Abstract
Microwaves at the ISM (Industrial, Scientific and Medical, reserved internationally) frequency of 2450 or 5800 MHz have been used to prepare FeCoNiCuAl, FeCrNiTiAl and FeCoCrNiAl2.5 high entropy alloys by direct heating of pressed mixtures of metal powders. The aim of this work is to explore a new microwave-assisted near-net-shape technology, using a powder metallurgy approach for the preparation of high entropy alloys, able to overcome the limits of current melting technologies (defects formation) or solid state ones (time demanding). High entropy alloy compositions have been selected so as to comprise at least one ferromagnetic element and one highly reactive couple, like Ni-Al, Ti-Al, Co-Al or Fe-Al. Results show that direct microwave heating of the powder precursors occurs, and further heating generation is favored by the ignition of exothermal reactions in the load. Microwaves have been applied both for the ignition and sustaining of such reactions, showing that by the proposed technique, it is possible to control the cooling rate of the newly-synthesized high entropy alloys. Results showed also that microwave heating in predominant magnetic field regions of the microwave applicator is more effective at controlling the cooling rate. The herein proposed microwave-assisted powder metallurgy approach is suitable to retain the shape of the load imparted during forming by uniaxial pressing. The homogeneity of the prepared high entropy alloys in all cases was good, without the dendritic segregation typical of arc melting, even if some partially-unreacted powders were detected in the samples.
1. Introduction
High entropy alloys (HEA) are a class of multi-component alloys composed of five or more principal constituent elements, none of which is predominant, and each with a concentration between 5 and 35 atomic % [1]. These alloys have a tendency to form simple structures, like face centered cubic (FCC) and body centered cubic (BCC), instead of intermetallic compounds [2]. This is because the high entropy of mixing reduces the free energy of the system and stabilizes these simple structures. HEA constitute an extremely vast and almost entirely unexplored research field. Assuming an arbitrary choice of a group of 10 mutually-miscible metallic elements, this would enable the design of 7099 systems with five to 13 elements in equimolar ratios alone, provided the rules for the formation of HEA are respected. These involve atomic size difference, mixing enthalpy, mixing entropy, electronegativity and valence electron concentration among constituent elements [3].
Regardless of the HEA composition, this family of alloys shows several interesting features; in particular, they tend to form simple solid solution phases with the possible presence of nanostructures or even amorphous structures [4], presenting Vickers hardness ranging from 100 to 1100 HV30, accompanied by a good thermal stability and excellent resistance to anneal softening [5]. Moreover, they can be heat treated to form precipitates (precipitation hardening, between 500 °C and 1000 °C); they possess excellent corrosion resistance, wear resistance, oxidation resistance and high compressive strengths, in excess of 3 GPa, and good ductility (more than 50% elongation in some cases) [6,7,8]. They present the sluggish diffusion phenomenon [9], which makes them extremely stable at high temperatures, but poses homogenization problems during their synthesis in the solid state. Due to this broad spectrum of properties, the HEA have many potential applications [10], including mechanical parts and furnace parts requiring high strength, thermal stability and wear and oxidation resistance, aircraft components susceptible to thermal stresses, anticorrosive high-strength materials in chemical plants, piping and pumping components for marine applications, functional hard-facing coatings for medical devices, anti-sticking coatings for molds, tools and dies and soft magnetic films for ultra-high frequency communications.
Since the introduction of the high entropy alloy concept in the literature, several production techniques have been tried to synthesize these materials [10]. According to the literature research, up to now, most of the production attempts followed one of the four following techniques: from the liquid state [11] (arc melting, induction melting), from the solid state [12] (mechanical alloying, powder metallurgy), from the gas state [13] (sputtering techniques, mainly for coatings) and from electrochemical process [14] (again, mainly for coatings). The most used technique to produce HEA is the arc melting technique, but for elements with a low melting point, which are easy to evaporate, e.g., Mg, Zn and Mn, this route may not be the best choice, because of the lack of control of the exact composition. In this case, resistance heating or induction heating may result in being more appropriate. Induction heating is used also as an auxiliary process for the production of HEA in two steps [15]. Minor research has been conducted on the mechanical alloying technique to produce HEA [16], using planetary ball mills and long milling times. Up to now, the arc melting is still one of the most promising technologies, as it guarantees a short alloying time (high energy density on the load, rapid melting and, hence, reduced contamination by the surrounding environment), efficient cooling (water-cooled crucibles are used to achieve higher cooling rates) and the capability to operate in a controlled atmosphere.
The same conditions can be achieved using higher frequency electromagnetic fields, like in microwave heating, provided the load is capable of coupling with the incident electric and magnetic field. Microwave heating of powdered metals has been known since the pioneering work of Roy et al. [17], and recently, it has found many applications in the field of sintering powder metallurgy products [18] or even in primary metallurgy [19,20]. The possibilities to achieve high power density in a powdered metal load, operating in a relatively cold environment and under a protective atmosphere, and to achieve selective heating of the load fulfill the requirements for the preparation of HEA. Scientific literature regarding the use of microwaves to prepare HEA is limited to only one contribution by Teng et al. [21], who synthesized FeCoNiCuAl HEA by microwave-assisted combustion synthesis, starting from oxidic precursors as raw materials. In this case, besides microwave dielectric heating of the precursors, the highly exothermal reaction among the reactants was used to rapidly increase the temperature of the system. The drawback of such aluminothermic reactions is that alumina is produced as a byproduct of the Al-assisted reduction. Instead, the use of metal precursors is expected to avoid contamination and to achieve much higher combustion temperatures.
Microwave-assisted combustion synthesis of pure metal powders as reactants has already been used during the last decade by some of the authors to prepare intermetallics [22], functionally-graded materials [23] or as a joining technique between dissimilar materials [24]. The advantage of applying microwaves to combustion synthesis reactions resulted in high purity of the products [25], rapid ignition of the reaction [26] and the possibility to control the products’ microstructure [27] and cooling rate after synthesis, especially in the presence of ferromagnetic reactants [28]. Given these premises, the aim of this work is to explore a new microwave-assisted near-net-shape technology, using the powder metallurgy approach for the preparation of HEA, able to overcome the limits of current melting technologies, which tend to originate defects in the cast products, like voids, unwanted porosity, segregation and surface contamination [29].
2. Experimental Section
In this work, different HEA have been prepared, comprised of at least one ferromagnetic element and one highly reactive element couple, like Al-Ti, Al-Ni and Al-Fe, in order to improve heat generation due to both the magnetic field contribution (microwave heating) and the exothermal reaction contribution (combustion synthesis). Elemental powders, supplied by Sigma-Aldrich, have been used as reactants and are shown in Table 1.

Table 1.
Composition of the metal powders used (BCC = body centered cubic; FCC = face centered cubic; HCP = Hexagonal close-packed arrangement).
| Element | Purity (%) | Particle Size (µm) | At Radius (pm) | Cell |
|---|---|---|---|---|
| Fe | 97.00 | <44 | 156 | BCC |
| Co | 99.80 | <2 | 152 | HCP |
| Ni | 99.70 | <5 | 149 | FCC |
| Cu | 99.00 | <10 | 145 | FCC |
| Ti | 99.90 | <44 | 176 | HCP |
| Cr | 99.00 | <44 | 166 | BCC |
| Al | 99.00 | <75 | 118 | FCC |
The proper amount of powders was weighed (2 to 8 g), depending on the HEA composition to be prepared, and mixed under vacuum in an Al2O3 ceramic jar for approximately 30 min. Uniaxial pressing was used at different forming pressures (200, 300 or 400 MPa) to form reactive disc-shaped specimens of different weights (3 to 8 g) and dimensions (3 to 10-mm radius). The selection of different forming pressures is due to the limited penetration depth of microwaves into metallic powder compacts as their green density increases and microwave frequency increases [30].
The following HEA (theoretical composition) have been investigated in this study:
- FeCoNiCuAl
- FeCrNiTiAl
- FeCoCrNiAl2.5
Synthesis experiments, which are expected to include one ignition step of a combustion synthesis, have been performed using microwaves at the ISM frequencies of 2450 and 5800 MHz. All of the experiments have been conducted in rectangular TE10n single-mode applicators (i.e., with a transverse electrical mode presenting only one semisinusoidal variation of the electric field in the x direction, 0 variations in the y direction and n variations in the z direction (n = 2 or 3 in the experimental setup used), whose geometry has been described in detail elsewhere [31]. Briefly, they consists of a magnetron generator (MKS-Alter, Reggio Emilia, Italy), with an output power level ranging from 100 to 800 W, connected to a three-port circulator and to a three-stub tuner (MKS-Alter). The cavity for heat treatments consisted of a rectangular resonant applicator, based on the standard WR-340 waveguide (in the case of a 2450-MHz frequency) or the WR-159 waveguide (in the case of a 5800-MHz frequency). A shorting plunger allows one to controllably modify the electromagnetic field distribution along the cavity. Figure 1 shows the two furnaces used for this study.
The choice of the single-mode applicator lies in its possibility to expose the load to regions of a predominant electric or magnetic field [32], even if both contributing to heating has to be considered, due to the perturbation of the electromagnetic field in the cavity due to the presence of the load [33]. In this kind of applicator, for loads of small dimensions and not introducing major perturbations, the central part of the applicator presents predominant electric field conditions, while the side walls present the predominant maximum magnetic field conditions. In order to avoid excessive oxidation, a constant Ar flux (20 NmL/min) was blown into the single-mode cavity during experiments.
