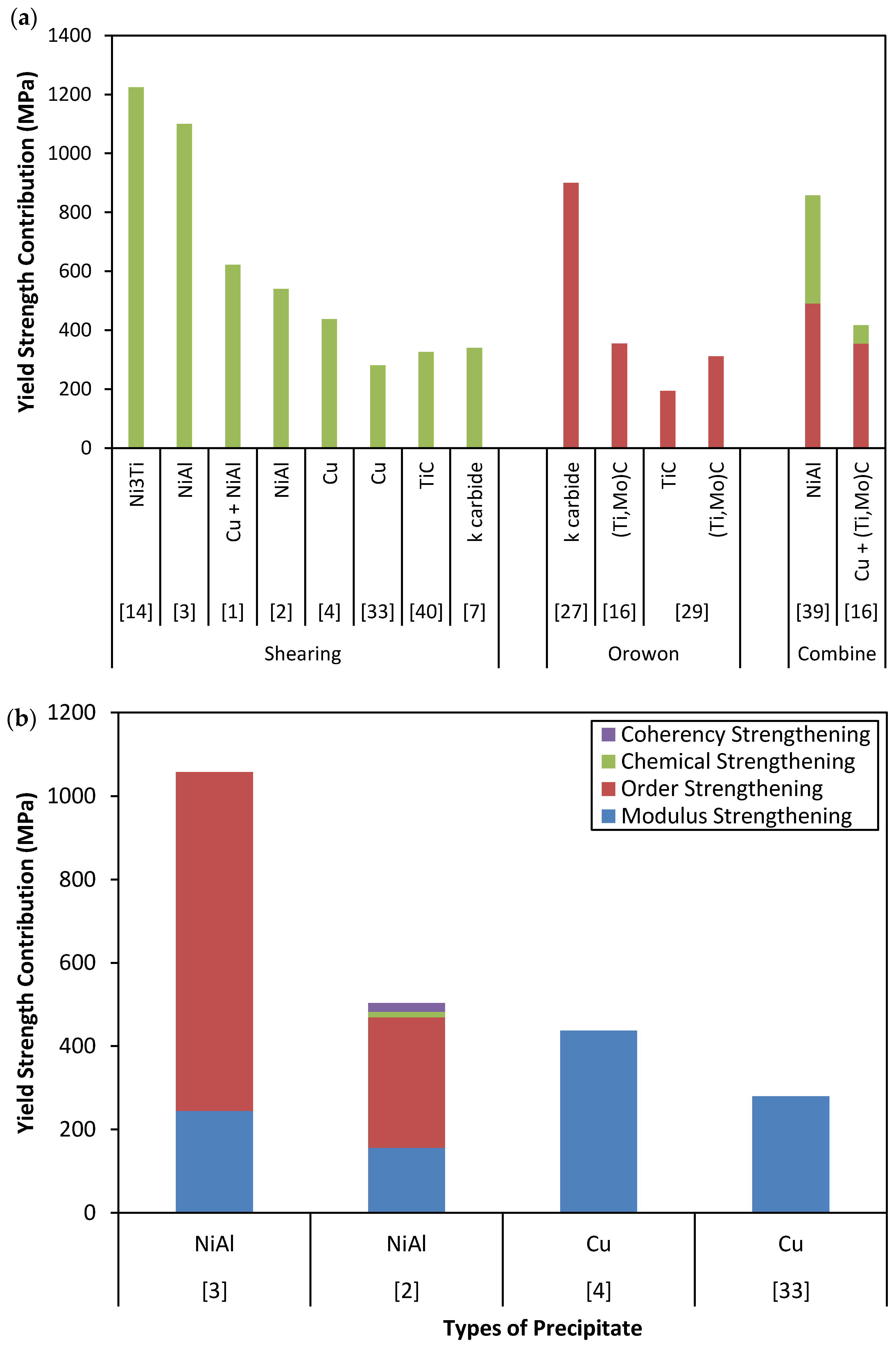
3. The Strengthening Mechanisms of Nano-Scale Precipitates Strengthened Steels
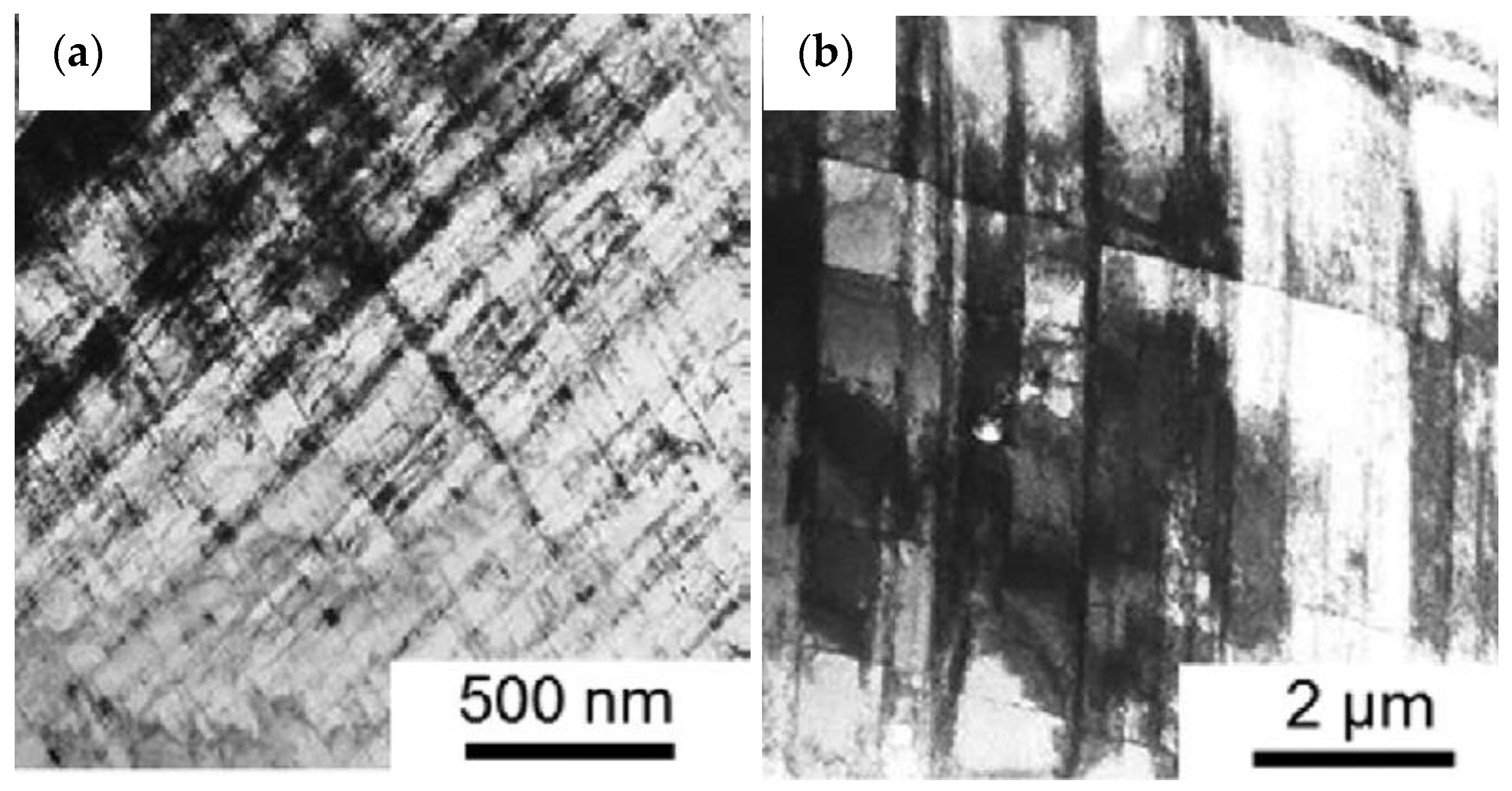
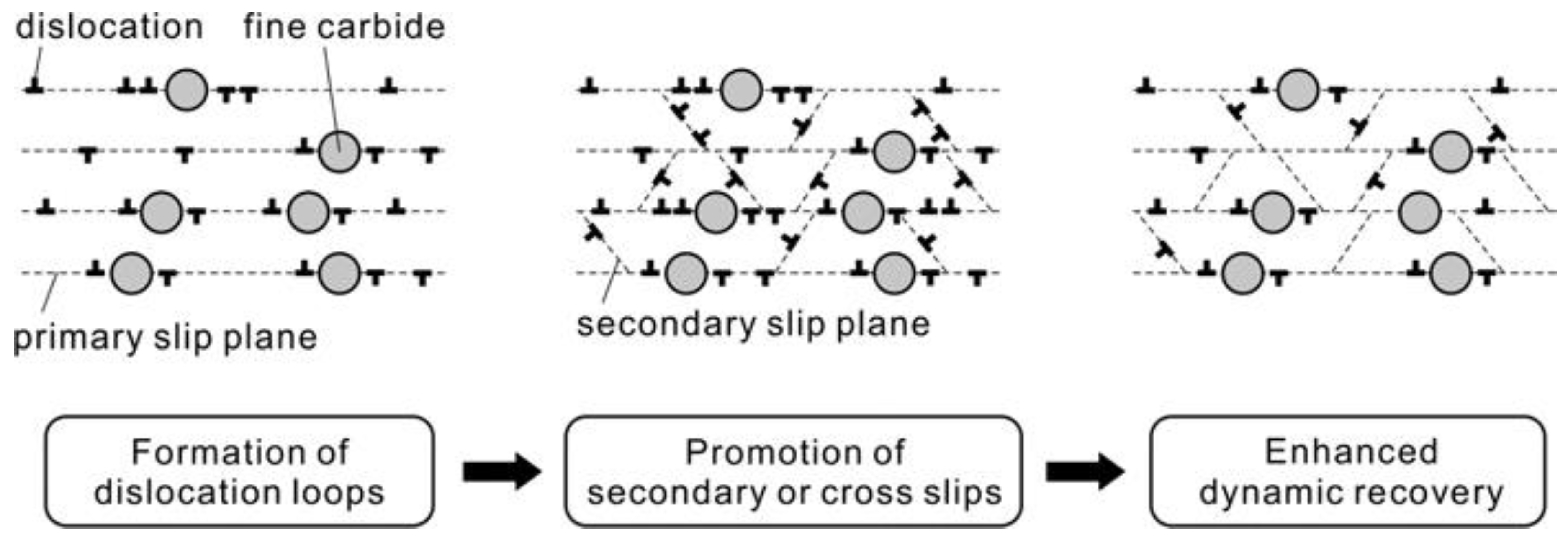
4. The Effects of Precipitates on Slip Systems and Dislocation-Precipitate Interactions
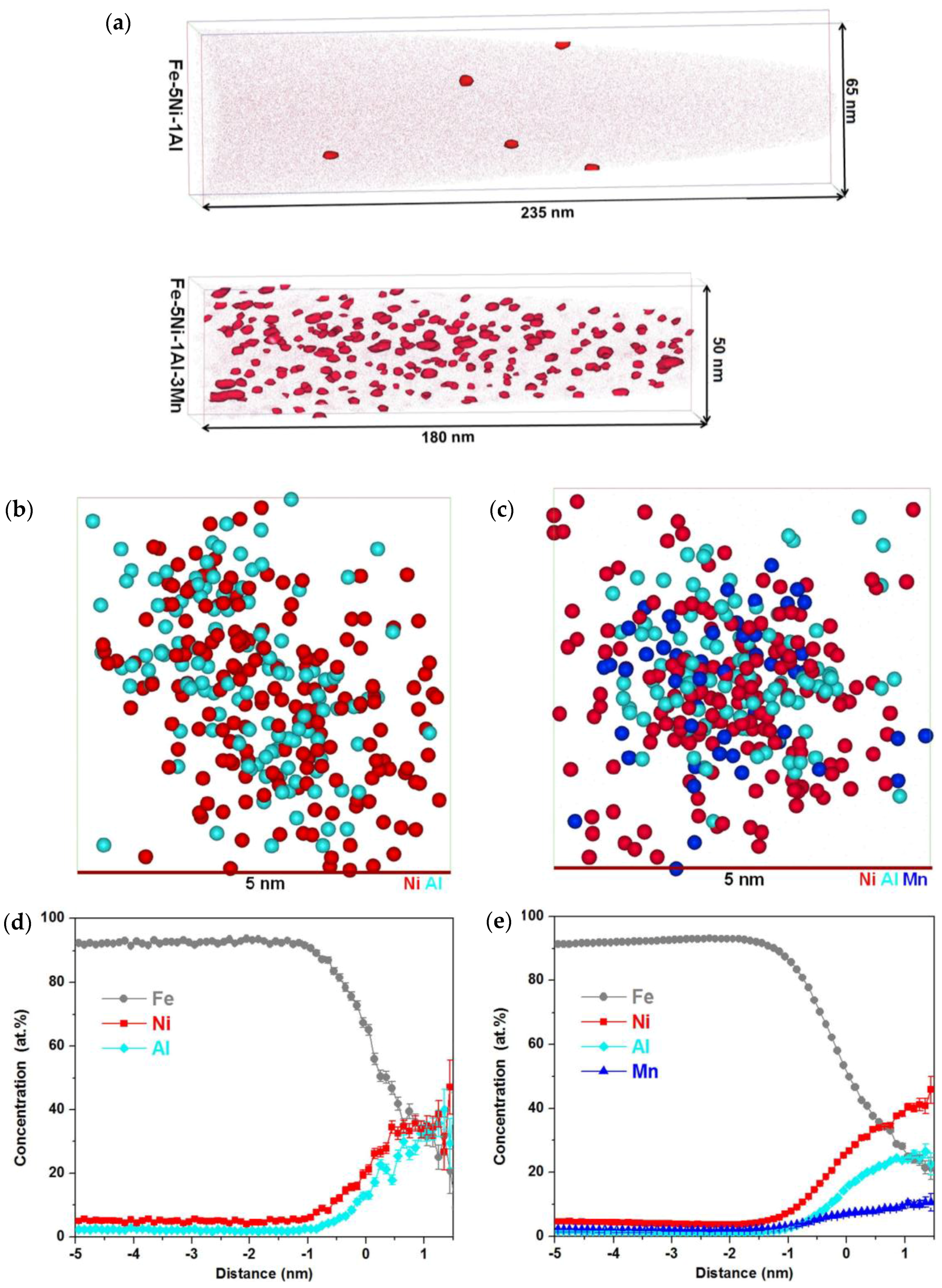
5. Nucleation of Precipitates
5.1. Nucleation Energy Barrier Reduction by Reducing the Surface and Elastic Strain Energy
5.2. Reduction of the Nucleation Energy Barrier by Increasing the Nucleation Driving Force
5.3. Nucleation Energy Barrier Reduction by Increasing the Number of Nucleation Sites
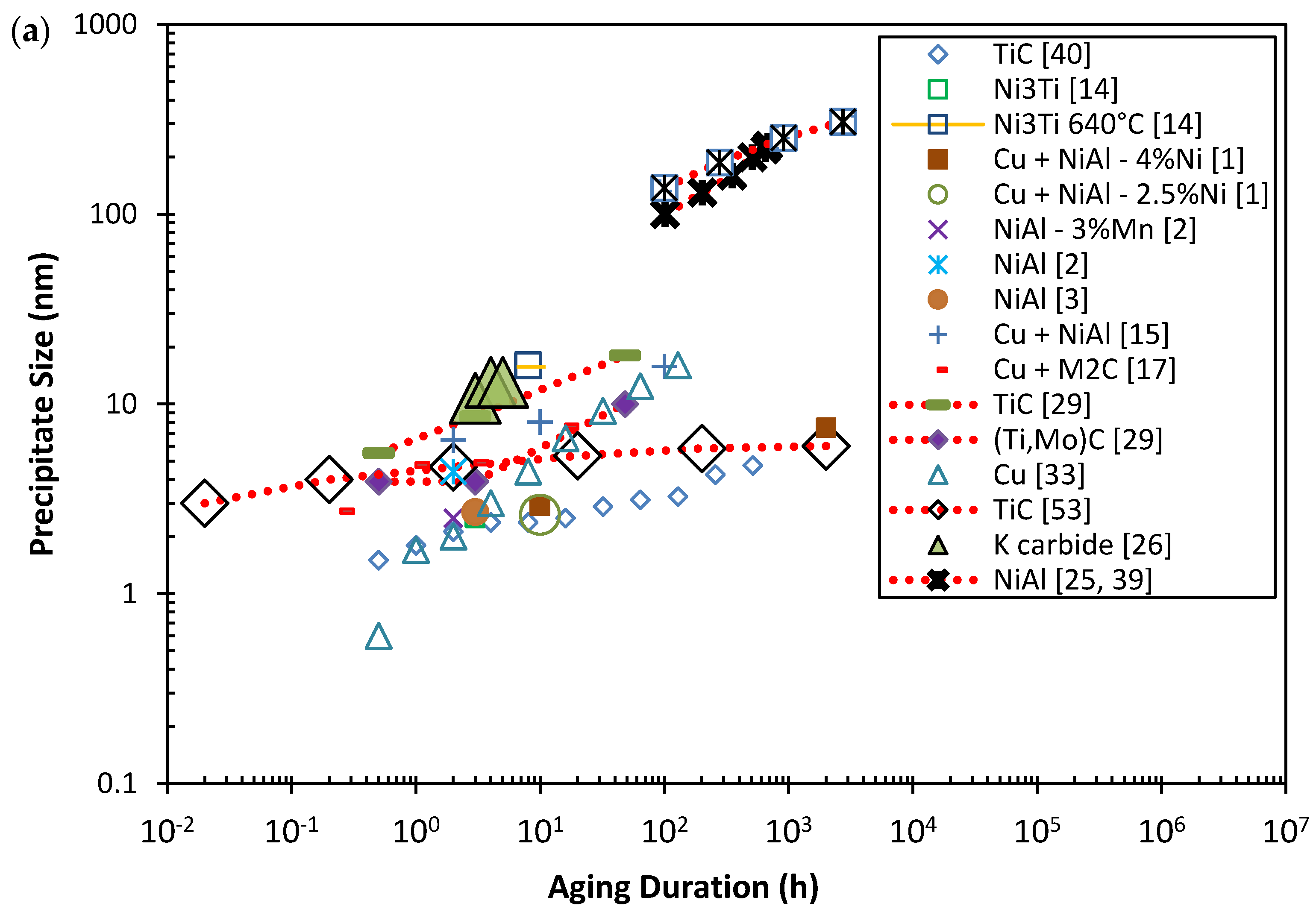
6. Stability of Precipitates
6.1. Stability Improvement by Reducing Interfacial Energy
6.2. Stability Improvement by Reducing the Diffusion Coefficient of Solutes
6.3. Stability Improvement by Reducing the Solubility Limit of Solutes
7. Future Challenges of Nano-Scale Precipitate Strengthened Steels
8. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Z.; Liu, C.T.; Miller, M.K.; Wang, X.-L.; Wen, Y.; Fujita, T.; Hirata, A.; Chen, M.; Chen, G.; Chin, B.A. A nanoscale co-precipitation approach for property enhancement of Fe-base alloys. Sci. Rep. 2013, 3, 1327. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Luan, J.; Miller, M.; Yu, C.; Liu, C. Effects of Mn partitioning on nanoscale precipitation and mechanical properties of ferritic steels strengthened by NiAl nanoparticles. Acta Mater. 2015, 84, 283–291. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.; Chen, H.; Yao, M.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Luan, J.; Zhang, Z.; Miller, M.; Ma, W.; Liu, C. Synergistic effects of Cu and Ni on nanoscale precipitation and mechanical properties of high-strength steels. Acta Mater. 2013, 61, 5996–6005. [Google Scholar] [CrossRef]
- Halfa, H. Recent Trends in Producing Ultrafine Grained Steels. J. Miner. Mater. Charact. Eng. 2014, 2, 428–469. [Google Scholar] [CrossRef]
- Kim, C.; Kwon, S.; Lee, B.; Moon, J.; Park, S.; Lee, J.; Hong, H. Atomistic study of nano-sized κ-carbide formation and its interaction with dislocations in a cast Si added FeMnAlC lightweight steel. Mater. Sci. Eng. A 2016, 673, 108–113. [Google Scholar] [CrossRef]
- Haase, C.; Zehnder, C.; Ingendahl, T.; Bikar, A.; Tang, F.; Hallstedt, B.; Hu, W.; Bleck, W.; Molodov, D.A. On the deformation behavior of κ-carbide-free and κ-carbide-containing high-Mn light-weight steel. Acta Mater. 2017, 122, 332–343. [Google Scholar] [CrossRef]
- Schneider, M.J.; The Timken Company; Chatterjee, B.M.S. Introduction to Surface Hardening of Steels. In ASM Handbook: Steel Heat Treating Fundamentals and Process; ASM International: Almere, The Netherlands, 2013; Volume 4A, pp. 389–398. [Google Scholar]
- Shinozaki, I.; Hasegawa, Y. Precipitation Strengthening by the Nitrides in High Cr Containing Ferritic Creep Resistant Steels. In Proceedings of the Advances in Materials Technology for Fossil Power Plants, Waikoloa, HI, USA, 22–25 October 2013. [Google Scholar]
