1. Introduction
Teaching Biochemistry is usually a difficult task since it requires a great deal of abstract thinking and different approaches should be used to enable the proper understanding of different subjects [
1,
2]. In particular, for the visually impaired or blind students, the understanding of Biochemistry can be far more complex, and important adjustments need to be done for their proper inclusion.
Many authors believe that visual literacy is essential in the teaching and learning process in general (for a review see ref. [
3]). Schönborn and Anderson [
4] emphasize the importance of visual literacy to the understanding of biochemical processes. Locatelli et al. [
5] consider visualization as a crucial element in learning chemistry, and suggest the need of “
a metavisual capability in all levels and modes of representation to really understand concepts in chemistry”. According to Teruya et al. [
6], chemistry knowledge is intrinsically multimodal, implying that for any verbal representation, there is a corresponding visual representation of the content. Costa et al. [
7], for instance, indorse that “
computational Biology and Bioinformatics are indispensable components in the training of life scientists”.
On the other hand, interesting suggestions in chemistry education for students with disabilities have been reported [
8,
9,
10,
11,
12]. As Miner et al. [
8] pointed out, visual impairment occurs in different forms and degrees of severity, which demands flexibility. These authors also call attention to the fact that visually impaired students are not helpless, and suggest that simple accommodations should be pursued for a full participation of the student.
Hiemenz and Pfeifer [
9] described an adaptation of a triple-beam balance where pre-determined weights are notched, causing a clicking noise as the weight slides over the notched beam, which allows the students to weight objects with an uncertainty of 0.2 g. Supalo et al. [
10,
11,
12] show that the instructors can use some simple resources to teach chemistry to the blind and visually impaired students. For instance, the use of a slate, tracing wheel, hot glue, and thermo pen to make tactile drawings [
10], or commercially available techniques to represent graphs or organic molecules [
11], in addition to a plastic syringe with calibrated volumes notched in the plastic pull tab [
12].
Computer based assisted technologies to teach science to visually impaired and blind students have been reported since the early 1980s [
13,
14,
15,
16,
17,
18]. The microcomputer-based Universal Laboratory Training and Research Aid (ULTRA) was developed to aid blind students in undergraduate chemistry laboratories, and could be interfaced with a wide variety of instruments and sensors that provided an analog signal for a chart recorder or a digital output [
13,
14]. Later on, Morrison et al. [
15] described extensions made to the ULTRA system by adapting it to accept voice commands, the Voice-Operated Isolated Command Entry (VOICE) terminal. Tomlinson et al. [
16] developed the software Graph and Number line Input and Exploration software (GNIE), of auditory graphs that present an alternative to visual graphs to teach mathematics. Successful applications of the Sci-Voice Talking LabQuest software to engage visual impaired and blind students in hands-on science learning activities have also been reported [
17,
18]. It has been suggested by Supalo et al. [
19] that the use of Job Access with Speech (JAWS), combined with data collection software to enable blind and visually impaired students to read data aloud. Nevertheless, Johnson and Ruppert [
20] reported a few problems related to JAWS that compromised its use by persons with disabilities.
In this work, we will focus on a case study of one undergraduate blind student of Biology from a distance education division of our University that enrolled Biochemistry 1, at the Center of Distance Education of the State of Rio de Janeiro (CEDERJ). CEDERJ offers 15 higher education courses in 33 core units widespread throughout the State of Rio de Janeiro. Tutoring support is provided from both distance and face-to-face interactions. The distance communication between students and tutors occurs in a learning platform based on Moodle, or by a toll-free number. There are two types of face-to-face components in these courses, and laboratory classes are compulsory activities.
Biochemistry 1 offers a weekly 4 hours face-to-face meeting (optional), and two laboratory classes (compulsory) during one academic term of one semester. It covers the basics of structure and function of macromolecules, and one of the laboratory classes is about enzymes. Here we show the adaptation of laboratory protocols and 3D graphical representations of enzyme properties to a blind student using low-cost materials. In order to check the enzyme activity, we used samples that could be analyzed by touching. The enzyme source was pineapple juice that is rich in bromelain [
21], a cysteine protease. As substrates, we used gelatin and reconstituted powdered milk, both good sources of protein.