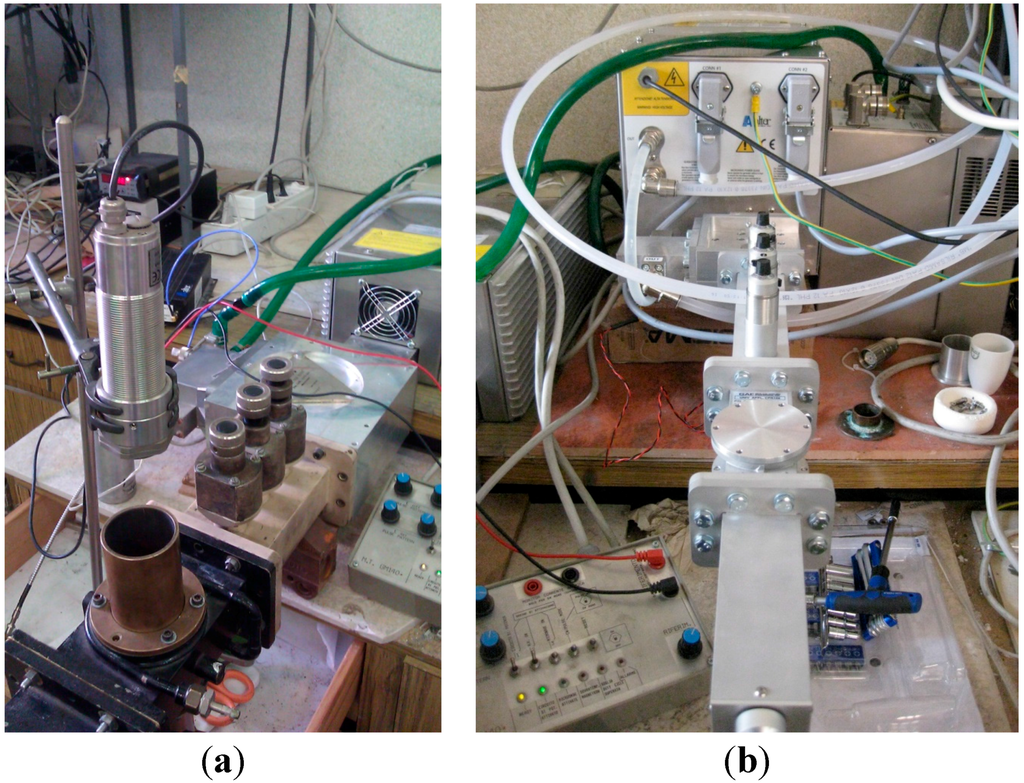
Figure 1.
(a) Single-mode applicator, 2450 MHz, with measuring port and optical pyrometer on top; (b) single-mode applicator, 5800 MHz, with measuring port temporarily closed.
Figure 1.
(a) Single-mode applicator, 2450 MHz, with measuring port and optical pyrometer on top; (b) single-mode applicator, 5800 MHz, with measuring port temporarily closed.
Temperature during heating tests was monitored and recorded at 1-s intervals, for load positions of the predominant electric field, using an optical pyrometer (IKS-T14-09, Sitel control Srl, Milan, Italy) (shown in Figure 1a) or by direct contact with a sapphire fiber connected to a MIKRON M680 (Mikron Infrared Inc., Oakland, NJ, USA) signal conditioner.
The obtained specimens were sectioned to expose the whole cross-section in order to investigate their microstructural and compositional features by means of scanning electron microscopy (ESEM Quanta-200 Fei, Hillsboro, OR, USA) and energy dispersive X-ray spectroscopy (EDS, INCA-350, Oxford Instruments, Abingdon, UK). X-ray diffraction ((XRD, X’ Pert PRO, PANAlytical, Almelo, The Netherlands) using Ni-filtered Cu-Kα radiation (λ = 1.5405 Å)) was used to verify the formation of the solid solutions typical of HEA, and microhardness testing (Wolpert group 402 MVD instrument, Vickers, Wilson Wolpert Instruments, Aachen, Germany; 300 g load for 15 s) was performed on selected regions of the samples, after polishing and etching with aqua regia for 10 s.
3. Results and Discussion
3.1. Heating and Cooling Curves
The Al and the Ti-containing HEA showed, during synthesis, a pronounced heating rate after a certain threshold temperature was reached, followed by cooling down despite the exposure to microwaves. Figure 2 shows the heating curves, in predominant electric field regions of the applicator, for the FeCoNiCuAl system, at 5800 MHz, with 240 W forward power.
Figure 2 shows two important phenomena occurring when microwave heating powder metal compacts, i.e., the dependence on the forming pressure (and hence, on the green density) and the different temperature readings depending on the contact-less or contact measurement method used. The latter is evident comparing the 200-MPa sample and the 200-MPa-f sample, with the “-f” indicating that the sapphire fiber has been used to monitor temperature, directly in contact with the sample. This latter reading is much more reliable than the pyrometric one, which provides information only on the surface layer of the load, despite the fact that readings start only above 600 °C. The heating curve shows that at a temperature slightly above the melting point of aluminum, a strong heating rate is encountered, and this is likely to occur due to combustion synthesis reactions taking place in the load. This is in agreement with the reaction mechanism of the formation of aluminides, which usually starts with the melting of the aluminum and its subsequent reaction with other metals, like Ni, Fe and Co [34]. When the reaction is completed, the heating rate decreases dramatically (actually, cooling occurs), indicating that the reaction contribution to heating is stopped and only microwave heating continues.
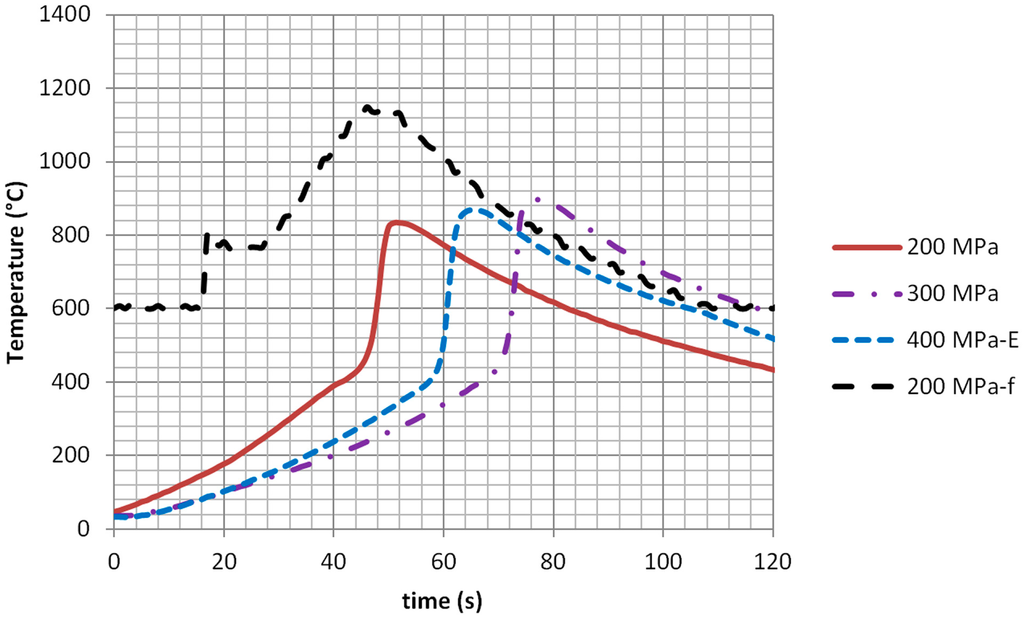
Figure 2.
Heating curves of the Fe + Co + Ni + Cu + Al powder compacts measured by optical pyrometer: microwave heating at 240 W forward power, 5800 MHz, in predominant electric field regions of the microwave applicator. Different forming pressures applied; suffix “-f” indicates temperature monitored by sapphire fiber; suffix “-E”, electric field.
Figure 2.
Heating curves of the Fe + Co + Ni + Cu + Al powder compacts measured by optical pyrometer: microwave heating at 240 W forward power, 5800 MHz, in predominant electric field regions of the microwave applicator. Different forming pressures applied; suffix “-f” indicates temperature monitored by sapphire fiber; suffix “-E”, electric field.
Figure 2 shows also that ignition of the combustion reaction is dependent on the forming pressure, as is the combustion temperature. This can be ascribed to the higher microwave penetration depth in case of lower green density samples [35] and, hence, a larger volume of the load undergoing direct microwave absorption, but also to the different thermal properties (specific heat) and reactivity of the pressed samples: from a kinetic point of view, reactions are favored by the intimate contact of the reactants, suggesting that a high forming pressure, which does not inhibit aluminum melt spreading during the reaction, should favor reaction propagation [36]. On the other side, a higher forming pressure causes the specific heat of the load to increase, hence a larger energy (and time) is required to lead the same volume of the load to ignition conditions. The lower ignition temperature is typical of combustion synthesis reactions propagated on poorly-heated loads, which use part of the heat of the reaction to heat the reactants to the ignition temperature, while in the case of homogenously-heated reactants, ignition can occur simultaneously in different parts of the load (thermal explosion regime) [37]. Because the tests were conducted at the same power level, with a similar reflection coefficient during testing, a longer ignition time means a higher energy conveyed to the load, hence a higher overall temperature of the reagents when the reaction is propagated.
A further aspect, peculiar to microwave heating, can be observed comparing the heating in predominant electric or magnetic field regions, immediately after reaction propagation, as shown in Figure 3.
