Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Analytical Chemicals
2.3. Preparation of Dried H. hirsuta L. Samples
2.4. Experimental Design
2.5. Determination of Physical Properties
2.6. Determination of Extractable Solid Content
2.7. Determination of Bioactive Compounds and Antioxidant Capacity of H. hirsuta L. Leaves
2.7.1. Bioactive Compounds
2.7.2. Antioxidant Capacity
2.8. Statistical Analyses
3. Results and Discussion
3.1. Effect of Solvents on Extractable Solid Content, Bioactive Compounds and Antioxidant Capacity of H. hirsuta L. Leaves
3.1.1. Effect on Extractable Solid Content

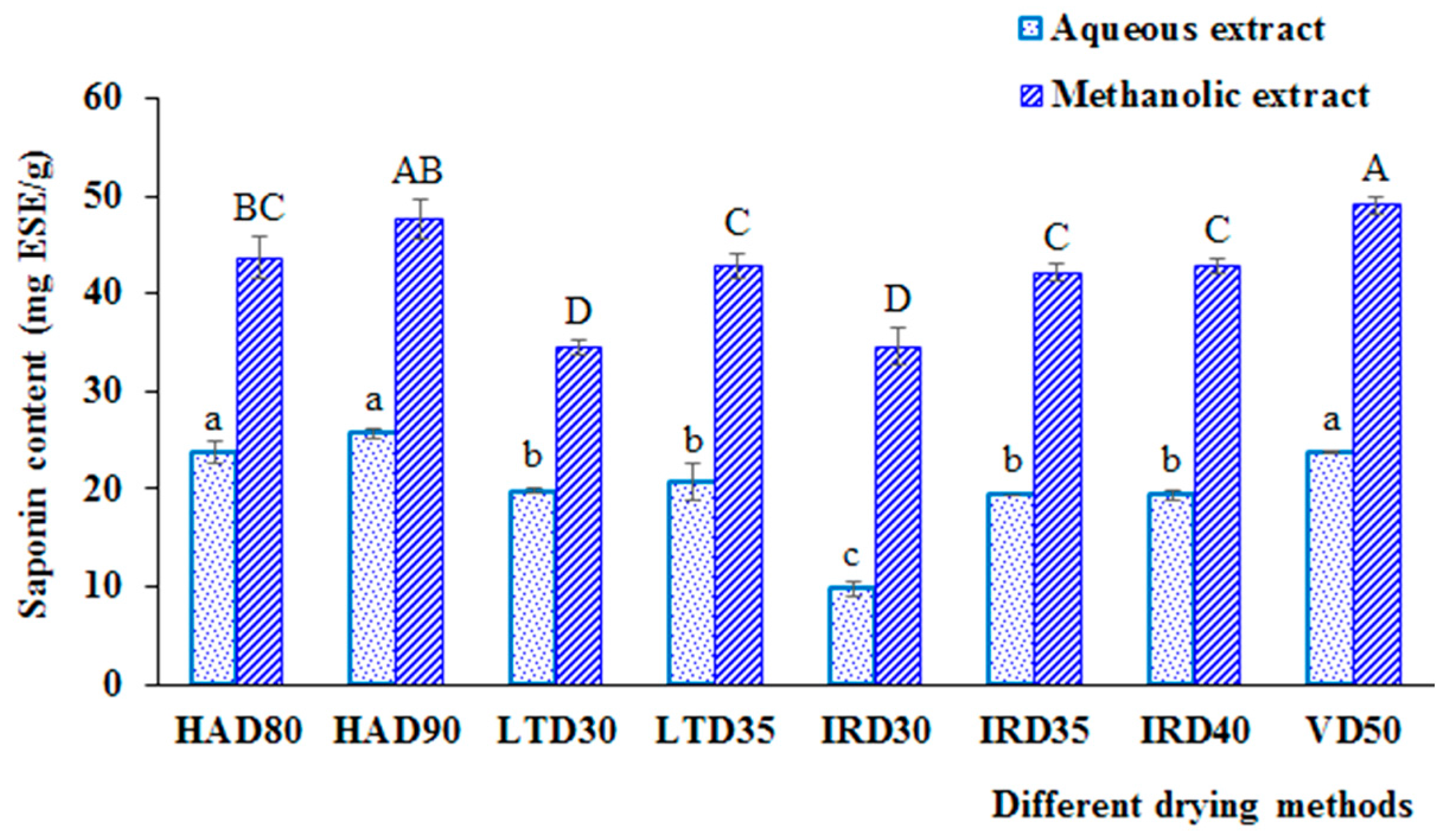
3.1.2. Effect on Bioactive Compounds
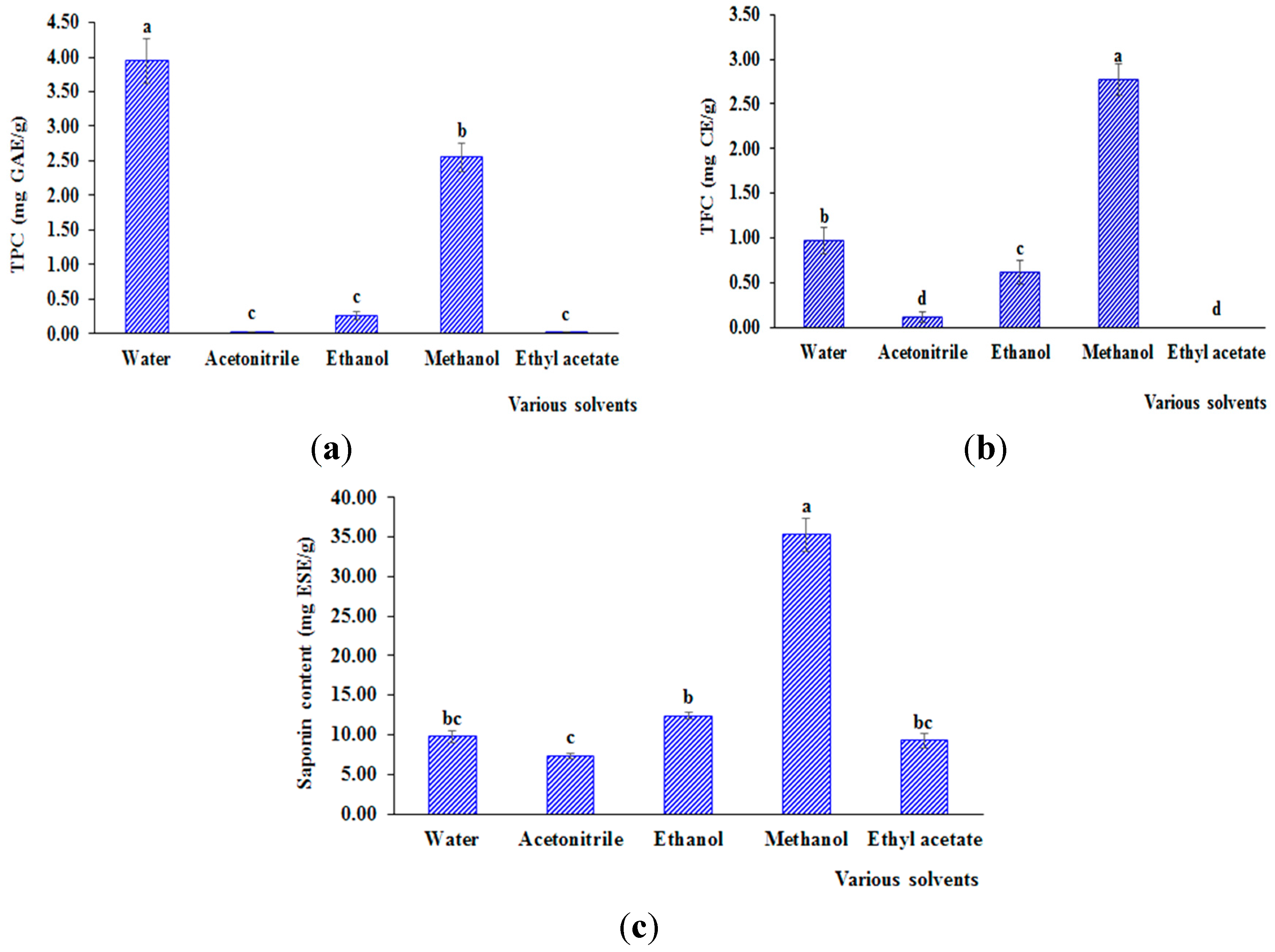
3.1.3. Effect on Antioxidant Capacity
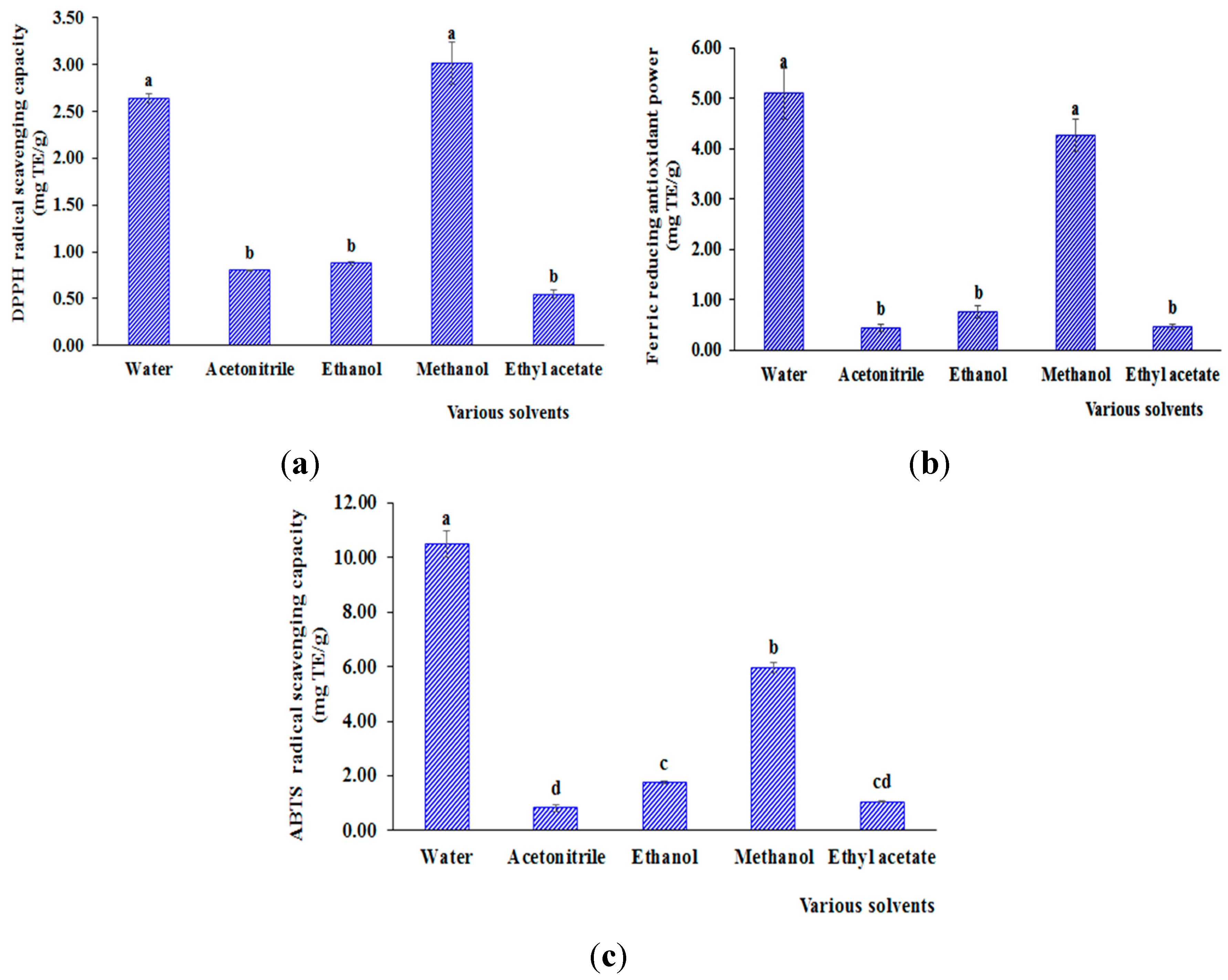
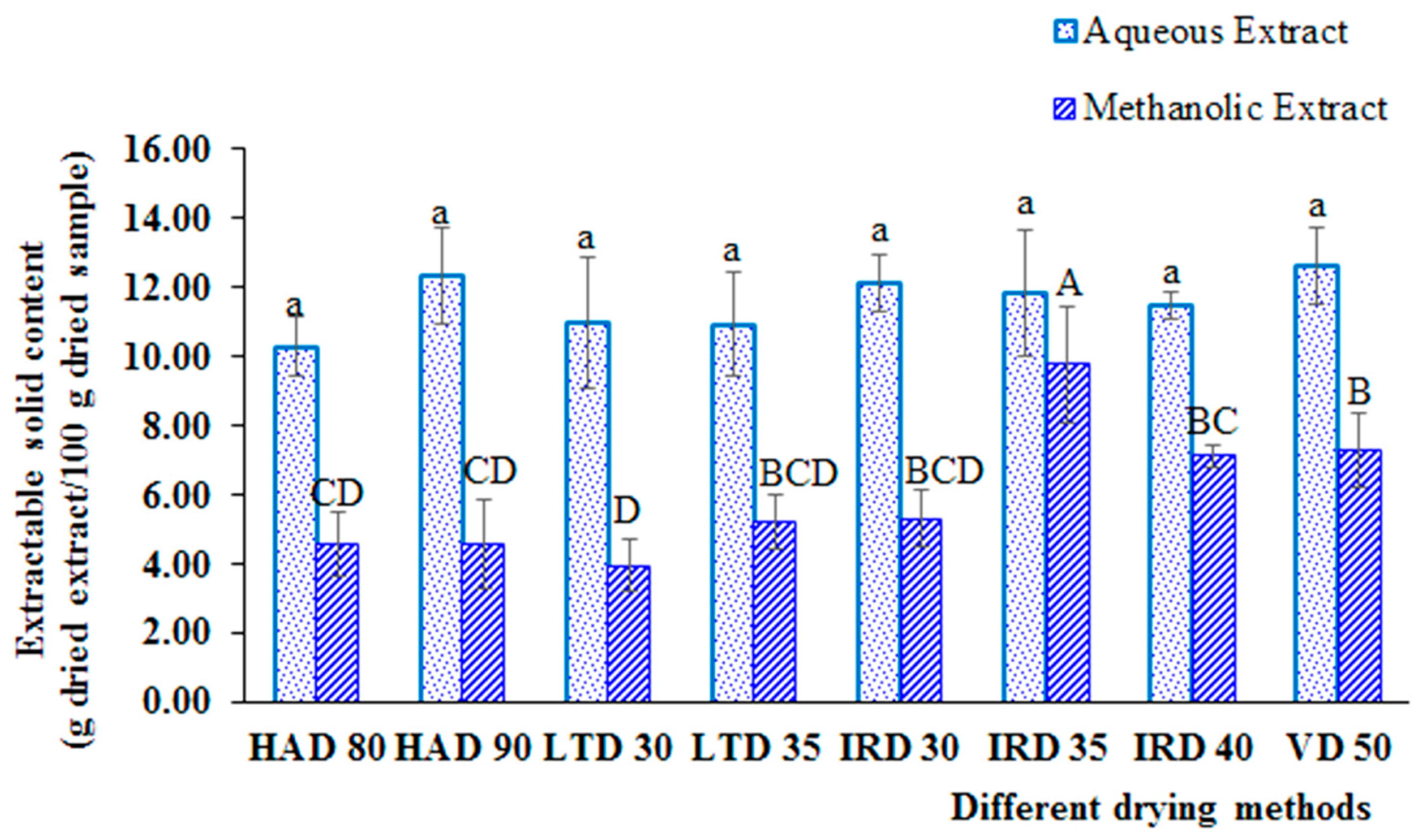
3.2. Impact of Drying Methods on Extractable Solid Content, Bioactive Compound Yield and Antioxidant Capacity of Aqueous and Methanol Extracts from H. hirsuta L. Leaves
3.2.1. Impact on Extractable Solid Content
3.2.2. Impact on Bioactive Compounds
| Drying Method | TPC (mg GAE/g) | TFC (mg CE/g) | ||
|---|---|---|---|---|
| Aqueous Extract | Methanol Extract | Aqueous Extract | Methanol Extract | |
| HAD80 | 7.77 ± 0.67 a | 3.57 ± 0.59 a | 5.79 ± 0.07 a | 4.75 ± 0.36 a |
| HAD90 | 6.58 ± 0.59 b | 2.34 ± 0.11 bc | 3.25 ± 0.23 c | 3.55 ± 0.26 ab |
| LTD30 | 4.52 ± 0.40 ed | 1.91 ± 0.07 c | 1.48 ± 0.09 e | 2.40 ± 0.23 b |