Gelatin is obtained by heat dissolution at alkaline or acidic pH, and partial hydrolysis of collagen in animal skins, bones and tendons [
22]. Gelatin presents colloidal properties, and it is a sol (resembles a liquid) at temperatures higher than 35 °C, and is a gel when cooled. An additional hydrolysis of the peptides in the gelatin solution by bromelain prevents gel formation, even at low temperatures.
Casein, the major protein in milk, is a complex set of different proteins that form large colloidal particles called casein micelles [
23]. Fluorescence light scattering suggested that submicellar aggregates containing 20–30 monomers associated to form micelles, in which hydrophobic interactions play an important role [
24]. Therefore, hydrolysis of the casein micelle exposes their hydrophobic interior, which, in turn, leads to milk clot.
2. Procedures
2.1. Preparation of Pineapple Juice and Gelatin
Pineapple chunks were blended until a smooth purée was formed (100 g pineapple flesh + 10 mL Milli-Q water). Large fibers were separated with a sieve and the remaining juice was filtered in a funnel containing two layers of silk or a Jelly strainer bag. This prep yields about 40 mL of pineapple juice (One medium pineapple yields ~400 mL of juice). The juice can be stored in the freezer for at least two months without significant changes in the bromelain activity.
2.2. Enzyme Activity Using Gelatin as Substrate
A 12 g pack (or two 0.25 oz. packs) (This is the amount in one pack of unflavored gelatin in Brazil. It can be substituted by 2 packs of 0.25 oz. each (~14 g)) of unflavored gelatin was added to 250 mL of boiling water in a 600 mL beaker. After the gelatin was well dissolved, 250 mL of ice-cold water was added. The final solution was at 30 °C and ready to be used in the experiments, in the sol state. Samples of 20 mL of gelatin solution (unset gelatin) were placed in 50 mL centrifuge tubes. In order to start the reaction, 3 mL of fresh pineapple juice was added. The samples were homogenized and then left in the fridge (8–10 °C) for 30 min. After hydrolysis, the gelatin was unable to set in the gel state in the refrigerator, remaining in a liquid state. The consistency of the gelatin was checked by the blind student in two different ways: by feeling the consistency of the samples with glass rods, or by shaking the closed tubes (up and down and/or back and forth). For the experiment of thermal denaturation of the enzyme, 10 mL of pineapple juice was placed in boiling water for 5 min in order to denature the enzyme, and transferred to crushed ice for 5 min before using. Control experiments were done by the addition of water instead of pineapple juice.
2.3. Milk Clot Procedure