It is evident that in case of heating in predominant magnetic-field regions (suffix -H), ignition occurs slightly earlier, but, most significantly, the subsequent cooling down phase is less pronounced. The two curves of Figure 3 show a change of slope during cooling, occurring when the microwave generator power is turned off. This occurred after 100 s in the case of heating in the predominant electric field and after 215 s in the case of heating in the predominant magnetic field. Taking the reading at 100 s, the H-field region position allowed limiting the cooling down to less than 90 °C (190 degrees/min average cooling rate), while in the case of the electric-field region (suffix -E), it surpassed 250 °C (530 degrees/min average cooling rate). In other words, processing the HEA precursors in predominant magnetic field regions allowed extending their permanence at high temperature, and this is expected to have positive effects on the elements’ diffusion and on the microstructural homogeneity of the resulting alloy.
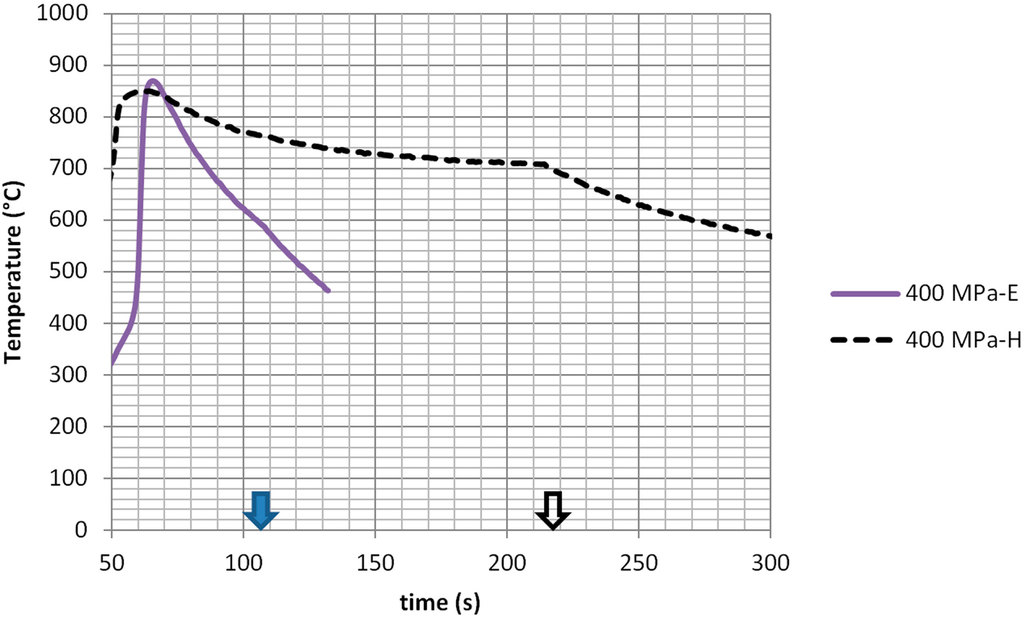
Figure 3.
Heating curves of the Fe + Co + Ni + Cu + Al powder compacts measured by optical pyrometer: microwave heating at 240 W forward power, 5800 MHz, in predominant electric field (suffix -E) or magnetic field (suffix -H) regions of the microwave applicator. Microwave exposure is prolonged after the reaction is terminated, until the time indicated by the arrow, resulting in a slope change during cooling.
Figure 3.
Heating curves of the Fe + Co + Ni + Cu + Al powder compacts measured by optical pyrometer: microwave heating at 240 W forward power, 5800 MHz, in predominant electric field (suffix -E) or magnetic field (suffix -H) regions of the microwave applicator. Microwave exposure is prolonged after the reaction is terminated, until the time indicated by the arrow, resulting in a slope change during cooling.
A similar behavior has been observed in other samples, for instance, as shown in Figure 4, in the synthesis of the FeCrNiTiAl high entropy alloy.
In this case (predominant H-field region, 700 W forward power, 2450 MHz), monitored by the contacting sapphire fiber, the reaction was triggered at a much higher temperature and occurred much slower than in the previous system. The application of microwaves after the small exothermal event (evident as sudden heating at time = 55 s) allowed mitigating the cooling phase, until microwaves are turned off at time = 120 s, with a subsequent increase in the cooling rate. The results of Figure 4 show also that in the case that a lower frequency is used, a higher forward power is required to achieve synthesis in similar times, in agreement with what is expected according to the power density equation (power density in the load results in being directly proportional to the frequency used, for a given E and H field strength) [38].
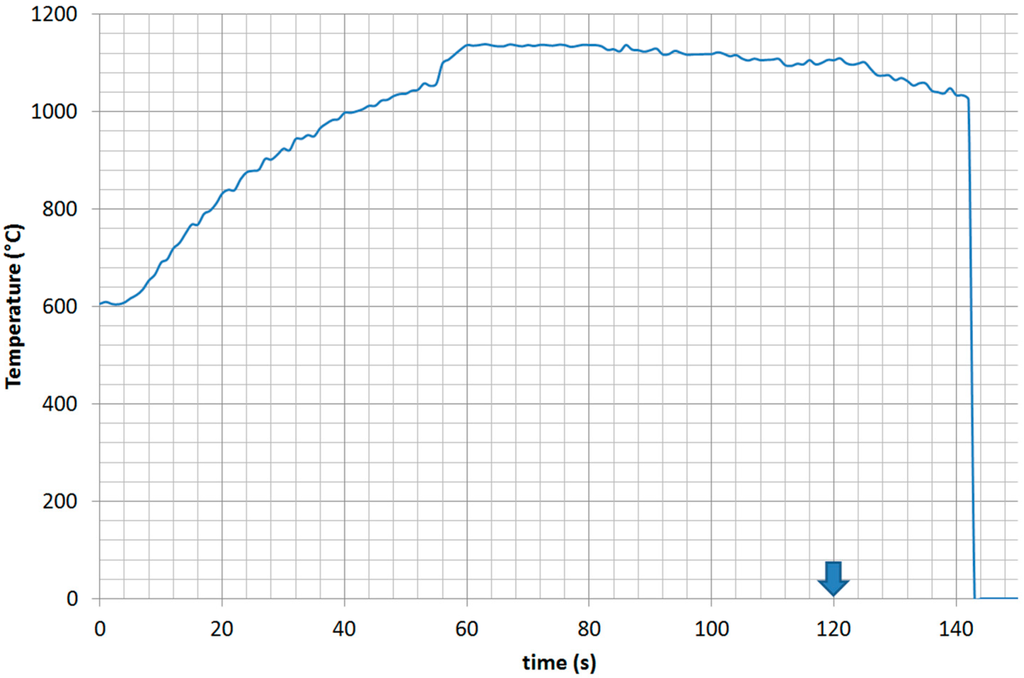
Figure 4.
Heating curve of the Fe + Cr + Ni + Ti + Al powder compacts measured by sapphire fiber: microwave heating at 700 W forward power, 2450 MHz, in predominant magnetic field regions of the microwave applicator. Microwave exposure is prolonged after the reaction is terminated, until the time indicated by the arrow, resulting in a slope change during cooling.
Figure 4.
Heating curve of the Fe + Cr + Ni + Ti + Al powder compacts measured by sapphire fiber: microwave heating at 700 W forward power, 2450 MHz, in predominant magnetic field regions of the microwave applicator. Microwave exposure is prolonged after the reaction is terminated, until the time indicated by the arrow, resulting in a slope change during cooling.
Noticeably, in all of the cases studied, the time required to prepare the high entropy alloy resulted in being of the order of a few minutes, with temperatures not exceeding 1200 °C. This is an interesting result, considering that other conventional powder metallurgy routes, for such systems, require heating at 10 °C/s up to 1800 °C and holding for 2 h, followed by slow cooling [39]. Considering arc melting, a typical synthetic route for HEA, it requires many repeated melting steps (at least five) to improve homogeneity, sometimes adding one element at a time, followed by cooling [40]; other solid state processes, like high energy milling, can require even more than 40 h to achieve supersaturated solid solutions [41], depending on the process parameters.
3.2. Samples Characterization
After microwave heating, the disc-shaped samples retained their shape, and their diameter resulted in being almost unaltered, indicating that sintering occurred without significant densification. Only in the samples processed at higher temperatures, like the FeCrNiTiAl, an average shrinkage of 9% was measured.
The as-synthesized samples have been subjected to X-ray diffraction to confirm the formation of a solid solution. Figure 5 shows the superimposed X-ray diffraction patterns in the case of the FeCoNiCuAl system, as an example.
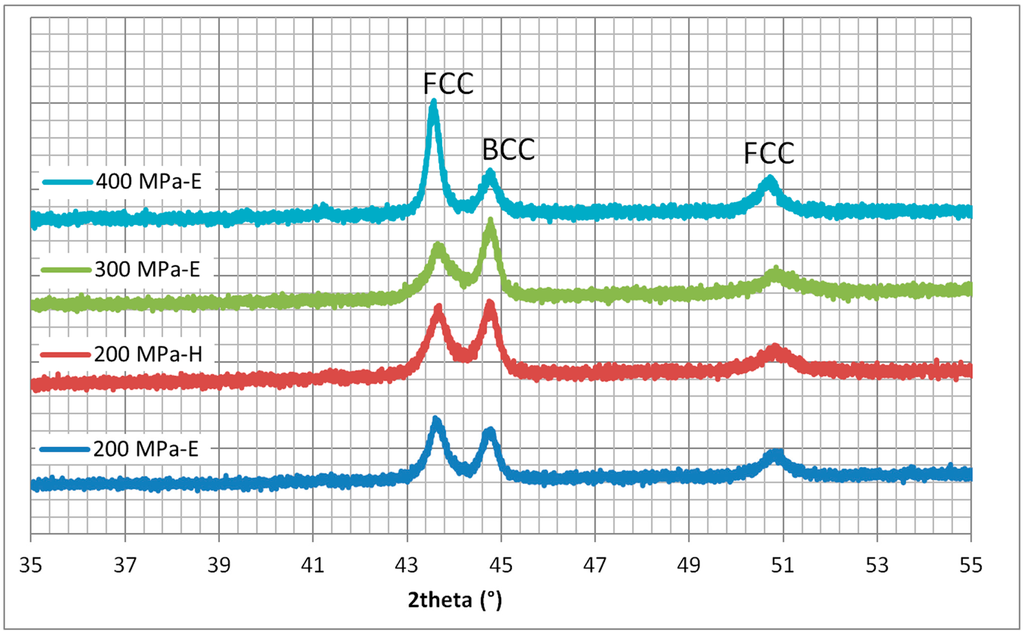
Figure 5.