| LTD35 | 5.70 ± 0.46 bc | 2.75 ± 0.08 b | 1.99 ± 0.02 d | 4.58 ± 0.31 a |
| IRD30 | 3.99 ± 0.33 f | 2.44 ± 0.17 bc | 0.97 ± 0.15 f | 2.86 ± 0.17 b |
| IRD35 | 4.41 ± 0.39 ed | 2.39 ± 0. 21 bc | 1.33 ± 0.01 e | 3.47 ± 0.14 ab |
| IRD40 | 5.35 ± 0.24 cd | 2.41 ± 0.15 bc | 1.87 ± 0.06 d | 3.38 ± 0.11 ab |
| VD50 | 8.33 ± 0.56 a | 4.06 ± 0.11 a | 4.62 ± 0.02 b | 4.76 ± 1.45 a |
3.2.3. Impact on Antioxidant Capacity
| Drying Method | DPPH Radical Scavenging Capacity (mg TE/g) | ABTS Radical Scavenging Capacity (mg TE/g) | Ferric Reducing Antioxidant Power (mg TE/g) | ||||
|---|---|---|---|---|---|---|---|
| Aqueous Extract | Methanol Extract | Aqueous Extract | Methanol Extract | Aqueous Extract | Methanol Extract | ||
| HAD80 | 14.23 ± 0.16 a | 3.78 ± 0.22 b | 18.14 ± 0.08 a | 7.22 ± 0.36 b | 15.09 ± 0.07 a | 7.31 ± 0.40 a | |
| HAD90 | 9.33 ± 0.57 b | 3.28 ± 0.16 bc | 12.83 ± 0.37 c | 5.15 ±0.45 cd | 8.41 ± 0.65 c | 5.11 ± 0.36 bcd | |
| LTD30 | 5.09 ± 0.38 e | 3.35 ± 0.41 bc | 9.19 ± 0.50 f | 4.29 ±0.62 d | 5.46 ± 0.39 e | 3.93 ± 0.60 e | |
| LTD35 | 7.76 ± 0.11 c | 3.78 ± 0.17 b | 12.64 ± 0.08 c | 5.83 ± 0.61 c | 7.22 ± 0.49 d | 5.66 ± 0.41 b | |
| IRD30 | 2.64 ± 0.05 f | 3.01 ± 0.23 c | 10.49 ± 0.48 e | 5.96 ± 0.21 c | 5.09 ± 0.51 e | 4.27 ± 0.32 de | |
| IRD35 | 4.67 ± 0.41 e | 3.23 ± 0.28 bc | 10.98 ± 0.44 de | 4.08 ± 0.42 d | 5.53 ± 0.46 e | 4.47 ± 0.25 cde | |
| IRD40 | 6.66 ± 0.49 d | 3.14 ± 0.23 bc | 12.08 ± 0.25 cd | 5.76 ± 0.26 c | 7.17 ± 0.37 d | 5.39 ± 0.03 bc | |