After filling ice candy bags with powdered whole milk with the help of a funnel, we added of Milli-Q water to each bag (see
Section 2.4 and
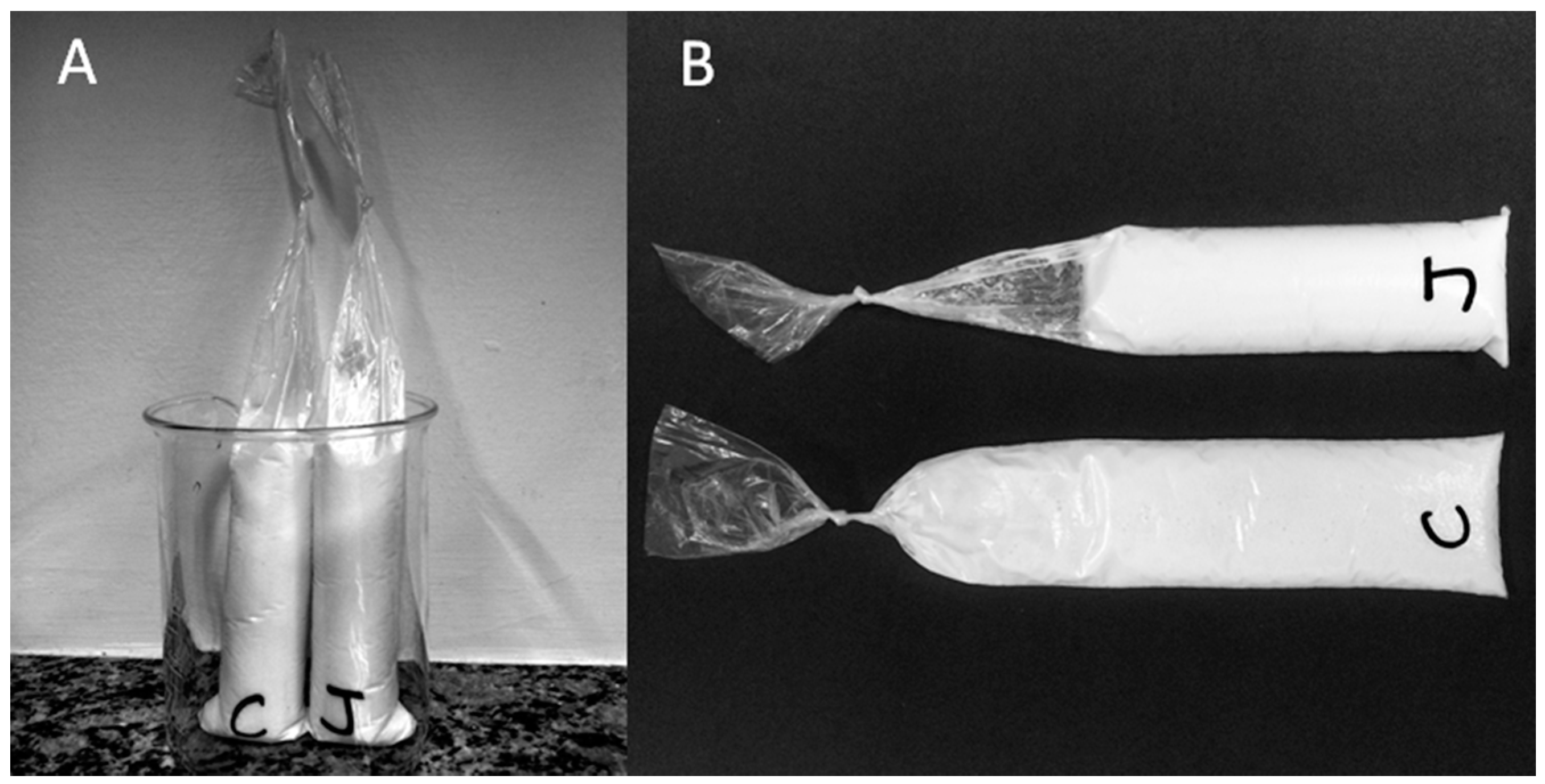
Section 2.5 for quantities). Reconstituted dry milk was obtained by hand mixing the samples until the milk was well dissolved. To each bag we added 5 mL of Mill-Q water (control experiments) or fresh pineapple juice. Hand mixing followed every addition until a homogeneous sample was obtained. Before knotting the bag, the excess of air was carefully removed. The bags of the samples containing pineapple juice were marked with a cut in the opening to enable the identification by the blind student (see
Figure 1B).
The samples were left inside of a beaker (
Figure 1A) on the bench for 20 min at room temperature (22 °C), and the milk consistency was checked by touching the bags. Milk clot was observed in the samples with native bromelain (positive reaction).
Figure 1B shows the consistency of the milk in two samples after the reaction took place: in the absence (C) and in the presence (J) of pineapple juice.
2.4. Effect of Enzyme Concentration
This experiment was done with the milk cloth assay. For the reconstitution of powdered milk, four solutions of enzyme in different concentrations were obtained in graduated glass cylinders, as follows: (1) Control: 30 mL of water; (2) 5% Bromelain: 28.5 mL Mill-Q water + 1.5 mL pineapple juice; (3) 10% Bromelain: 27 mL Milli-Q water + 3 mL pineapple juice; and (4) 20% Bromelain: 24 mL Milli-Q water + 6 mL pineapple juice. The solutions were transferred from the cylinders to 4 ice candy bags, containing 20 g of powdered whole milk each. After homogenization, milk consistency was checked every 5 min. It is important to notice that, since the results relies on the verification of the milk consistency by touch, and the student needs to compare all the samples at the same time, this procedure needs the help of other students—we had four students adding the solutions at the same time to the four bags, so the time of the reaction was the same in each sample.
2.5. Effect of Substrate Concentration
Three ice candy bags were filled with 12 g, 16 g, and 20 g of powdered whole milk with the help of a funnel. To each bag, 20 mL of Milli-Q water was added. Reconstituted dry milk was obtained by hand mixing the samples until the milk was well dissolved. To each bag, we added 5 mL fresh juice. The same procedure was done with control samples where 5 mL Milli-Q water was added. Hand mixing followed every addition until a homogeneous sample was obtained. As described above, we carefully took the air out of the bag before tying a knot. The consistency of the milk was checked every 5 min by touching the bags.