X-ray diffraction patterns of FeCoNiCuAl samples (Y-axis: arbitrary units) processed in predominant electric (E) or magnetic (H) field regions of the applicator (240 W, 5800 GHz), confirming the formation of FCC and BCC solid solutions.
Figure 5.
X-ray diffraction patterns of FeCoNiCuAl samples (Y-axis: arbitrary units) processed in predominant electric (E) or magnetic (H) field regions of the applicator (240 W, 5800 GHz), confirming the formation of FCC and BCC solid solutions.
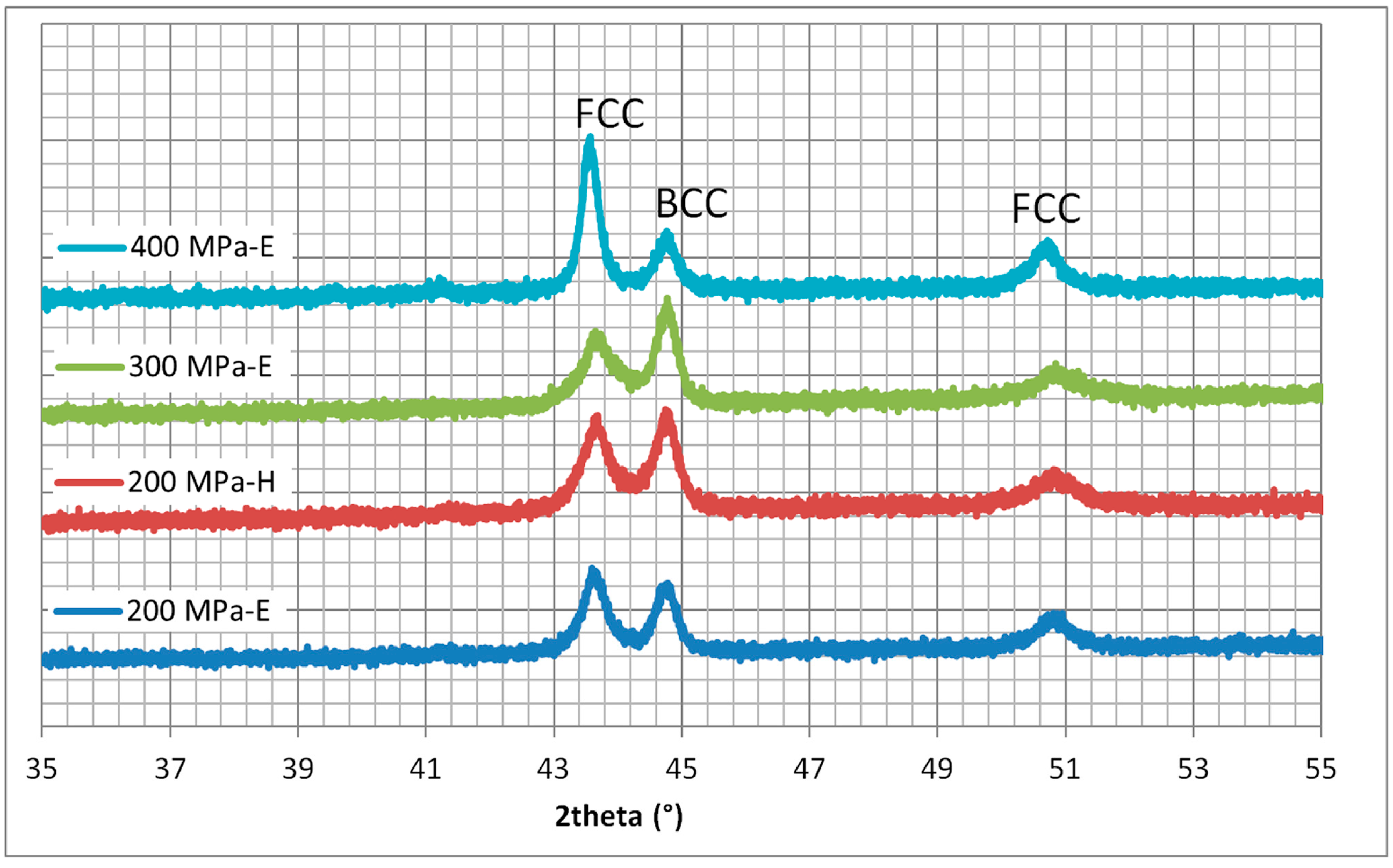
Diffraction results are consistent with the formation of two solid solutions, one FCC and one BCC, in agreement with the literature results on similar systems [42]. The other studied systems show similar diffraction patterns, but with the formation of a single solid solution The heating in a predominant electric or magnetic field did not significantly affect the phase formation, but only the proportion (the ratio between the (111) peak of the FCC phase, at 43.5°, and the (100) peak of the BCC phase, at 44.6°). However, this effect is smaller than the one induced by a different forming pressure applied.
The microstructural difference between the condition existing prior to synthesis and after is evident in Figure 6, where backscattered electrons have been used to identify the element type using a grey scale.
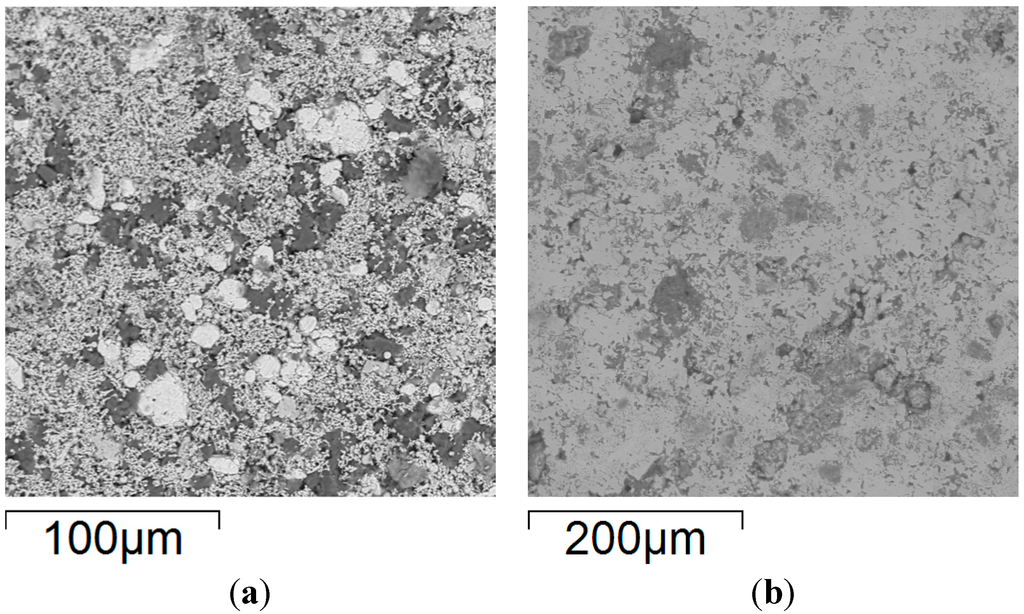
Figure 6.
Backscattered electron SEM micrographs of FeCoNiCuAl samples’ surface: (a) after uniaxial pressing, 300 MPa; and (b) after microwave heating at 240 W, 5800 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
Figure 6.