- Zhang, Z.W.; Yao, L.; Wang, X.-L.; Miller, M.K. Vacancy-controlled ultrastable nanoclusters in nanostructured ferritic alloys. Sci. Rep. 2015, 5, 10600. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-J.; Li, J.; Darling, K.A.; Wang, W.Y.; Vanleeuwen, B.K.; Liu, X.L.; Kecskes, L.J.; Dickey, E.C.; Liu, Z.-K. Nano-sized Superlattice Clusters Created by Oxygen Ordering in Mechanically Alloyed Fe Alloys. Sci. Rep. 2015, 5, 11772. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kang, B.S.-J.; Alvin, M.A.; Huang, C.C. Characterization of Oxide-Dispersion-Strengthened (ODS) Alloy Powders Processed by Mechano-Chemical-Bonding (MCB) and Balling Milling (BM). KONA Powder Part. J. 2014, 31, 146–155. [Google Scholar] [CrossRef]
- Jiao, Z.; Luan, J.; Miller, M.; Chung, Y.; Liu, C. Co-precipitation of nanoscale particles in steels with ultra-high strength for a new era. Mater. Today 2017, 20, 142–154. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Dey, G.K.; Asundi, M.K. Precipitation hardening in 350 grade maraging steel. Metall. Trans. A 1993, 24, 2429–2442. [Google Scholar] [CrossRef]
- Kapoor, M.; Isheim, D.; Ghosh, G.; Vaynman, S.; Fine, M.E.; Chung, Y.-W. Aging characteristics and mechanical properties of 1600 MPa body-centered cubic Cu and B2-NiAl precipitation-strengthened ferritic steel. Acta Mater. 2014, 73, 56–74. [Google Scholar] [CrossRef]
- Phaniraj, M.; Shin, Y.-M.; Lee, J.; Goo, N.H.; Kim, D.-I.; Suh, J.-Y.; Jung, W.-S.; Shim, J.-H.; Choi, I.-S. Development of high strength hot rolled low carbon copper-bearing steel containing nanometer sized carbides. Mater. Sci. Eng. A 2015, 633, 1–8. [Google Scholar] [CrossRef]
- Jain, D.; Isheim, D.; Hunter, A.H.; Seidman, D.N. Multicomponent High-Strength Low-Alloy Steel Precipitation-Strengthened by Sub-nanometric Cu Precipitates and M2C Carbides. Metall. Mater. Trans. A 2016, 47, 3860–3872. [Google Scholar] [CrossRef]
- Carinci, G.M.; Hetherington, M.G.; Olson, G.B. M2C Carbide Precipitation in Af1410 Steel. J. Phys. Colloq. 1988, 49, C6-311–C6-316. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, W.; Liu, Z.; Wang, G. Direct observations on the crystal structure evolution of nano Cu-precipitates in an extremely low carbon steel. Mater. Lett. 2017, 187, 49–52. [Google Scholar] [CrossRef]
- Han, G.; Xie, Z.; Li, Z.; Lei, B.; Shang, C.; Misra, R. Evolution of crystal structure of Cu precipitates in a low carbon steel. Mater. Des. 2017, 135, 92–101. [Google Scholar] [CrossRef]
- Monzen, R.; Jenkins, M.L.; Sutton, A.P. The bcc-to-9R martensitic transformation of Cu precipitates and the relaxation process of elastic strains in an Fe-Cu alloy. Philos. Mag. A 2000, 80, 711–723. [Google Scholar] [CrossRef]
- Othen, P.J.; Jenkins, M.L.; Smith, G.D.W.; Phythian, W.J. Transmission electron microscope investigations of the structure of copper precipitates in thermally-aged Fe-Cu and Fe-Cu-Ni. Philos. Mag. Lett. 1991, 64, 383–391. [Google Scholar] [CrossRef]
- Lozano-Perez, S.; Jenkins, M.L.; Titchmarsh, J.M. Evidence for deformation-induced transformations of Cu-rich precipitates in an aged FeCu alloy. Philos. Mag. Lett. 2006, 86, 367–374. [Google Scholar] [CrossRef]
- Hu, S.; Schmauder, S.; Chen, L. Atomistic Simulations of Interactions between Cu Precipitates and an Edge Dislocation in a B.C.C. Fe Single Crystal. Phys. Status Solidi 2000, 220, 845–846. [Google Scholar] [CrossRef]