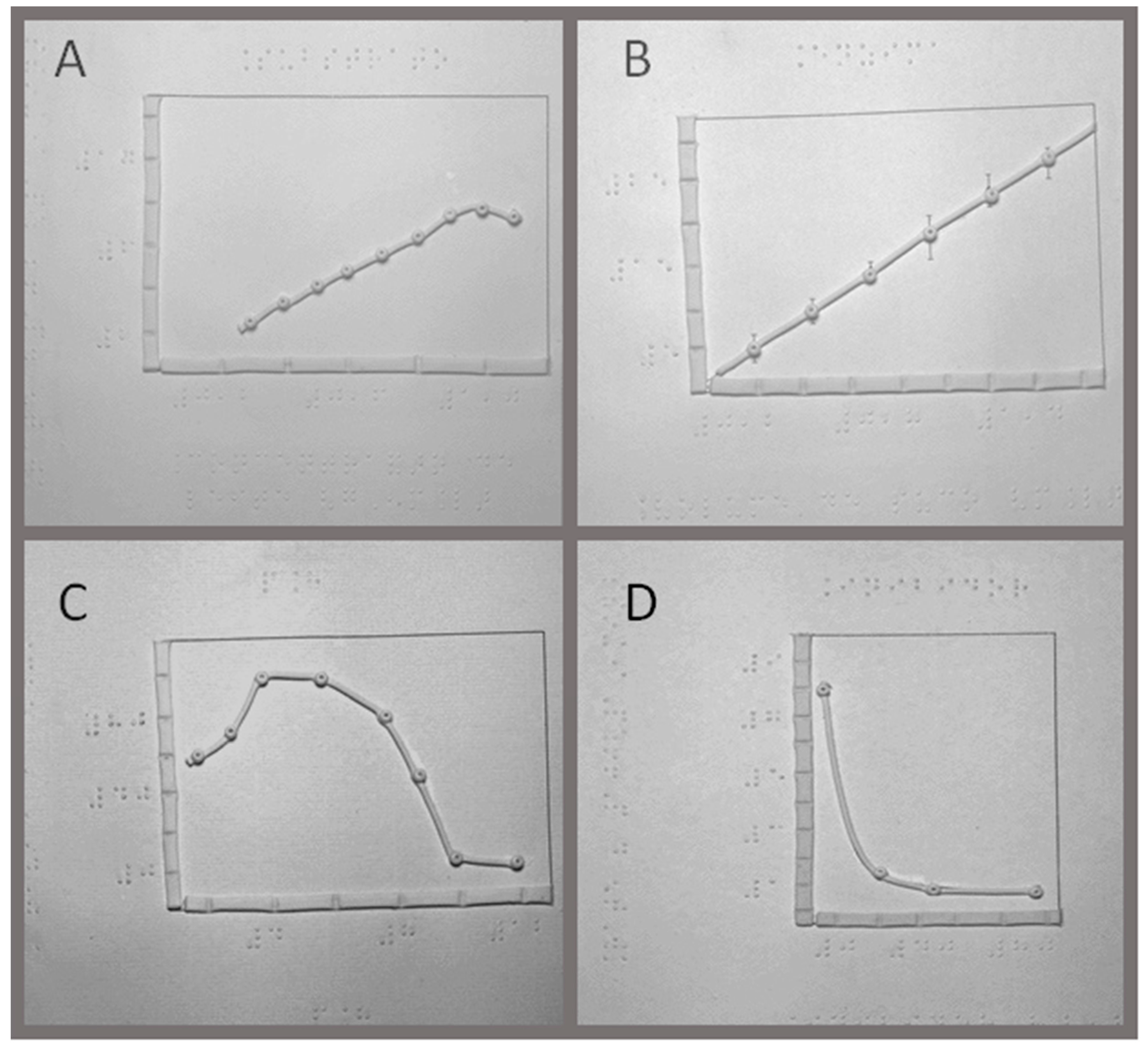
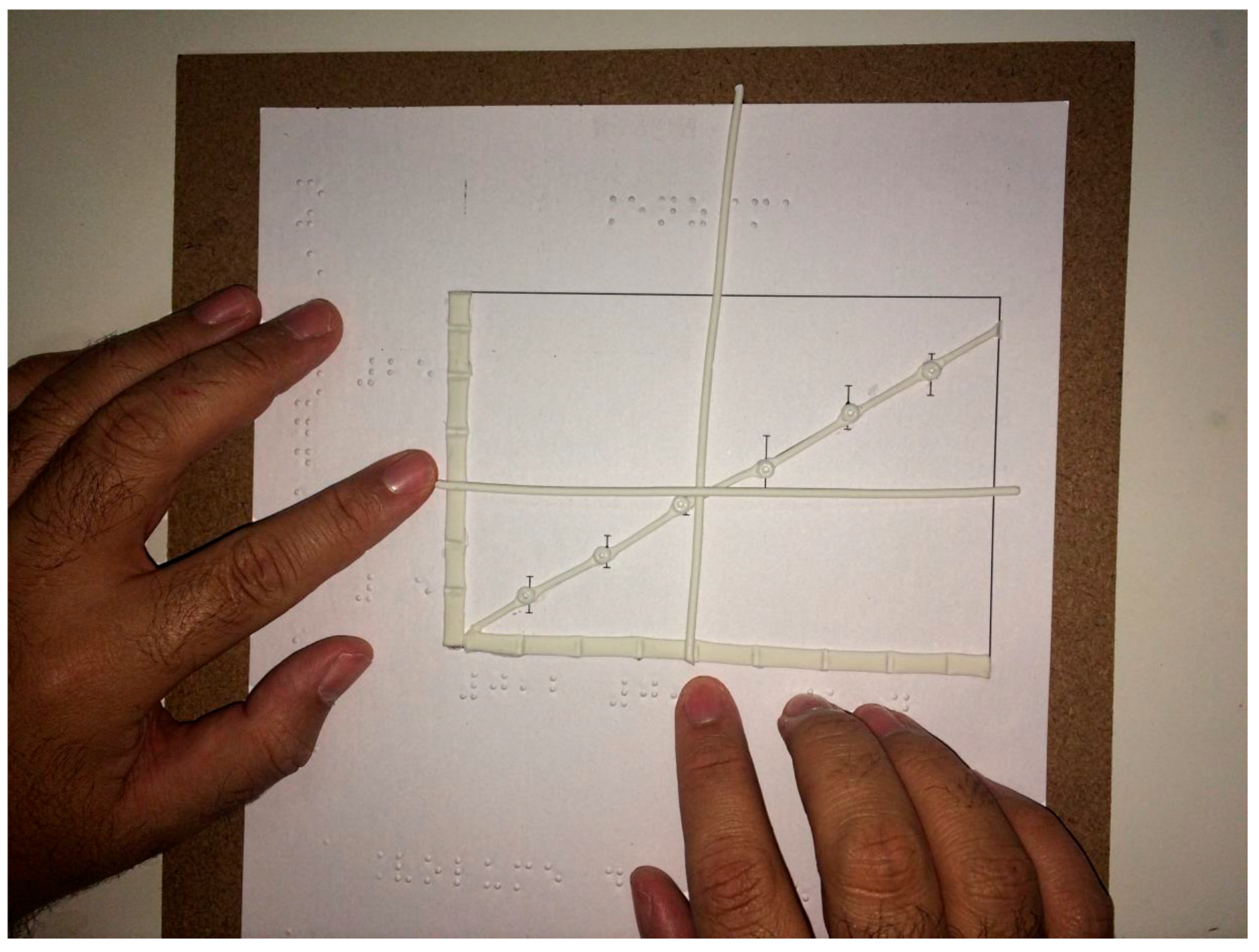
2.6. Graphical Representations of the Data
Plots were done with cold porcelain (Lycin Mercantil Industrial, Ltda, São Paulo, SP, Brazil), and glued on 10 × 10 inches boards of medium-density fiberboard (MDF) over graph representations of different enzyme properties, such as the effect of substrate concentration, enzyme concentration, pH, and inhibitor. The graphs were plotted in Origin® 7 (MicroCal, Northampton, MA, USA) and printed without the numbers. The axis and curves were covered with cold porcelain and let dry for 24 h at room temperature (22 °C). The numbers and the legends were written in Braille, with a positive slate from TECE (Tecnologia e Ciência Educacional Ltda). This is a reverse slate that unlike the conventional instrument that allows the writing from left to right in high-relief, when a stylus is pushed against the paper between the two slates. After gluing the paper to the boards, the axes and the curves were done with the help of an extruder for cold porcelain so that every piece was standardized, and the pieces were glued over the drawings.