| VD50 | 13.45 ± 0.18 a | 4.73 ± 0.16 a | 16.77 ± 0.53 b | 8.50 ± 0.75 a | 13.54 ± 0.19 b | 7.98 ± 0.59 a | |
3.2.4. Correlation of Bioactive Compounds in Dried H. hirsuta L. Leaves and Antioxidant Capacity
| Bioactive Compounds | R-Squared Value * | ||
|---|---|---|---|
| DPPH Radical Scavenging Capacity | ABTS Radical Scavenging Capactity | Ferric Reducing Antioxidant Power | |
| TPC | 0.97 | 0.95 | 0.92 |
| TFC | 0.96 | 0.96 | 0.97 |
| Saponins | 0.56 | 0.55 | 0.48 |
3.3. Impact of Drying Methods on the Physical Properties of H. hirsuta L. Leaves
| Drying Method | Drying Time (Hours) | Moisture Content (%) | Water Activity |
|---|---|---|---|
| HAD80 | 4.0 | 5.51 ± 0.53 c | 0.49 ± 0.01 e |
| HAD90 | 2.0 | 3.09 ± 0.35 d | 0.41 ± 0.01 f |
| LTD30 | 10.5 | 8.18 ± 0.44 a | 0.59 ± 0.00 c |
| LTD35 | 9.0 | 6.93 ± 0.35 b | 0.57 ± 0.01 d |
| IRD30 | 18.0 | 8.67 ± 0.15 a | 0.68 ± 0.01 a |
| IRD35 | 12.0 | 8.35 ± 0.25 a | 0.59 ± 0.00 c |
| IRD40 | 6.0 | 8.51 ± 0.35 a | 0.61 ± 0.00 b |
| VD50 | 19.0 | 6.13 ± 0.58 bc | 0.49 ± 0.01 e |
| Drying Method | Color Characteristic | |||
|---|---|---|---|---|
| Lightness | Chroma | Hue Angle (°) | Browning Index | |
| HAD80 | 8.88 ± 0.06 d | 3.78 ± 0.07 e | 92.46 ± 0.80 c | 51.99 ± 0.85 a |
| HAD90 | 10.39 ± 0.16 b | 4.36 ± 0.03 a | 93.94 ± 0.48 b | 50.21 ± 1.32 b |
| LTD30 | 9.57 ± 0.18 c | 3.39 ± 0.05 g | 90.14 ± 1.13 d | 42.44 ± 0.70 e |