- Sun, Z.; Song, G.; Ilavsky, J.; Ghosh, G.; Liaw, P.K. Nano-sized precipitate stability and its controlling factors in a NiAl-strengthened ferritic alloy. Sci. Rep. 2015, 5, 16081. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Chao, C.G.; Bor, H.Y.; Liu, T.F. Relationship between Microstructures and Tensile Properties of an Fe-30Mn-8.5Al-2.0C Alloy. Mater. Trans. 2010, 51, 1084–1088. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Raabe, D. High strength and ductile low density austenitic FeMnAlC steels: Simplex and alloys strengthened by nanoscale ordered carbides. Mater. Sci. Technol. 2014, 30, 1099–1104. [Google Scholar] [CrossRef]
- Yao, M.; Dey, P.; Seol, J.-B.; Choi, P.; Herbig, M.; Marceau, R.; Hickel, T.; Neugebauer, J.; Raabe, D. Combined atom probe tomography and density functional theory investigation of the Al off-stoichiometry of κ-carbides in an austenitic Fe-Mn-Al-C low density steel. Acta Mater. 2016, 106, 229–238. [Google Scholar] [CrossRef]
- Kamikawa, N.; Abe, Y.; Miyamoto, G.; Funakawa, Y.; Furuhara, T. Tensile Behavior of Ti, Mo-added Low Carbon Steels with Interphase Precipitation. ISIJ Int. 2014, 54, 212–221. [Google Scholar] [CrossRef]
- Thomson, R. Characterization of Carbides in Steels Using Atom Probe Field-Ion Microscopy. Mater. Charact. 2000, 44, 219–233. [Google Scholar] [CrossRef]
- Kolli, R.P.; Seidman, D.N. Co-Precipitated and Collocated Carbides and Cu-Rich Precipitates in a Fe-Cu Steel Characterized by Atom-Probe Tomography. Microsc. Microanal. 2014, 20, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yen, H.; Kao, F.; Li, W.; Huang, C.; Yang, J.; Wang, S. Precipitation hardening of high-strength low-alloy steels by nanometer-sized carbides. Mater. Sci. Eng. A 2009, 499, 162–166. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y. Consideration of particle-strengthening mechanism of copper-precipitation-strengthened steels by atom probe tomography analysis. Mater. Sci. Eng. A 2012, 535, 144–152. [Google Scholar] [CrossRef]
- Isheim, D.; Gagliano, M.S.; Fine, M.E.; Seidman, D.N. Interfacial segregation at Cu-rich precipitates in a high-strength low-carbon steel studied on a sub-nanometer scale. Acta Mater. 2006, 54, 841–849. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Yu, C.Y.; Liu, C.T. Group precipitation and age hardening of nanostructured Fe-based alloys with ultra-high strengths. Sci. Rep. 2016, 6, 21364. [Google Scholar] [CrossRef] [PubMed]
- Vanderwalker, D.M. The precipitation sequence of Ni3Ti in Co-free maraging steel. Metall. Trans. A 1987, 18, 1191–1194. [Google Scholar] [CrossRef]
- Heo, Y.-U.; Takeuchi, M.; Furuya, K.; Lee, H.-C. Dislocation Assisted Phase Transformation Observed in Iron Alloys. In Advanced Steels; Springer: Berlin/Heidelberg, Germany, 2011; pp. 103–107. [Google Scholar]
- Teng, Z.; Miller, M.; Ghosh, G.; Liu, C.; Huang, S.; Russell, K.; Fine, M.; Liaw, P. Characterization of nanoscale NiAl-type precipitates in a ferritic steel by electron microscopy and atom probe tomography. Scr. Mater. 2010, 63, 61–64. [Google Scholar] [CrossRef]
- Sun, Z.; Song, G.; Ilavsky, J.; Liaw, P.K. Duplex Precipitates and Their Effects on the Room-temperature Fracture Behaviour of a NiAl-Strengthened Ferritic Alloy. Mater. Res. Lett. 2015, 3, 128–134. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takahashi, J.; Kawakami, K. Experimental evaluation of the particle size dependence of the dislocation–particle interaction force in TiC-precipitation-strengthened steel. Scr. Mater. 2012, 67, 854–857. [Google Scholar] [CrossRef]
