Mass Proportion, Bioactive Compounds and Antioxidant Capacity of Carrot Peel as Affected by Various Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analytical Chemicals
2.3. Determination of Mass Proportion of Carrot Root
2.4. Preparation of Carrot Peel Extracts
2.5. Determination of Bioactive Compounds of Carrot Peel
2.5.1. Total Phenolic Content (TPC)
2.5.2. Saponin Content
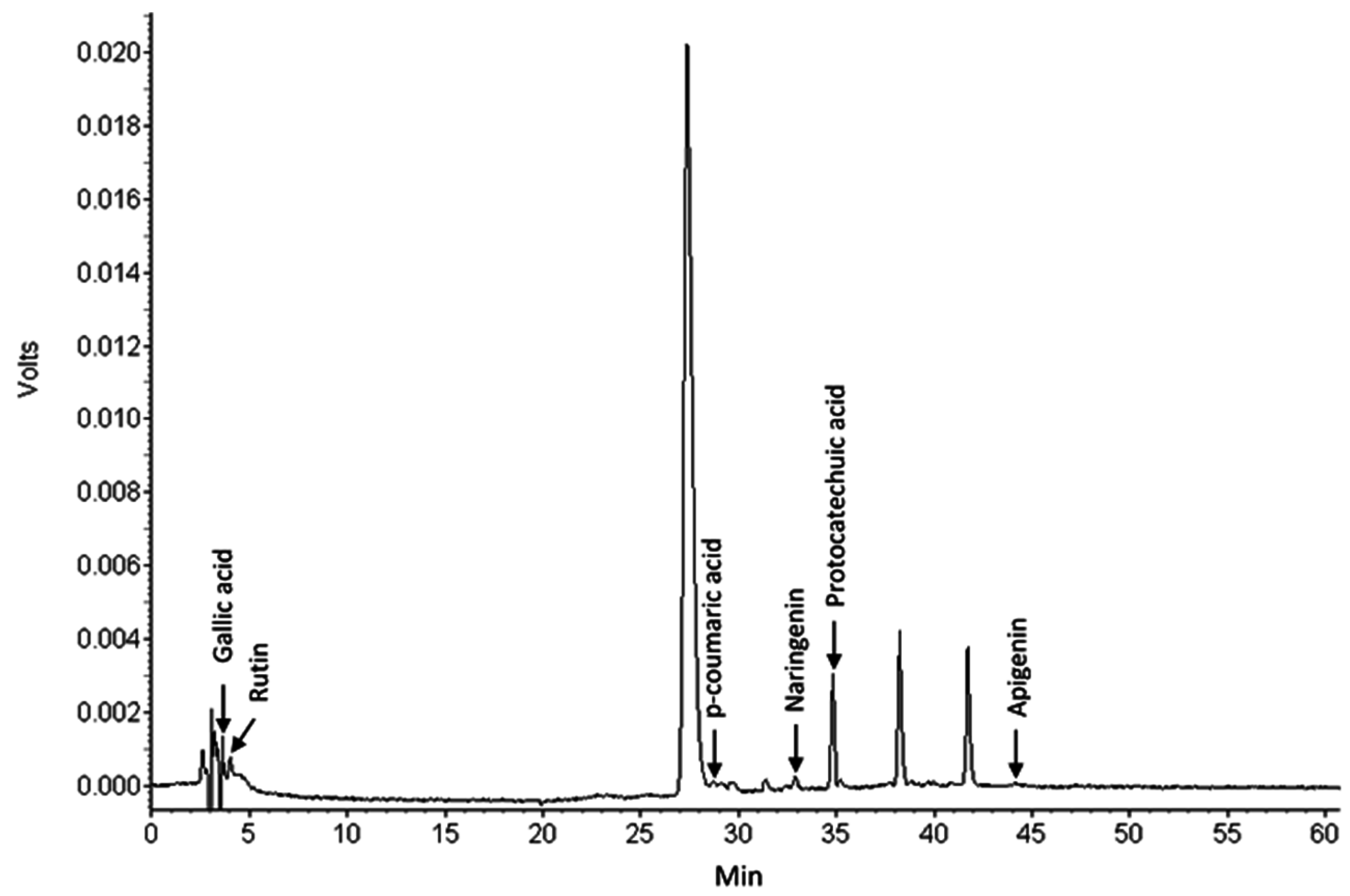
2.5.3. Identification of Individual Bioactive Compounds within Carrot Root Extractn
2.6. Determination of Antioxidant Capacity of Carrot Root
2.6.1. ABTS Radical Scavenging Capacity (ARSC)
2.6.2. DPPH Radical Scavenging Capacity (DRSC)
2.6.3. Determination of Cupric Ion Reducing Antioxidant Capacity (CUPRAC)
2.6.4. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Mass Proportion of Carrot Root
3.2. Effect of Solvents on Extraction Yield and Bioactive Compounds of Carrot Peel
3.2.1. Extraction Yield
3.2.2. Total Phenolic Content
3.2.3. Saponin Content
3.2.4. Individual Bioactive Compounds within Carrot Root
3.3. Effect of Solvents on Antioxidant Capacity of Carrot Peel
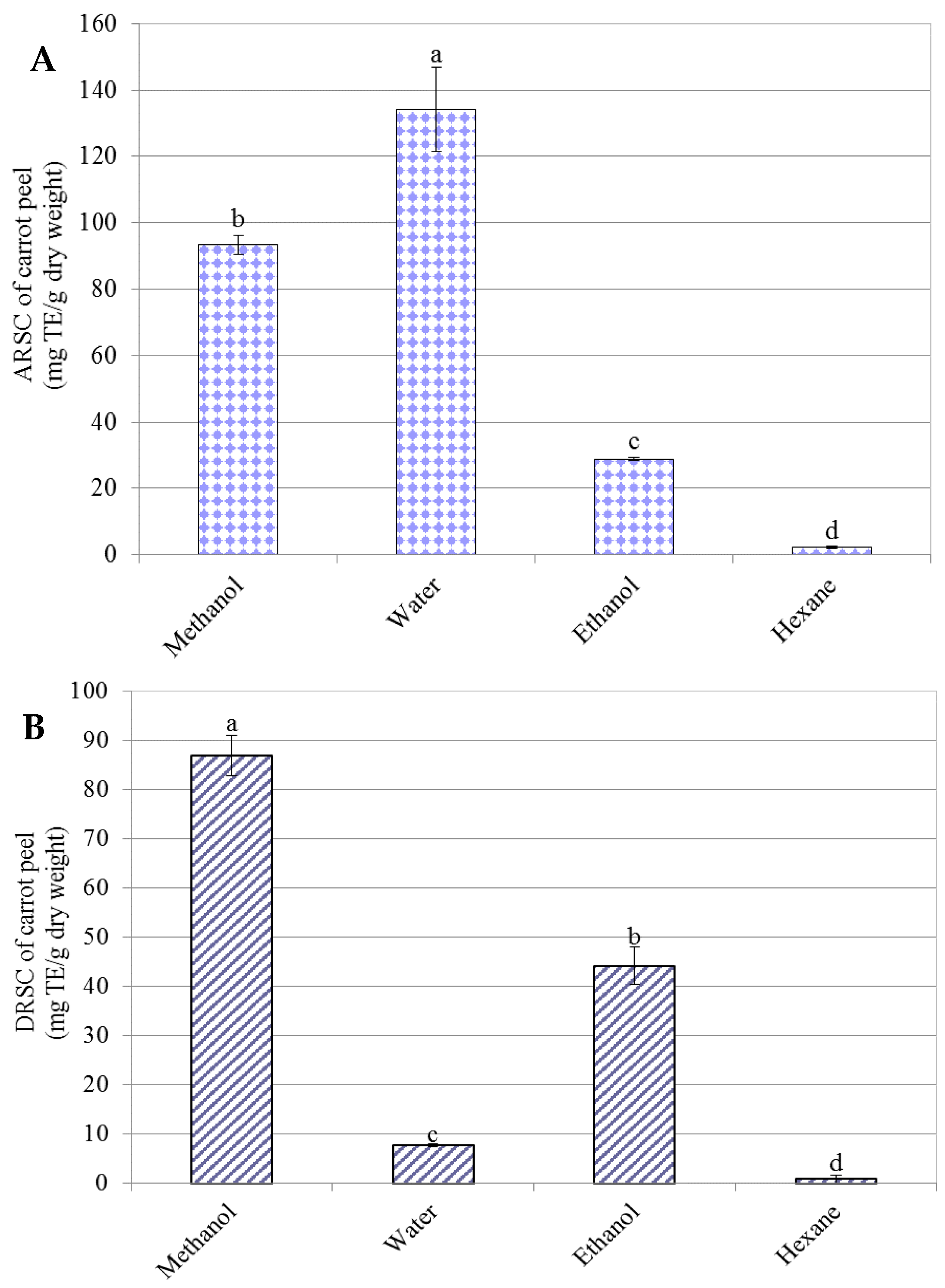
3.3.1. ABTS Radical Scavenging Capacity (ARSC)
3.3.2. DPPH Radical Scavenging Capacity
3.3.3. Cupric Ion Reducing Antioxidant Capacity
3.3.4. Ferric Reducing Antioxidant Power
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naumovski, N. Bioactive composition of plants and plant foods. In Plant Bioactive Compounds for Pancreatic Cancer Prevention and Treatment; Scarlett, C.J., Vuong, Q.V., Eds.; Nova Science Publishers: New York, NY, USA, 2014; pp. 81–115. [Google Scholar]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Pham, N.M.Q.; Vuong, Q.V.