Backscattered electron SEM micrographs of FeCoNiCuAl samples’ surface: (a) after uniaxial pressing, 300 MPa; and (b) after microwave heating at 240 W, 5800 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
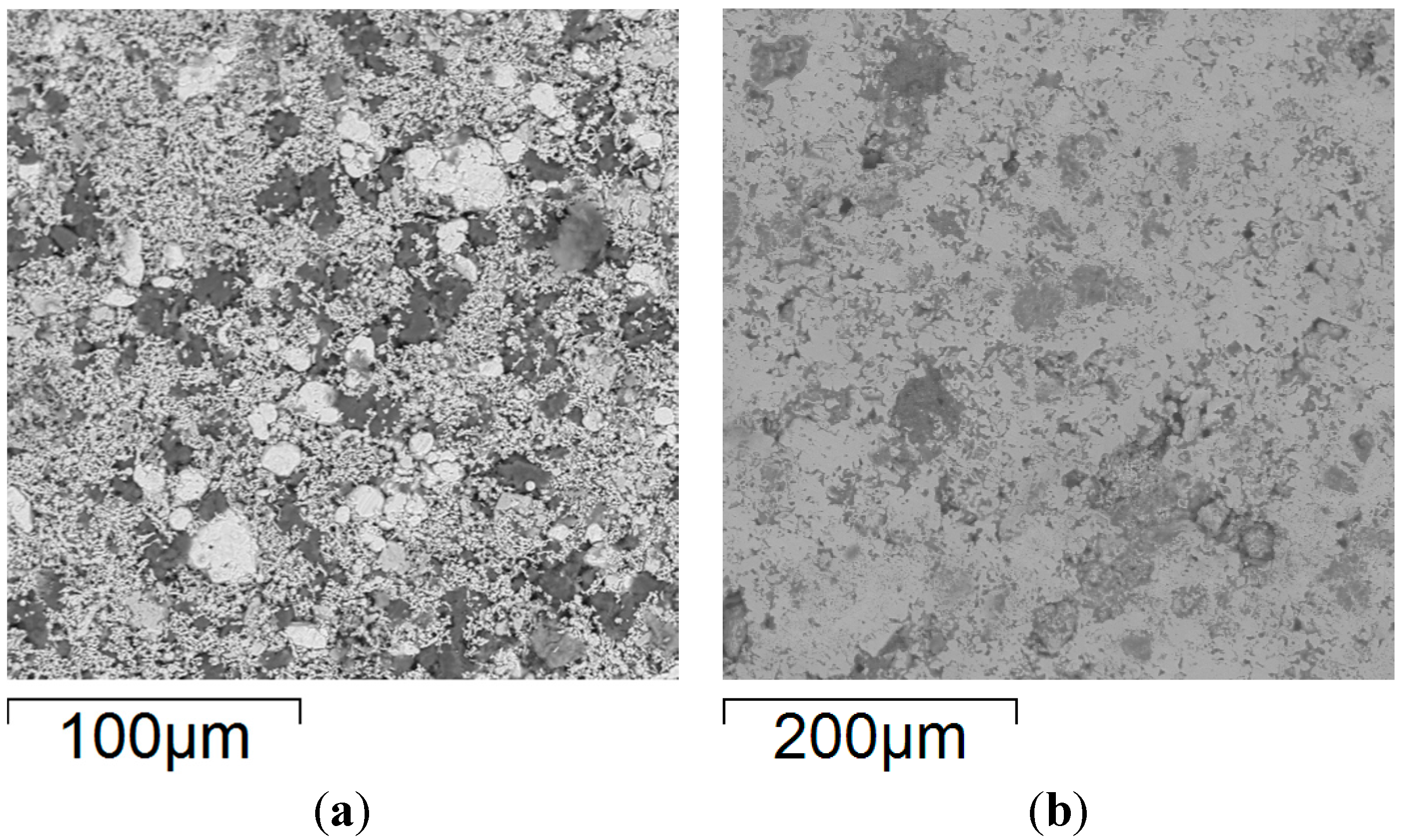
Rather interestingly, the sample exposed to microwaves still presents regions with different grey scales, indicating a microstructure that is not perfectly homogenous, as evidenced in detail in the elemental maps of Figure 7.
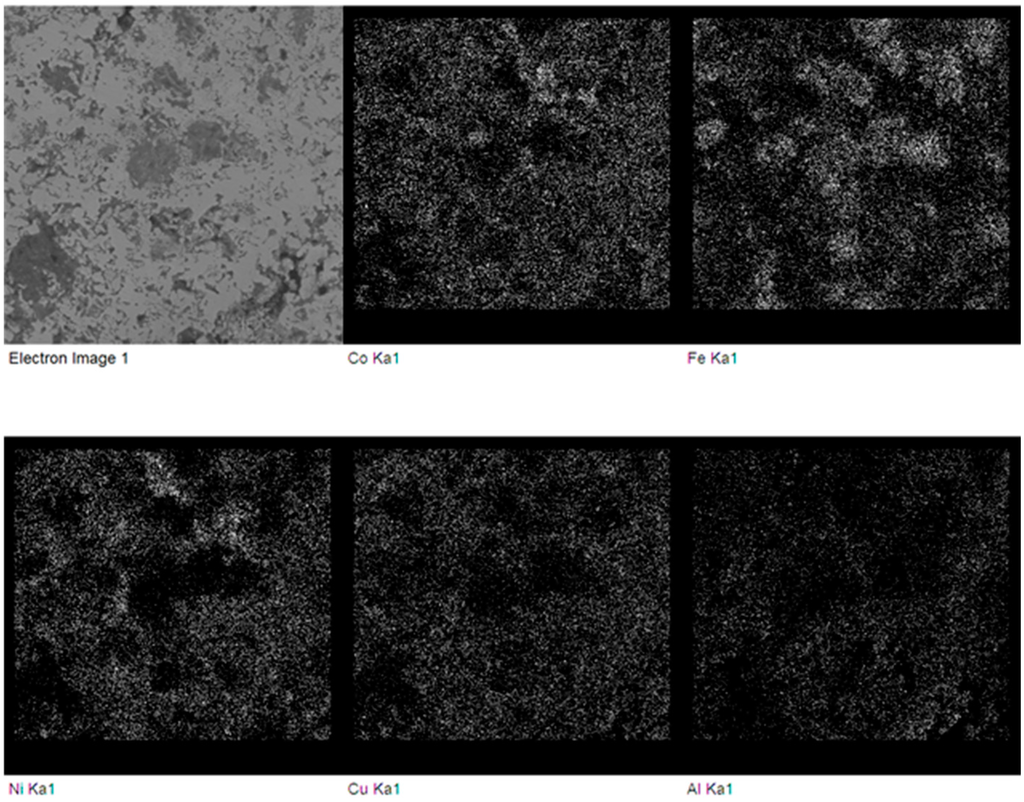
Figure 7.
Backscattered electron SEM micrograph and elemental maps of the FeCoNiCuAl sample after microwave heating at 240 W, 5800 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa). White dots indicate a higher concentration of the element specified below each map.
Figure 7.
Backscattered electron SEM micrograph and elemental maps of the FeCoNiCuAl sample after microwave heating at 240 W, 5800 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa). White dots indicate a higher concentration of the element specified below each map.
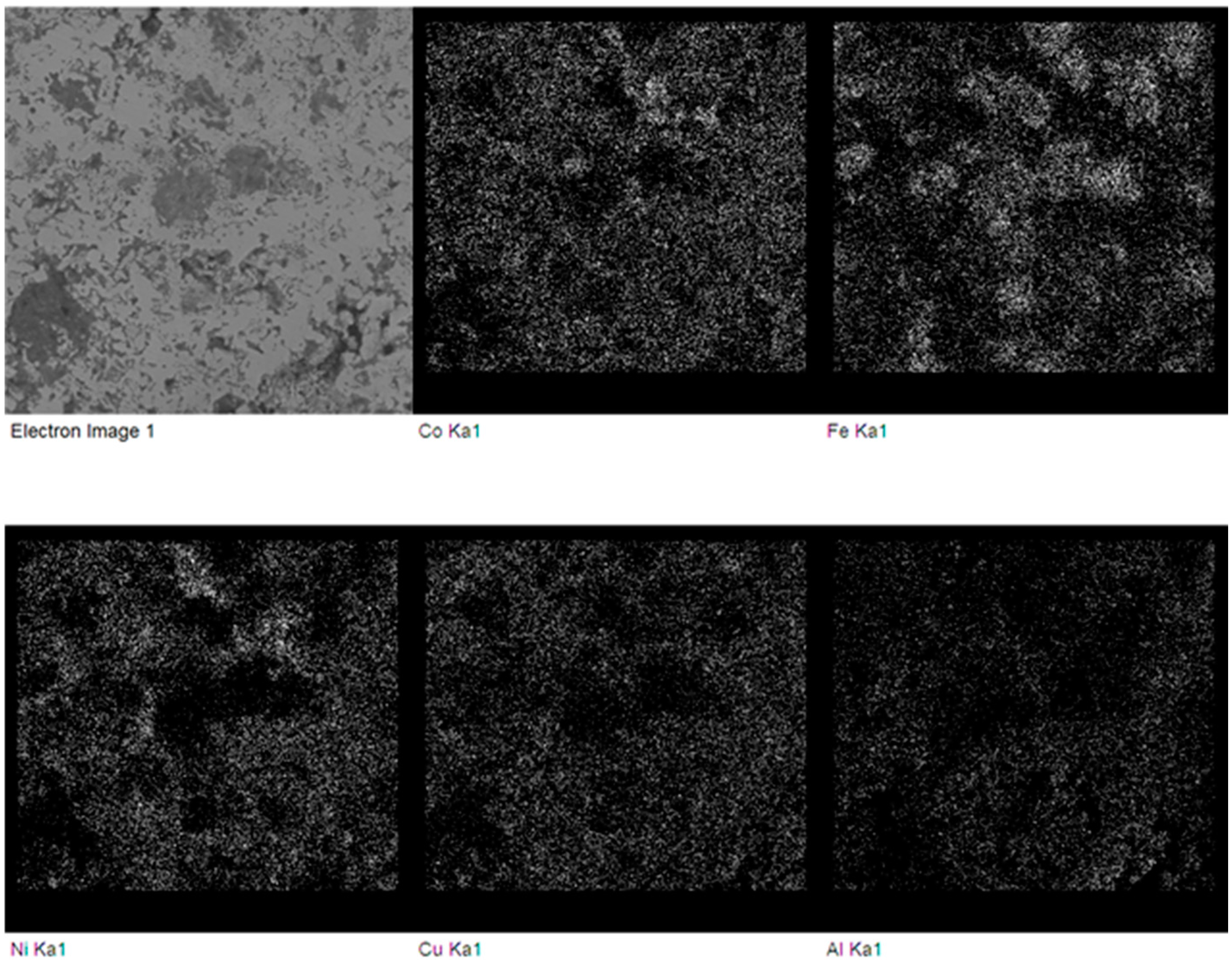
In particular, the presence of partially-unreacted iron is evident, probably due to the much larger particle size of the Fe reactant powders (light grey spheres in Figure 6a) compared to the other ones (except for Al (darker regions in Figure 6a), but the latter is in the molten state during synthesis).