- Jiao, Z.; Luan, J.; Miller, M.; Liu, C. Precipitation mechanism and mechanical properties of an ultra-high strength steel hardened by nanoscale NiAl and Cu particles. Acta Mater. 2015, 97, 58–67. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W. Quantification of Precipitation Hardening and Evolution of Precipitates. Mater. Trans. 2002, 43, 1273–1282. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation hardening in metals. Mater. Sci. Technol. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Ardell, A.J. Precipitation Hardening. Metall. Trans. A 1985, 16, 2131–2165. [Google Scholar] [CrossRef]
- Orowon, E. Symposium on Internal Stresses in Metals and Alloys. Monogr. Rep. Ser. 1948, 5, 451–453. [Google Scholar]
- Martin, J.W. Precipitation Hardening; Butterworth-Heinemann: Oxford, UK, 1998. [Google Scholar]
- Hornbogen, E.; Gahr, K.-H.Z. Distribution of plastic strain in alloys containing small particles. Metallography 1975, 8, 181–202. [Google Scholar] [CrossRef]
- Russell, K.C.; Brown, L. A dispersion strengthening model based on differing elastic moduli applied to the iron-copper system. Acta Metall. 1972, 20, 969–974. [Google Scholar] [CrossRef]
- Fine, M.; Isheim, D. Origin of copper precipitation strengthening in steel revisited. Scr. Mater. 2005, 53, 115–118. [Google Scholar] [CrossRef]
- Choi, K.; Seo, C.-H.; Lee, H.; Kim, S.; Kwak, J.H.; Chin, K.G.; Park, K.-T.; Kim, N.J. Effect of aging on the microstructure and deformation behavior of austenite base lightweight Fe-28Mn-9Al-0.8C steel. Scr. Mater. 2010, 63, 1028–1031. [Google Scholar] [CrossRef]
- Stoloff, N.S.; Davies, R.G. The Mechanical Properties of Ordered Alloys. Prog. Mater. Sci. 1968, 13, 1–84. [Google Scholar] [CrossRef]
- Kapoor, M.; Isheim, D.; Vaynman, S.; Fine, M.; Chung, Y.-W. Effects of increased alloying element content on NiAl-type precipitate formation, loading rate sensitivity, and ductility of Cu- and NiAl-precipitation-strengthened ferritic steels. Acta Mater. 2016, 104, 166–171. [Google Scholar] [CrossRef]
- Fine, M.E.; Vaynman, S.; Isheim, D.; Chung, Y.-W.; Bhat, S.P.; Hahin, C.H. A New Paradigm for Designing High-Fracture-Energy Steels. Metall. Mater. Trans. A 2010, 41, 3318–3325. [Google Scholar] [CrossRef]
- He, B.B.; Hu, B.; Yen, H.W.; Cheng, G.J.; Wang, Z.K.; Luo, H.W.; Huang, M.X. High dislocation density–induced large ductility in deformed and partitioned steels. Science 2017, 357, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R. Diffusive Phase Transformations in the Solid State. In Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 1996; pp. 1363–1505. [Google Scholar]
- O’Hayre, R. Liquid-Solid and Solid-Solid Phase Transformations. In Materials Kinetics Fundamentals: Principles, Processes, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Wang, Z.; Zhang, H.; Guo, C.; Liu, W.; Yang, Z.; Sun, X.; Zhang, Z.; Jiang, F. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Miyamoto, G.; Shinbo, K.; Furuhara, T.; Ohmura, T.; Suzuki, T.; Tsuzaki, K. Effects of transformation temperature on VC interphase precipitation and resultant hardness in low-carbon steels. Acta Mater. 2015, 84, 375–384. [Google Scholar] [CrossRef]