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Phytochemical retention and antioxidant capacity of xao tam phan (Paramignya trimera) root as prepared by different drying methods. Dry. Technol. Int. J. 2016, 34, 324–334. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Pham, H.N.T.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Influence of solvents and novel extraction methods on bioactive compounds and antioxidant capacity of Phyllanthus amarus. Chem. Pap. 2016, 70, 556–566. [Google Scholar] [CrossRef]
- Arlorio, M.; Coïsson, J.D.; Travaglia, F.; Varsaldi, F.; Miglio, G.; Lombardi, G.; Martelli, A. Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res. Int. 2005, 38, 1009–1014. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Bhakyaraj, R.; Singaravadive, K. Minerals and bioactive compounds in cashew apple (Anacardium occidentale L.). J. Food Res. Sci. 2012, 1, 32–36. [Google Scholar]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Rubilar, M.; Scheuermann, E.; Cancino, B.; Uquiche, E.; Garcés, M.; Inostroza, K.; Shene, C. Bioactive compounds of spent coffee grounds, a coffee industrial residue. In Proceedings of the Symposium on Agricultural and Agroindustrial Waste Management (III Siger), Sao Pedro, Brazil, 12–14 March 2013; pp. 1–4.
- Atindana, J.N.; Zhong, F.; Mothibe, K.J.; Bangoura, M.L.; Lagnika, C. Quantification of total polyphenolic content and antimicrobial activity of cocoa (Theobroma cacao L.) bean shells. Pak. J. Nutr. 2012, 11, 574–579. [Google Scholar]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; de Souza Sant’Ana, A.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Kalia, K.; Sharma, K.; Singh, H.P.; Singh, B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008, 56, 10129–10134. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Nguyen, V.T. Mass proportion, proximate composition and effects of solvents and extraction parameters on pigment yield from cacao pod shell (Theobroma cacao L.). J. Food Process. Preserv. 2014, 39, 1414–1420. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vuong, Q.V.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Effects of different drying methods on bioactive compound yield and antioxidant capacity of Phyllanthus amarus. Dry. Technol. Int. J. 2015, 33, 1006–1017. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Bowyer, M.C.; Vuong, Q.V.; van Altena, I.A.; Scarlett, C.J. Phytochemicals and antioxidant capacity of xao tam phan (Paramignya trimera) root as affected by various solvents and extraction methods. Ind. Crops Prod. 2015, 67, 192–200. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Bilen, F.D.; Gonzales, G.B.; Grootaert, C.; Van de Wiele, T.; Van Camp, J. Bioaccessibility of polyphenols from plant-processing byproducts of black carrot (Daucus carota L.). J. Agric. Food Chem. 2015, 64, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization Statistics (FAOSTAT). FAO Statistic Division; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Available online: http://faostat3.fao.org/home/E (accessed on 10 August 2016).