| LTD35 | 10.83 ± 0.43 a | 4.04 ± 0.09 c | 93.67 ± 0.67 b | 43.36 ± 2.02 de |
| IRD30 | 10.70 ± 0.27 a | 3.86 ± 0.11 d | 91.68 ± 0.78 c | 42.58 ± 0.34 e |
| IRD35 | 9.75 ± 0.19 c | 3.65 ± 0.02 f | 90.58 ± 0.68 d | 45.29 ± 1.05 c |
| IRD40 | 10.31 ± 0.21 b | 3.79 ± 0.08 de | 90.49 ± 1.03 d | 44.27 ± 0.91 cd |
| VD50 | 10.87 ± 0.29 a | 4.24 ± 0.07 b | 96.39 ± 1.34 a | 44.01 ± 1.11 d |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chin, Y.W.; Jones, W.P.; Rachman, I.; Riswan, S.; Kardono, L.B.; Chai, H.B.; Farnsworth, N.R.; Cordell, G.A.; Swanson, S.M.; Cassady, J.M.; et al. Cytotoxic lignans from the stems of Helicteres hirsuta collected in Indonesia. Phytother. Res. 2006, 20, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Chuakul, W.; Saralamp, P.; Boonpleng, A. Medicinal plants used in the Kutchum District, Yasothon Province, Thailand. Thai J. Phytopharm. 2002, 9, 22–49. [Google Scholar]
- Nóbrega, E.M.; Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. The impact of hot air drying on the physical-chemical characteristics, bioactive compounds and antioxidant activity of acerola (Malphigia emarginata) residue. J. Food Process. Preserv. 2015, 39, 131–141. [Google Scholar] [CrossRef]
- Rabeta, M.S.; Vithyia, M. Effect of different drying methods on the antioxidant properties of Vitex negundo Linn. tea. Int. Food Res. J. 2013, 20, 3171–3176. [Google Scholar]