- Lee, H.M.; Allen, S.M.; Grujicic, M. Coarsening resistance of M2C carbides in secondary hardening steels: Part II. Alloy design aided by a thermochemical database. Metall. Trans. A 1991, 22, 2869–2876. [Google Scholar] [CrossRef]
- Kesternich, W. Dislocation-controlled precipitation of TiC particles and their resistance to coarsening. Philos. Mag. A 1985, 52, 533–548. [Google Scholar] [CrossRef]
- Hardy, H.K.; Heal, T.J. Nucleation-and-Growth Processes in Metals and Alloys. In Proceedings of the Symposium on the Mechanism of Phase Transformations in Metals, London, UK, 9 November 1955. [Google Scholar]
- Wilson, D. Effects of plastic deformation on carbide precipitation in steel. Acta Metall. 1957, 5, 293–302. [Google Scholar] [CrossRef]
- Mulholland, M.D.; Seidman, D.N. Multiple dispersed phases in a high-strength low-carbon steel: An atom-probe tomographic and synchrotron X-ray diffraction study. Scr. Mater. 2009, 60, 992–995. [Google Scholar] [CrossRef]
- Cemal, M.; Cevik, S.; Uzunonat, Y.; Diltemiz, F. ALLVAC 718 Plus™ Superalloy for Aircraft Engine Applications. In Recent Advance in Aircraft Technology; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Song, G.; Sun, Z.; Li, L.; Xu, X.; Rawlings, M.; Liebscher, C.H.; Clausen, B.; Poplawsky, J.; Leonard, D.N.; Huang, S.; et al. Ferritic Alloys with Extreme Creep Resistance via Coherent Hierarchical Precipitates. Sci. Rep. 2015, 5, 16327. [Google Scholar] [CrossRef]
- Tortorelli, P.; Unocic, K.; Wang, H.; Santella, M.; Shingledecker, J. Ni-Based Alloys for Advanced Ultrasupercritical Steam Boilers. In Proceedings of the Fossil Energy Crosscutting Research Program Review, Pittsburgh, PA, USA, 25 April 2015. [Google Scholar]
- Gleiter, H. Microstructure. In Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 1996; Volume 1, pp. 844–942. [Google Scholar]
- Balliger, N.K.; Honeycombe, R.W.K. Coarsening of vanadium carbide, carbonitride, and nitride in low-alloy steels. Met. Sci. 1981, 14, 121–133. [Google Scholar] [CrossRef]
- Jiao, Z.; Luan, J.; Guo, W.; Poplawsky, J.; Liu, C. Effects of welding and post-weld heat treatments on nanoscale precipitation and mechanical properties of an ultra-high strength steel hardened by NiAl and Cu nanoparticles. Acta Mater. 2016, 120, 216–227. [Google Scholar] [CrossRef]
- West-East Gas Pipeline Project. Available online: http://www.hydrocarbons-technology.com/projects/west-east/ (accessed on 16 March 2018).
- Line Pipe Deals Afoot for China’s WEPP-2. Available online: http://www.texreport.co.jp/xenglish/eng-genryou/200708/200708161059Thu-1.html (accessed on 16 March 2018).
- High Strength Low Alloy (HSLA) Steel. Available online: http://www.imoa.info/molybdenum-uses/molybdenum-grade-alloy-steels-irons/high-strength-low-alloy-steel.php (accessed on 2 November 2017).
- Base Metals Investing—Prices, Mining Stocks and News. Available online: http://www.infomine.com/investment/base-metals/ (accessed on 6 November 2017).
- Minor Metals Prices. Available online: http://original.metal.com/metals/minor-metals/prices (accessed on 7 November 2017).