4. Discussion
Educational technologies (ET) seem to be related to the applications of mechanical and material tools, such as computers and software. However, according to Lakhana [
28], “
a more complex conception of ET includes immaterial tools, such as processes and ways of thinking, (…), whereby technology is not merely processes and tools, but is understood systematically”. This author [
20] suggests a broader interpretation of technologies, dismissing myths that limit ET to matters of hardware. In addition, the definition of ET by the Association for Educational Communication & Technology includes both hard and soft technologies, the latter meaning “intellectual processes”, i.e., transformative methods or actions that facilitate learning and performance [
28]. In this way, the protocols described here for a blind student can be defined as soft technology.
In order to check the enzyme activity, we used the samples in ice candy bags that could be analyzed by touching. The use of plastic bags to show chemical reactions was already described by Wedler et al. [
29] in which the instructors demonstrated a series of endothermic/exothermic, as well as acid−base reactions through the so-called “baggie experiments”.
The enzyme source was pineapple juice that is rich in bromelain [
21], a cysteine protease. As substrates, we used gelatin and reconstituted powdered milk. Nevertheless, in the short time to develop the protocols, we decided to have the assistance of a tutor in the preparation of the samples (weighting and/or measuring volumes). However, it would be interesting to use the adapted triple-beam balance [
9] and the syringe with notched values in the plastic pull tab [
12].
The gelatin, used as a substrate forms a colloidal solution when properly dissolved in water. As a colloid, the gelatin is a sol (liquid) at 35 °C and higher, and a gel at low temperatures. In the absence of bromelain, the gelatin was able to form a gel after 30 min in the refrigerator. However, when hydrolysis took place, the gelatin was unable to form the gel, and the consistency of the sample was liquid. On the contrary, when reconstituted powdered milk was used, casein hydrolysis by bromelain caused precipitation (milk clotting), while the control sample remained liquid.
This is very interesting since opposite effects are seen after hydrolysis of the substrates. The tactile approach allowed for a perfect understanding of the effects of the protease in different proteins, such as the gelatin and casein in milk.
The proper understanding of a graphical representation requires a first step related to data analysis, and a choice of the best plot type (scatter/line/column/bar) that helps to accurately communicate the meaning of the data. Patterson and Leonard [
30] suggest that graphical representations involve two different types of thinking: analytical, logical thinking, and creative, holistic thinking.
The ability to read and interpret graphics, for instance, is one of the most difficult tasks for the instructors to teach. Students, in general, are not able to understand the general aspects of a graph and the interpretation of the data in several types of graphical representations. This is far more difficult to the blind or visually impaired student. Therefore, it is crucial that new instructional tools are developed in order to help these students.
Supalo [
31] draws attention to the fact that although many new instructional materials, and/or teaching strategies are being developed, they are not intended towards the blind student. Software and models are commonly found, but graphical representations need to be drawn in a paper using an embossed design, and specialized printers are expensive and are never always available. A low-cost strategy suggests the use of a tracing wheel or hot glue to draw a graph [
10]. Poon et al. [
24] suggested the use of wax-covered string manipulatives to represent molecular formulas, orbitals, and graphs. These authors used a commercial product known as Wikki Stix (Omnicor, Inc., Phoenix, AZ, USA), a pliable combination of wax and yarn [
32]. Draftsman, from the American Printing House for the Blind (APH), is a commercial product that can be used to draw graphs with a stylus over a sheet of plastic paper placed on its rubber surface [
11].
The computer based technologies to assist blind and visual impaired students [
13,
14,
15,
16,
17,
18,
19] are very useful. However, the use of these tools requires not only the computer itself, but also the expertise many visually impaired and blind students do not have [
17]. In addition, the high cost of some software can be a limitation in certain Schools and Colleges. This is the case of CEDERJ, a Public University that usually faces serious financial problems, and one of the reasons why we developed low-cost experiments and graphical representations.
Teachers are not usually prepared to adapt their practice to teaching students with visual impairment or blindness in inclusive classrooms. An actual inclusion represents a real challenge for the instructors and for the other students. It is our understanding that the many approaches described in the literature are valid and should be encouraged. Nevertheless, the possibility of using low-cost materials increase the chance of instructors to adapt their classes to blind students.