- Nguyen, V.T.; Vuong, Q.V.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Effects of different drying methods on bioactive compound yield and antioxidant capacity of Phyllanthus amarus. Dry. Technol. 2015, 33, 1006–1017. [Google Scholar] [CrossRef]
- Karaman, S.; Toker, O.S.; Çam, M.; Hayta, M.; Doğan, M.; Kayacier, A. Bioactive and physicochemical properties of persimmon as affected by drying methods. Dry. Technol. 2014, 32, 258–267. [Google Scholar] [CrossRef]
- Dev, S.R.S.; Geetha, P.; Orsat, V.; Gariepy, Y.; Raghavan, G.S.V. Effects of microwave-assisted hot air drying and conventional hot air drying on the drying kinetics, color, rehydration, and volatiles of Moringa oleifera. Dry. Technol. 2011, 29, 1452–1458. [Google Scholar] [CrossRef]
- Jain, A.; Sinha, P.; Desai, N.S. Estimation of flavonoid, phenol content and antioxidant potential of Indian screw tree (Helicteres isora L.). Int. J. Pharm. Sci. Res. 2014, 5, 1320–1330. [Google Scholar]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Effect of Different Solvents on Polyphenolic Content, Antioxidant Capacity and Antibacterial Activity of Irish York Cabbage. J. Food Biochem. 2012, 36, 344–358. [Google Scholar] [CrossRef]
- Kalia, K.; Sharma, K.; Singh, H.P.; Singh, B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008, 56, 10129–10134. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC Internationalno. 930.04 and 934.01, 19th ed.; AOAC International: Rockville, MD, USA, 2012; Volume 1. [Google Scholar]