- AOAC. Official Methods of Analysis, 16th ed.; Assoc. Official Anal. Chemists: Washington, DC, USA, 1998. [Google Scholar]
- Nguyen, V.T.; Ueng, J.P.; Tsai, G.J. Proximate composition, total phenolic content, and antioxidant activity of seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, C950–C958. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hossain, S.; Rahaman, A.; Fatima, N.; Nahar, T.; Uddin, B.; Basunia, M.A. Antioxidant activity of Centella asiatica (Linn.) urban: Impact of extraction solvent polarity. J. Pharmacol. Phytochem. 2013, 1, 27–32. [Google Scholar]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Chisté, R.C.; Benassi, M.D.T.; Mercadante, A.Z. Efficiency of different solvents on the extraction of bioactive compounds from the Amazonian fruit Caryocar villosum and the effect on its antioxidant and colour properties. Phytochem. Anal. 2014, 25, 364–372. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Poh-Hwa, T.; Yoke-Kqueen, C.; Indu Bala, J.; Son, R. Bioprotective properties of three Malaysia Phyllanthus species: An investigation of the antioxidant and antimicrobial activities. Int. Food Res. J. 2011, 18, 887–893. [Google Scholar]
- Sen, A.; Batra, A. The study of in vitro and in vivo antioxidant activity and total phenolic content of Phyllanthus amarus Schum. & Thonn.: A medicinally important plant. Int. J. Pharm. Pharm. Sci. 2013, 5, 942–947. [Google Scholar]
- Shahriar, M.; Hossain, M.I.; Sharmin, F.A.; Akhter, S.; Haque, M.A.; Bhuiyan, M.A. In vitro antioxidant and free radical scavenging activity of Withania somnifera root. Iosr J. Pharm. 2013, 3, 38–47. [Google Scholar]
- Ibtissem, H.S.; Fatma, Z.R.; Iness, B.R.; Soumaya, B.; Ferid, L.; Brahim, M. Total phenolics, flavonoids, and antioxidant activity of Sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 2013, 6, 806–817. [Google Scholar]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins—Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Doughari, J.H. Phytochemicals—A global perspective of their role in nutrition and health. In Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents; Rao, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 1–32. [Google Scholar]
- Patel, J.R.; Tripathib, P.; Sharmaa, V.; Chauhana, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmarcol. 2011, 138, 286–313. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2013. [Google Scholar] [CrossRef]
- Kamonwannasit, S.; Nantapong, N.; Kumkrai, P.; Luecha, P.; Kupittayanant, S.; Chudapongse, N. Antibacterial activity of Aquilaria crassna leaf extract against Staphylococcus epidermidis by disruption of cell wall. Ann. Clin. Microbiol. Antimicrob. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Kumar, P.P.; Jeyaraj, B. Antioxidant activity of Illicium griffithi Hook. f. & Thoms seeds—In vitro. Asian J. Pharm. Clin. Res. 2013, 6, 269–273. [Google Scholar]
- Maity, S.; Chatterjee, S.; Variyar, P.S.; Sharma, A.; Adhikari, S.; Mazumder, S. Evaluation of antioxidant activity and characterization of phenolic constituents of Phyllanthus amarus root. J. Agric. Food Chem. 2013, 61, 3443–3450. [Google Scholar] [CrossRef] [PubMed]


| Solvent | Extraction Yield | |
|---|---|---|
| % by fresh weight | % by dry weight | |
| Water | 4.46 ± 0.51 ab | 46.53 ± 5.29 ab |
| Ethanol | 4.48 ± 0.48 ab | 46.67 ± 5.02 ab |
| Methanol | 5.18 ± 0.56 a* | 54.02 ± 5.81 a |
| Hexane | 4.13 ± 0.37 b | 43.04 ± 3.85 b |
| Correlations * (R2) | ARSC | DRSC | CUPRAC | FRAP |
|---|---|---|---|---|
| TPC | 0.25 | 0.79 | 0.93 | 0.96 |
| Saponins | 0.78 | 0.17 | 0.36 | 0.43 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.T.; Scarlett, C.J. Mass Proportion, Bioactive Compounds and Antioxidant Capacity of Carrot Peel as Affected by Various Solvents. Technologies 2016, 4, 36. https://doi.org/10.3390/technologies4040036
Nguyen VT, Scarlett CJ. Mass Proportion, Bioactive Compounds and Antioxidant Capacity of Carrot Peel as Affected by Various Solvents. Technologies. 2016; 4(4):36. https://doi.org/10.3390/technologies4040036
Chicago/Turabian StyleNguyen, Van Tang, and Christopher J. Scarlett. 2016. "Mass Proportion, Bioactive Compounds and Antioxidant Capacity of Carrot Peel as Affected by Various Solvents" Technologies 4, no. 4: 36. https://doi.org/10.3390/technologies4040036
APA StyleNguyen, V. T., & Scarlett, C. J. (2016). Mass Proportion, Bioactive Compounds and Antioxidant Capacity of Carrot Peel as Affected by Various Solvents. Technologies, 4(4), 36. https://doi.org/10.3390/technologies4040036