- Dhua, S.K.; Mukerjee, D.; Sarma, D.S. Influence of tempering on the microstructure and mechanical properties of HSLA-100 steel plates. Metall. Mater. Trans. A 2001, 32, 2259–2270. [Google Scholar] [CrossRef]
- Kuzmina, M.; Ponge, D.; Raabe, D. Grain boundary segregation engineering and austenite reversion turn embrittlement into toughness: Example of a 9 wt % medium Mn steel. Acta Mater. 2015, 86, 182–192. [Google Scholar] [CrossRef]
- Xie, Z.; Yuan, S.; Zhou, W.; Yang, J.; Guo, H.; Shang, C. Stabilization of retained austenite by the two-step intercritical heat treatment and its effect on the toughness of a low alloyed steel. Mater. Des. 2014, 59, 193–198. [Google Scholar] [CrossRef]
- Hickey, J.; Bulloch, J. The role of reverse temper embrittlement on some low and high temperature crack extension processes in low carbon, low alloy steels: A review. Int. J. Press. Vessels Pip. 1992, 49, 339–386. [Google Scholar] [CrossRef]
- Eliaz, N.; Shachar, A.; Tal, B.; Eliezer, D. Characteristics of hydrogen embrittlement, stress corrosion cracking and tempered martensite embrittlement in high-strength steels. Eng. Failure Anal. 2002, 9, 167–184. [Google Scholar] [CrossRef]
- Singh, R. Weld Cracking in Ferrous Alloys; Woodhead Publishing Limited: Cambridge, UK, 2008. [Google Scholar]
- Tsuji, N.; Okuno, S.; Koizumi, Y.; Minamino, Y. Toughness of Ultrafine Grained Ferritic Steels Fabricated by ARB and Annealing Process. Mater. Trans. 2004, 45, 2272–2281. [Google Scholar] [CrossRef]
- Watanabe, T. Grain boundary design and control for high temperature materials. Mater. Sci. Eng. A 1993, 166, 11–28. [Google Scholar] [CrossRef]
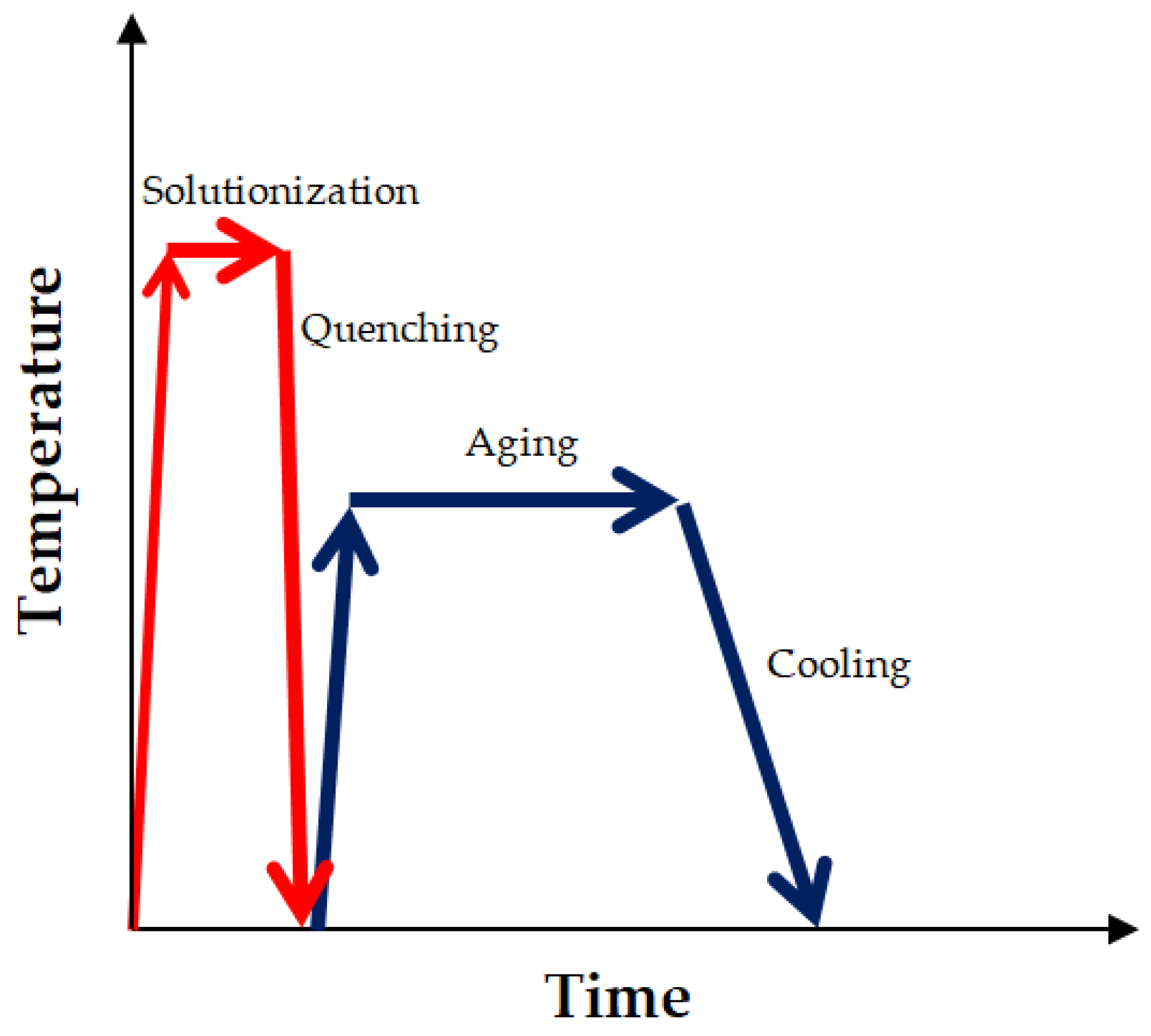
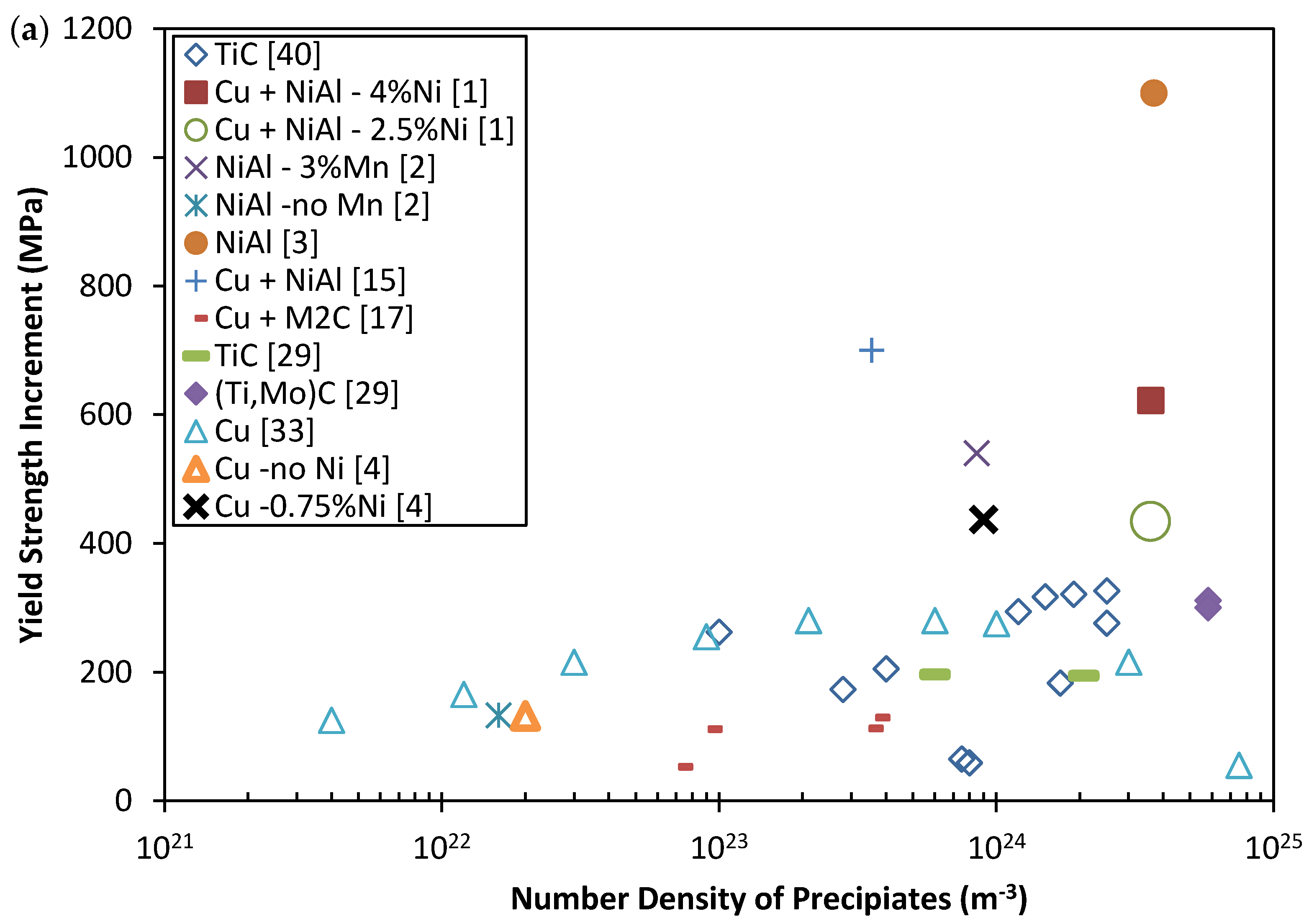
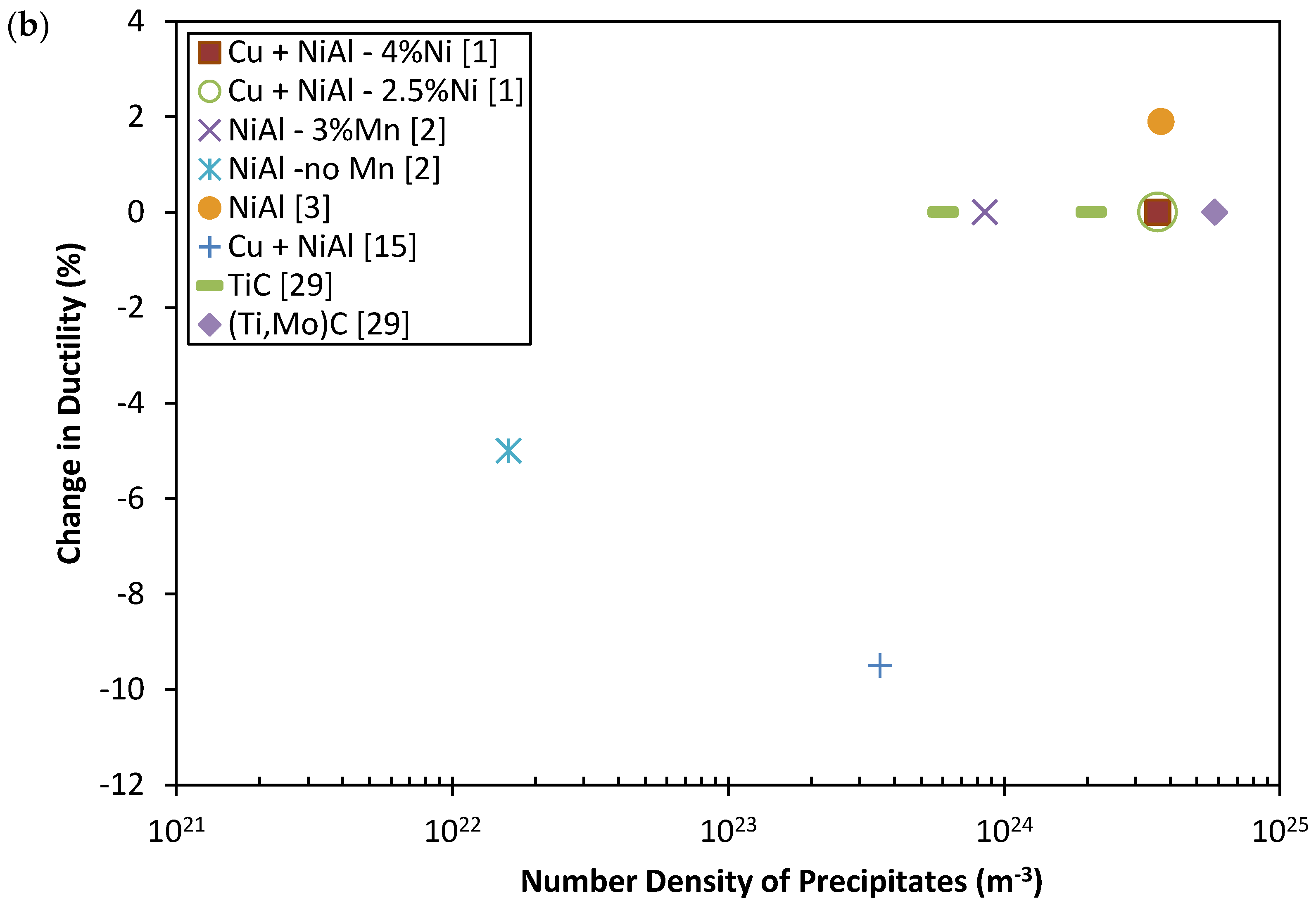