- Oliveira, S.M.; Ramos, I.N.; Brandão, T.R.S.; Silva, C.L.M. Effect of air-drying temperature on the quality and bioactive characteristics of dried Galega kale (Brassica oleracea L. var. Acephala). J. Food Process. Pres. 2015. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Kamonwannasit, S.; Nantapong, N.; Kumkrai, P.; Luecha, P.; Kupittayanant, S.; Chudapongse, N. Antibacterial activity of Aquilaria crassna leaf extract against Staphylococcus epidermidis by disruption of cell wall. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Bowyer, M.C.; Vuong, Q.V.; van Altena, I.A.; Scarlett, C.J. Phytochemicals and antioxidant capacity of Xao tam phan (Paramignya trimera) root as affected by various solvents and extraction methods. Ind. Crops Prod. 2015, 67, 192–200. [Google Scholar] [CrossRef]
- Rezaie, M.; Farhoosh, R.; Iranshahi, M.; Sharif, A.; Golmohamadzadeh, S. Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem. 2015, 173, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Yang, J.; Ye, Q.; Li, H.; Wang, H. Infrared, convective, and sequential infrared and convective drying of wine grape pomace. Dry. Technol. 2014, 32, 686–694. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Gekas, V. Effect of drying method on the phenolic content and antioxidant capacity of Spearmint. Czech J. Food Sci. 2013, 31, 509–513. [Google Scholar]
- Hung, P.V.; Duy, T.L. Effects of drying methods on bioactive compounds of vegetables and correlation between bioactive compounds and their antioxidants. Int. Food Res. J. 2012, 19, 327–332. [Google Scholar]