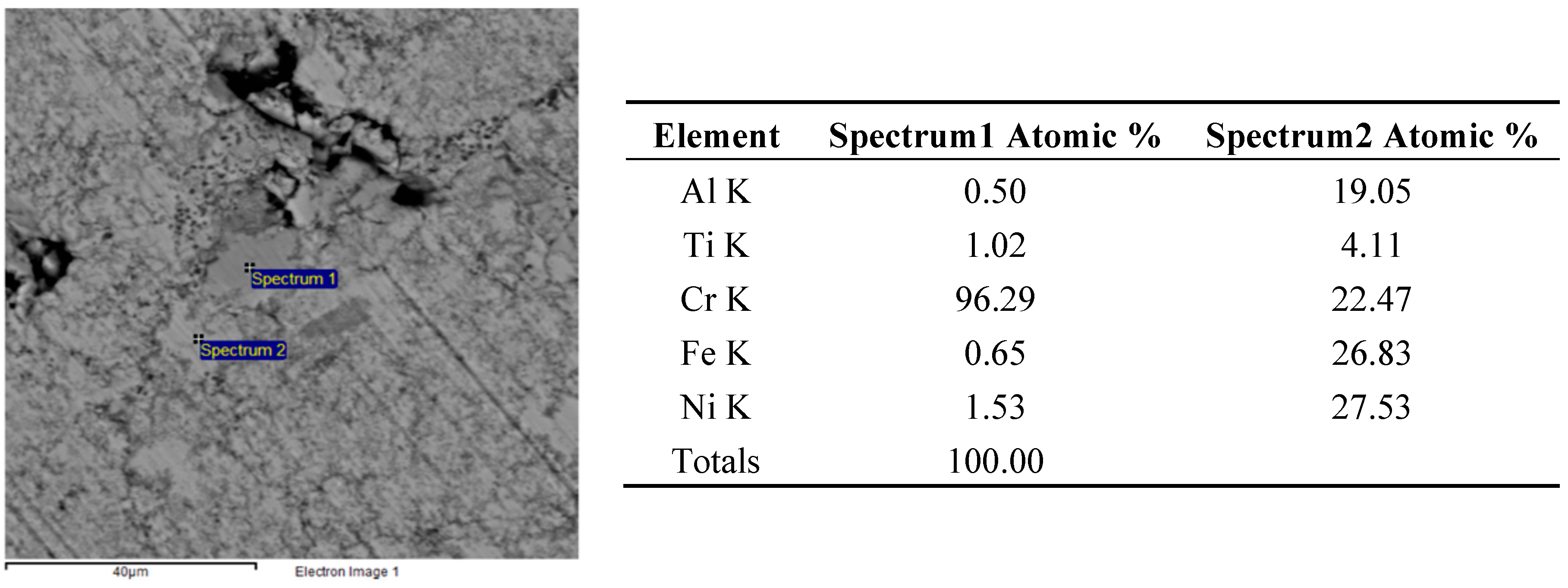
The higher reactivity in the FeCrNiTiAl system allowed reducing such effects, as shown in the much more homogenous microstructure of Figure 8. However, some unreacted particles still remain, like the Cr particles shown in Figure 9. Also in this case, the starting particle size of Cr powders is too large to ensure a complete homogenization, suggesting that a smaller particle size should be used or a supplementary high energy milling stage should be performed prior to forming. In some other cases, local depletion of a single element was detected, as well (Spectrum 2 of Figure 9).
Figure 8 and Figure 9 show another important aspect of the microstructure of the HEA manufactured by microwave heating, i.e., the presence of porosity. This is typical of sintered products, like the powder metallurgy ones, and can be further reduced by hot isostatic pressing or increasing the green density or the processing temperature. However, this topic is outside the scope of the present paper.
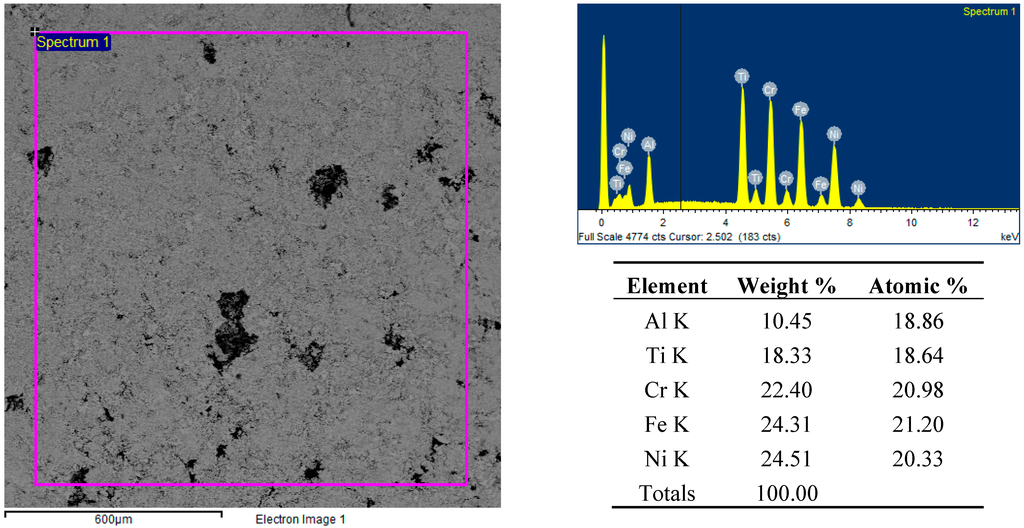
Figure 8.
Backscattered electron SEM micrographs, EDS spectra and elemental analysis of the FeCrNiTiAl sample’s surface after microwave heating at 700 W, 2450 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
Figure 8.
Backscattered electron SEM micrographs, EDS spectra and elemental analysis of the FeCrNiTiAl sample’s surface after microwave heating at 700 W, 2450 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
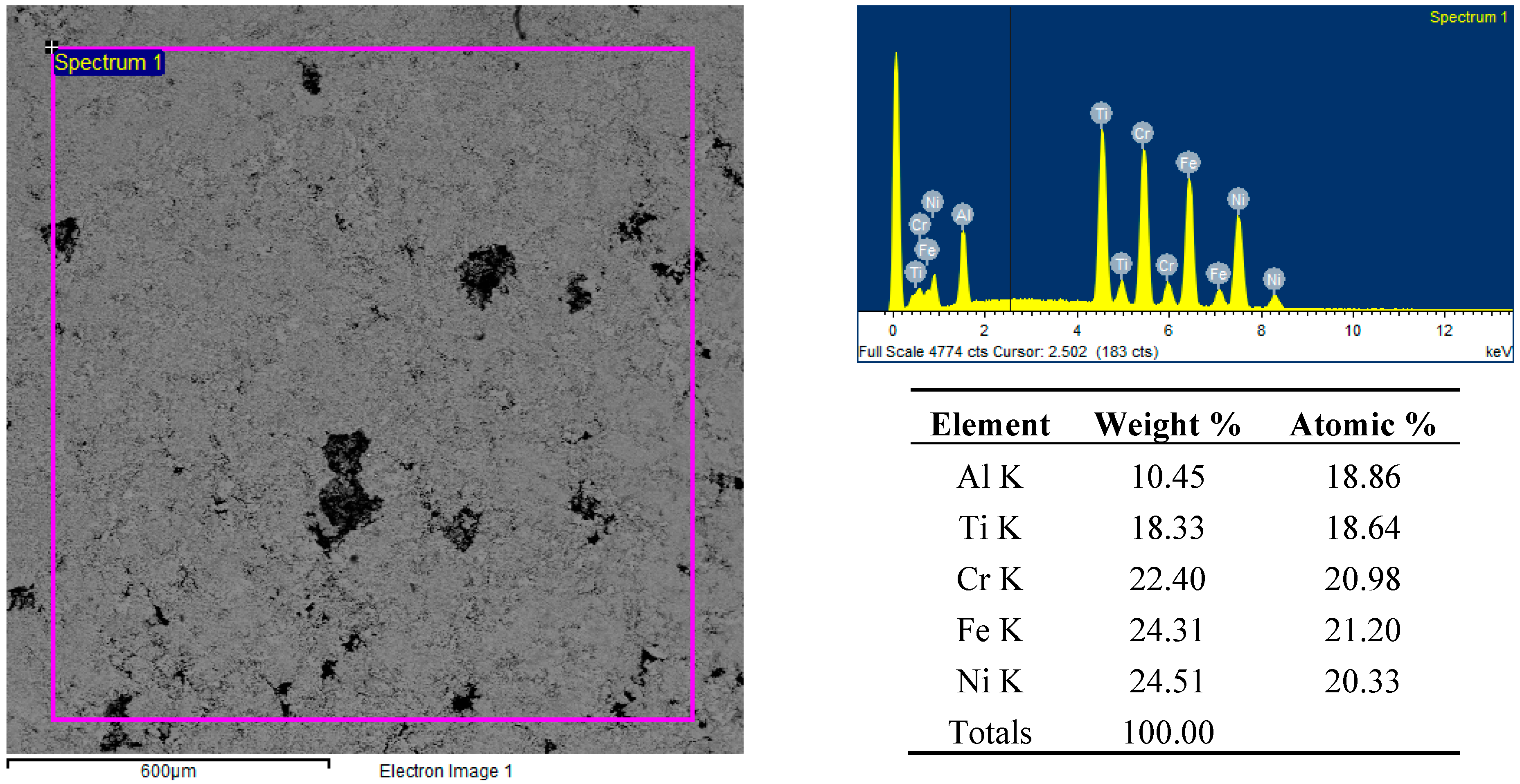
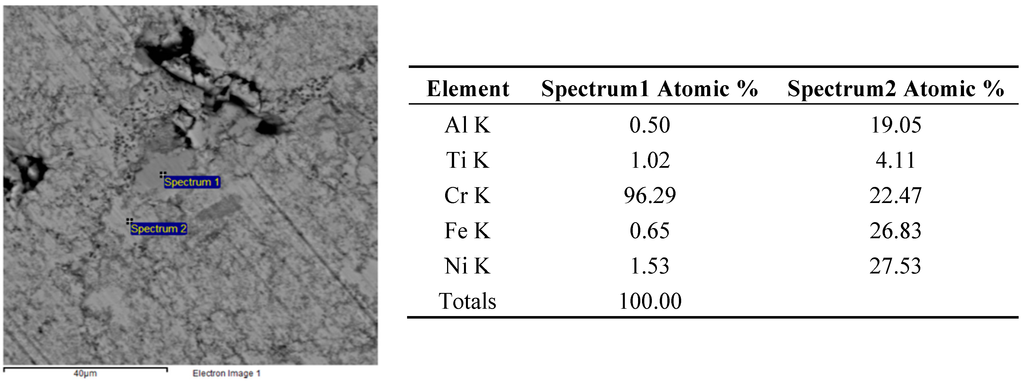
Figure 9.