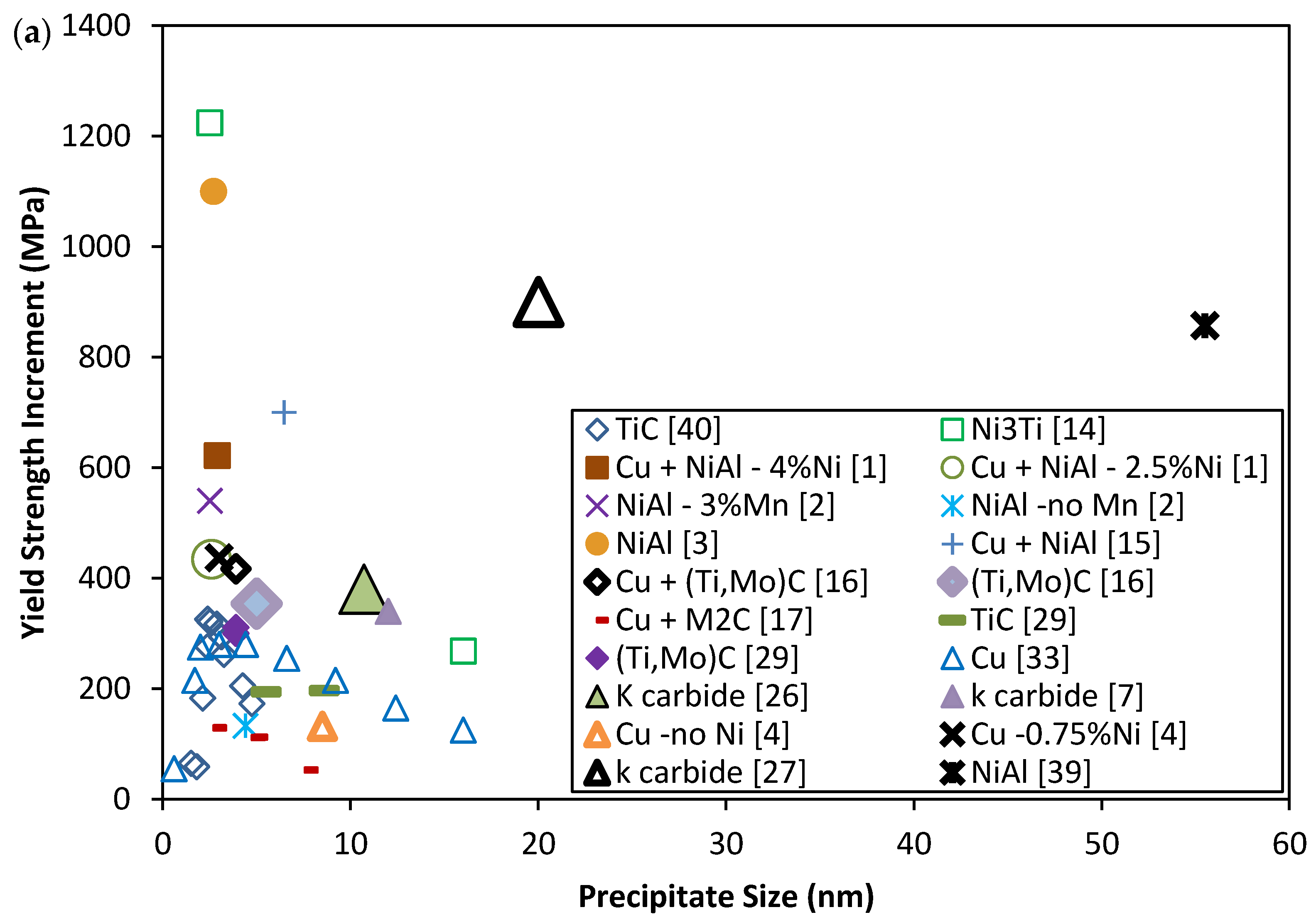

| Parameters | |||||
|---|---|---|---|---|---|
| Solutionization | Quench Medium | Aging | Ref. | ||
| Temperature (°C) | Duration (h) | Temperature (°C) | Duration (h) | ||
| 900 | 1 | Water | 500 | 10 | [1] |
| 900 | 0.5 | Water | 550 | 2 | [2] |
| 950 | 0.25 | Water | 500 | 3 | [3] |
| 900 | 0.5 | Argon Gas | 550 | 2 | [4] |
| 1050 | 2 | Water | 550 | 100 | [6] |
| 1200 | 2 | Water | 550 | 3 | [26] |
| 1150 | 5 | Water | 550 | 16 | [7] |
| 1110 | 2 | Water | 600 | 24 | [27] |
| Types | Structure | Precipitate Composition | Distribution | Shape |
|---|---|---|---|---|
| κ-carbides | L’12 [28] | (Fe2Mn1)(Mn0.09Al0.91)(C0.61Vac0.39) [28] (Fe,Mn)3AlC0.38 [6], (Fe,Mn)3AlC0.51 [6] | Homogeneously throughout matrix [6,26]; Grain boundaries (overaged) [26] | Cuboidal [6]; Plate-like (at grain boundaries) [26] |
| MC carbides | NaCl-type FCC [29] | TiC [29]; (Ti,Mo)C [29]; NbC [30,31]; VC [30]; (Ti,Nb)C [32] | Distributed in rows within a ferrite grain [29]; Co-located with Cu precipitates [31] | Disk-like [29,31] |
| Cu | BCC (<2 nm) [20,33] → 9R (>4 nm) * [20,33] → Detwinned 9R (24–26 nm) [20] or 3R [22] → FCC (37 nm) [20]; BCC → 9R → twinned FCC [19] | Particles enriched with Cu, Fe, Al, Ni and Mn with Ni, Al, and Mn segregation at the particle/matrix interface [4,15,34,35] | Homogenously throughout matrix [4,33,35]; Co-located with M2C on lath boundaries and dislocations [17]; Co-located with NbC, grain boundaries, and Fe3C [31]; Co-located with NiAl [15,35] | Spherical (B2 structure) [15,35]; Elongated (9R structure) [15] |
| M2C carbides | Hexagonal [18] | (Mo,Cr)2C [17]; (Mo,Cr,V)2C [30] | Co-located with Cu on lath boundaries and dislocations [17] | Rod (coherent) [17]; Irregular (incoherent) [17] |