- Chirife, J.; Buera, M.D.P. Water activity, glass-transition and microbial stability in concentrated/semimoist food systems. J. Food Sci. 1994, 59, 921–927. [Google Scholar] [CrossRef]
- Chirife, J.; Fontana, A.J. Introduction: Historical Highlights of Water Activity Research. In Water Activity in Foods: Fundamentals and Applications, 1st ed.; Barbosa-Cánovas, G.V., Fontana, A.J., Schmidt, S.J., Labuza, T.P., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 3–13. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, H.N.T.; Nguyen, V.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves. Technologies 2015, 3, 285-301. https://doi.org/10.3390/technologies3040285
Pham HNT, Nguyen VT, Vuong QV, Bowyer MC, Scarlett CJ. Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves. Technologies. 2015; 3(4):285-301. https://doi.org/10.3390/technologies3040285
Chicago/Turabian StylePham, Hong Ngoc Thuy, Van Tang Nguyen, Quan Van Vuong, Michael C. Bowyer, and Christopher J. Scarlett. 2015. "Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves" Technologies 3, no. 4: 285-301. https://doi.org/10.3390/technologies3040285
APA StylePham, H. N. T., Nguyen, V. T., Vuong, Q. V., Bowyer, M. C., & Scarlett, C. J. (2015). Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves. Technologies, 3(4), 285-301. https://doi.org/10.3390/technologies3040285