Backscattered electron SEM micrographs and EDS elemental analysis of the FeCrNiTiAl areas indicated by “Spectrum 1” and “Spectrum 2” after microwave heating at 700 W, 2450 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
Figure 9.
Backscattered electron SEM micrographs and EDS elemental analysis of the FeCrNiTiAl areas indicated by “Spectrum 1” and “Spectrum 2” after microwave heating at 700 W, 2450 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
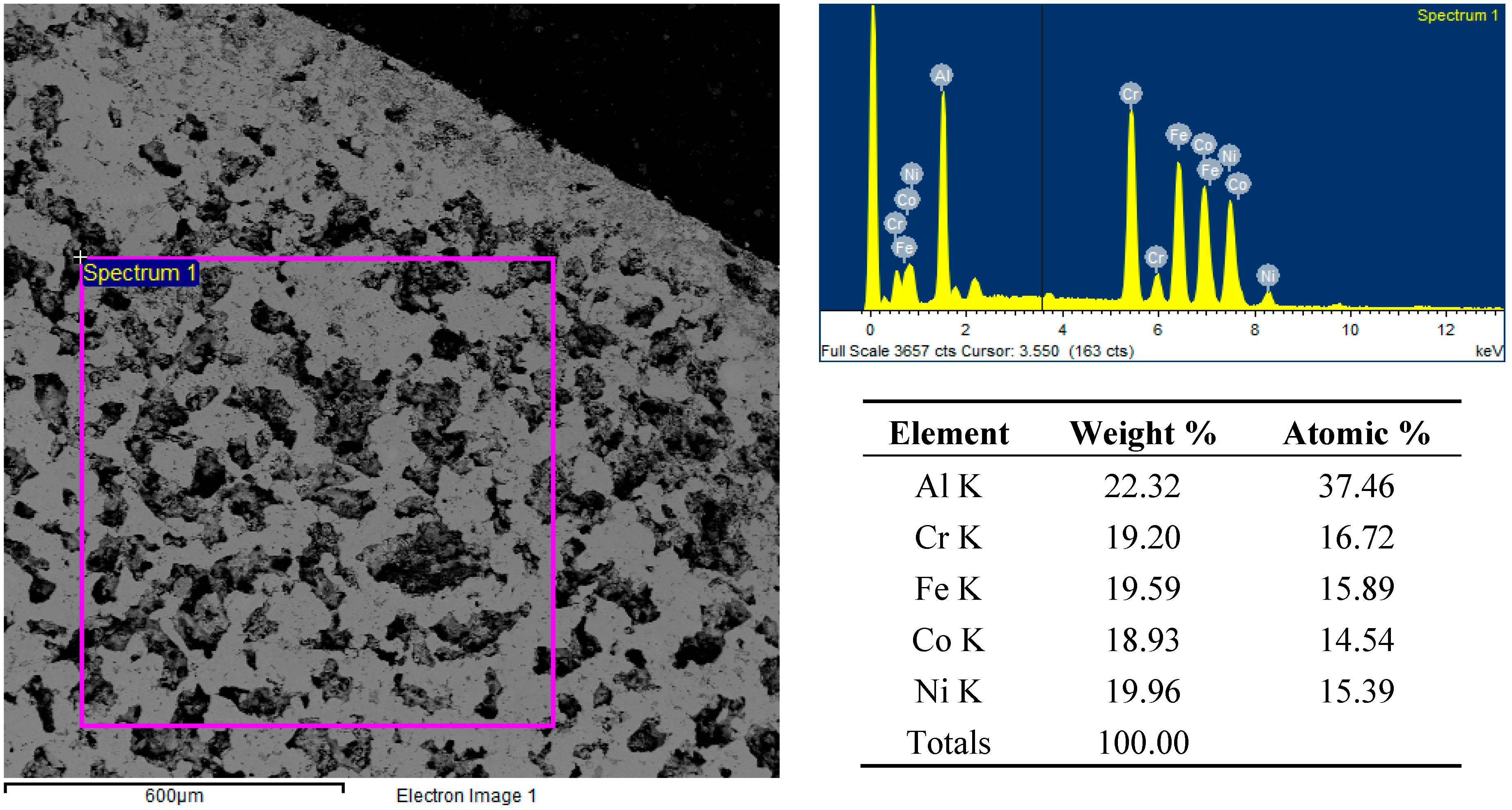
In the case of the FeCoCrNiAl2.5 system, the abundance of aluminum allowed minimizing the segregation phenomena, due to the formation of an Al-rich liquid phase during heating, able to wet the remaining powders and to promote reactions and diffusion. Nevertheless, the porosity remains quite high, but more round shaped, as shown in Figure 10.
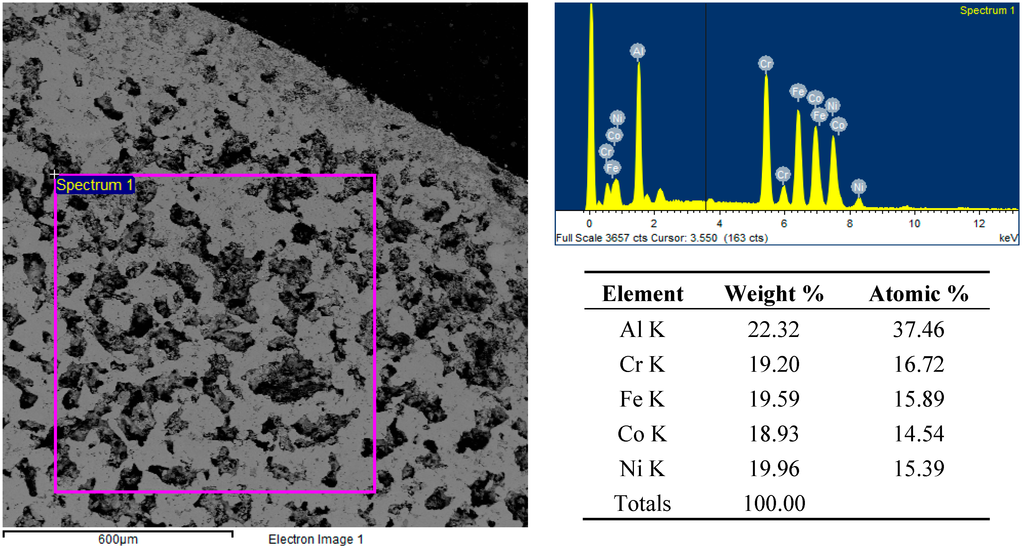
Figure 10.
Backscattered electron SEM micrographs and EDS spectra and elemental analysis of the FeCoCrNiAl2.5 sample’s surface after microwave heating at 700 W, 2450 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
Figure 10.
Backscattered electron SEM micrographs and EDS spectra and elemental analysis of the FeCoCrNiAl2.5 sample’s surface after microwave heating at 700 W, 2450 MHz, in regions of the predominant electric field of the applicator (forming pressure = 300 MPa).
Micro-hardness measurements, conducted only in the dense regions of the sample, provided a value of 638 ± 69 HV03, slightly lower than literature results regarding similar high entropy alloys, but Si-modified and deposited by laser cladding and annealed at 1000 °C [43].
4. Conclusions
High entropy alloys containing at least one ferromagnetic component and one highly reactive element, like Al or Ti, have been successfully prepared by microwave heating of metallic powder compacts. In this study, FeCoNiCuAl, FeCrNiTiAl and FeCoCrNiAl2.5 high entropy alloys have been synthesized using microwave-assisted combustion synthesis ignited and sustained in predominant electric or magnetic field regions of rectangular single-mode applicators, operating at 2450 and 5800 MHz. Results show that direct microwave heating of the powder precursors occurs, until the ignition conditions are reached. They roughly correspond to the melting point of aluminum, indicating that the reaction mechanism is between the Al-rich phase and the remaining metal particles. After ignition, the heat generated by the reaction is enough to rapidly heat the load, which then starts cooling when the reaction stops. Microwave heating proceeds, in the studied systems, allowing one to alter the cooling curve and, hence, to extend the time at high temperature. This is expected to improve the homogeneity of the final product, but this was not directly observed in this study, probably due to the too short microwave exposure time after synthesis (lower than 50 s).