| Ni3Ti | Hexagonal (DO24) [36,37] → L12 [37] → FCC [37] | (Ni,Fe,Co)3(Ti, Mo) [14] | Homogeneously throughout matrix, heterogeneous nucleation on interphases, dislocations and grain boundaries [36] | Disc and rod [14,36] |
| NiAl | B2 [3,15] | Ni, Al, Mn, Fe, Cu [15,35]; Ni, Al, Fe, Mn [2]; Ni(Al,Fe) [3]; Ni, Al, Fe, Cr, Mo [38] | Co-located with Cu [15]; Mostly homogeneously distributed in matrix and some elongated particles on dislocations [3] | Spherical [2,3]; Elongated [3] |
| Precipitate | Steel Composition (wt %) | Yield Strength (MPa) | Uniform Elongation (%) | Reference |
| NiAl | Fe-18Ni-3Al-4Mo-0.8Nb-0.08C-0.01B | 1947 | 3.8 | [3] |
| Cu and NiAl | Fe-2.5Cu-2.1Al-1.5Mn-4Ni | 1363 | 12 | [1] |
| NiAl | Fe-5Ni-1Al-3Mn | 1225 | 14 | [2] |
| Cu | Fe-0.75Cu-2Cu-0.75Mn-0.3Al-2.25Cr-1Mo-0.25V-0.07Ti-0.3Si-0.01B-0.08C | 1042 | 9 | [4] |
| NiAl | Fe-6.5Al-10Ni-10Cr-3.4Mo-0.25Zr-0.005B | 1015 | <1 | [39] |
| κ-carbides | Fe-30.5Mn-8Al-1.2C | 990 | 37 | [27] |
| κ-carbides | Fe-29.8Mn-7.65Al-1.11C-0.093Si-0.0083Ni | 880 | 26 | [7] |
| Cu and (Ti,Mo)C | Fe-1.53Mn-1.17Cu-0.34Si-0.21Mo-0.09Ti-0.04Al-0.07C | 732 | 13 | [16] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.J.; Liu, C.T. A Review on Nano-Scale Precipitation in Steels. Technologies 2018, 6, 36. https://doi.org/10.3390/technologies6010036
Kong HJ, Liu CT. A Review on Nano-Scale Precipitation in Steels. Technologies. 2018; 6(1):36. https://doi.org/10.3390/technologies6010036
Chicago/Turabian StyleKong, Hao Jie, and Chain Tsuan Liu. 2018. "A Review on Nano-Scale Precipitation in Steels" Technologies 6, no. 1: 36. https://doi.org/10.3390/technologies6010036
APA StyleKong, H. J., & Liu, C. T. (2018). A Review on Nano-Scale Precipitation in Steels. Technologies, 6(1), 36. https://doi.org/10.3390/technologies6010036