Results showed also that microwave heating in predominant magnetic field regions allows heating more of the products, after the reaction occurred, thus further reducing the cooling rate. Noticeably, the temperature and duration of the microwave-assisted process, in pressureless conditions, results in being much lower than other conventional powder metallurgy routes, but at the cost of a high residual porosity.
Sample characterization confirmed that the powder metallurgy approach is suitable to retain the shape of the load imparted during forming by uniaxial pressing. The homogeneity of the samples resulted in being good in all cases, without the typical dendritic segregation occurring by arc melting, even if some partially-unreacted powders were detected in the samples. This has been ascribed to the too large particle size of some of the reactants, suggesting that finer ones should be used in future studies or that a pre-milling stage should be introduced. Some lack of homogeneity, like local depletion of some elements, can be ascribed to the sluggish diffusion phenomenon, typical of many high entropy alloys. Hence, a practically solid-state synthetic route (with some minor transient liquid phase) like the one proposed in this study has to take into account this issue, which can be overcome by subsequent annealing treatments.
Acknowledgments
The authors are extremely grateful to Marco Garuti, MKS, Alter Products, Reggio Emilia, Italy, for the use of the 5800-MHz generator and applicator and for the reflected power measurements.
Author Contributions
Paolo Veronesi wrote the major part of the paper, collected part of the experimental data and coordinated the research activity. Cristina Leonelli revised the manuscript and coordinated the research activity. Roberto Rosa elaborated the experimental data and revised the manuscript. Elena Colombini collected part of the experimental data. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, Y.-L.; Lin, S.-J.; Chen, S.-K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hong, U.T.; Shih, H.C.; Yeh, J.W.; Duval, T. Electrochemical kinetics of the high entropy alloys in aqueous environments—A comparison with type 304 stainless steel. Corros. Sci. 2005, 47, 2679–2699. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 1–2, 74–78. [Google Scholar] [CrossRef]
- Shun, T.-T.; Du, Y.-C. Age hardening of the Al0.3CoCrFeNiC0.1 high entropy alloy. J. Alloy. Compd. 2009, 478, 269–272. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tsai, C.W.; Juan, C.C.; Chuang, M.H.; Yeh, J.W.; Chin, T.S.; Chen, S.K. Amorphization of equimolar alloys with HCP elements during mechanical alloying. J. Alloy. Compd. 2010, 506, 210–215. [Google Scholar] [CrossRef]
- Dolique, V.; Thomann, A.L.; Brault, P. High-entropy alloys deposited by magnetron sputtering. IEEE Trans. Plasma Sci. 2011, 39, 2478–2479. [Google Scholar] [CrossRef]
- Yao, C.Z.; Zhang, P.; Liu, M.; Li, G.R.; Ye, J.Q.; Liu, P.; Tong, Y.X. Electrochemical preparation and magnetic study of Bi-Fe-Co-Ni-Mn high entropy alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Microstructure and compressive properties of multicomponent Alx(TiVCrMnFeCoNiCu)100−x high-entropy alloys. Mater. Sci. Eng. A 2007, 454–455, 260–265. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B.S. Processing and properties of nanocrystalline CuNiCoZnAlTi high entropy alloys by mechanical alloying. Mater. Sci. Eng. A 2010, 527, 1027–1030. [Google Scholar] [CrossRef]
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 668–670. [Google Scholar]
- Gupta, M.; Wong, E.W. Microwaves and Metals; John Wiley & Sons: Singapore, 2007. [Google Scholar]
- Kingman, S.W. Recent developments in microwave processing of minerals. Int. Mater. Rev. 2006, 51, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Huang, M.; Peng, J. Electromagnetic fields. In Microwave Heating for Metallurgical Engineering; Kwang, M.-H., Yoon, S.-O., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2013. [Google Scholar]
- Teng, W.; Kong, J.; Bingxuan, C. Microstructure and mechanical properties of FeCoNiCuAl high-entropy alloy prepared by microwave-assisted combustion synthesis. Powder Metall. Technol. 2011, 29, 435–438. (In Chinese) [Google Scholar]
- Veronesi, P.; Rosa, R.; Colombini, E.; Leonelli, C.; Poli, G.; Casagrande, A. Microwave assisted combustion synthesis of non-equilibrium intermetallic compounds. J. Microw. Power Electromagn. Energy 2010, 44, 46–56. [Google Scholar]
- Rosa, R.; Veronesi, P. Functionally graded materials obtained by combustion synthesis techniques: A review. In Functionally Graded Materials; Reynolds, N.J.M., Ed.; Nova Science Publishers: New York, NY, USA, 2012; Chapter 2; pp. 93–122. [Google Scholar]
- Colombini, E.; Rosa, R.; Veronesi, P.; Cavallini, M.; Poli, G.; Leonelli, C. Microwave ignited combustion synthesis as a joining technique for dissimilar materials: Modeling and experimental results. Int. J. Self Propag. High Temp. Synth. 2012, 21, 25–31. [Google Scholar] [CrossRef]
- Poli, G.; Sola, R.; Veronesi, P. Microwave-assisted combustion synthesis of NiAl intermetallics in a single mode applicator: Modeling and optimization. Mater. Sci. Eng. A 2006, 441, 149–156. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Leonelli, C. A review on combustion synthesis intensification by means of microwave energy. Chem. Eng. Process. Process Intensif. 2013, 71, 2–18. [Google Scholar] [CrossRef]
- Veronesi, P.; Leonelli, C.; Poli, G.; Casagrande, A. Enhanced reactive NiAl coatings by microwave-assisted SHS. COMPEL 2008, 27, 491–499. [Google Scholar]
- Rosa, R.; Veronesi, P.; Leonelli, C.; Corradi, A.B. Alternative Sintering Processes: Microwave (MW)-Assisted Combustion Synthesis of Micrometric Metallic Powders for the Preparation of Intermetallic-Based Materials. In Proceedings of the PM2010 Powder Metallurgy World Congress, Florence, Italy, 10–14 October 2010; The European Powder Metallurgy Association: Shrewsbury, UK, 2010. [Google Scholar]
- Jablonski, P.D.; Licavoli, J.J.; Gao, M.C.; Hawk, J.A. Manufacturing of High Entropy Alloys. JOM 2015, 67, 2278–2287. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.A.; Krikun, E.V. Microwave Sintering: Fundamentals and Modeling. J. Am. Ceram. Soc. 2013, 96, 1003–1020. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Michelazzi, M.; Leonelli, C.; Boccaccini, A.R. Combination of electrophoretic deposition and microwave-ignited combustion synthesis for the preparation of ceramic coated intermetallic-based materials. Surf. Coat. Technol. 2012, 206, 3240–3249. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Leonelli, C.; Corradi, A.B.; Ferraris, M.; Casalegno, V.; Salvo, M.; Han, S.H. Microwave activated combustion synthesis and compaction in separate E and H fields: Numerical simulation and experimental results. Adv. Sci. Technol. 2011, 63, 197–202. [Google Scholar] [CrossRef]
- Dube, D.C.; Ramesh, P.D.; Cheng, J.; Lanagan, M.T.; Agrawal, D.; Roy, R. Experimental Evidence of Redistribution of Fields During Processing in a High-Power Microwave Cavity. Appl. Phys. Lett. 2004, 85, 3632–3624. [Google Scholar] [CrossRef]
- Cammarota, G.P.; Casagrande, A.; Poli, G.; Veronesi, P. Ni-Al-Ti coatings obtained by microwave assisted SHS: Effect of annealing on microstructural and mechanical properties. Surf. Coat. Technol. 2009, 203, 1429–1437. [Google Scholar] [CrossRef]
- Suzuki, M.; Ignatenko, M.; Yamashiro, M.; Tanaka, M.; Sato, M. Numerical study of microwave heating of micrometer size metal particles. ISIJ Int. 2008, 48, 681–684. [Google Scholar] [CrossRef]
- Morsi, K. Review: Reaction synthesis processing of Ni-Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15. [Google Scholar] [CrossRef]
- Itin, V.I.; Bratchikov, A.D.; Postnikova, L.N. Use of combustion and thermal explosion for the synthesis of intermetallic compounds and their alloys. Powder Metall. Met. Ceram. 1980, 19, 315–318. [Google Scholar] [CrossRef]
- Metaxas, A.A.; Meredith, R.J. Industrial Microwave Heating; The Institution of Engineering and Technology (IET): Herts, UK, 1983. [Google Scholar]
- Qiu, X.-W. Microstructure and properties of AlCrFeNiCoCu high entropy alloy prepared by powder metallurgy. J. Alloy. Compd. 2013, 555, 246–249. [Google Scholar] [CrossRef]
- Couzinié, J.P.; Dirras, G.; Perrière, L.; Chauveau, T.; Leroy, E.; Champion, Y.; Guillot, I. Microstructure of a near-equimolar refractory high-entropy alloy. Mater. Lett. 2014, 126, 285–287. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Nanocrystalline CoCrFeNiCuAl high-entropy solid solution synthesized by mechanical alloying. J. Alloy. Compd. 2009, 485, L31–L34. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, Y.L.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Tsau, C.H.; Lin, S.J.; Chang, S.Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Hui, Z.; Ye, P.; Yizhu, H. Laser cladding FeCoNiCrAl2Si high entropy alloy coating. Acta Metall. Sin. 2011, 47, 1075–1079. (In Chinese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
