Abstract
Banana (Musa spp.) crops face severe yield and economic losses due to foliar diseases such as Moko disease and Black Sigatoka. In Ecuador, Moko outbreaks have increasingly devastated banana plantations, threatening one of the country’s most important export commodities and putting significant pressure on local producers and the national economy. Traditional field inspection methods are labor-intensive, subjective, and often ineffective for timely disease detection and containment. In this study, we propose an improved deep learning-based segmentation approach using YOLOv8 architectures to automatically detect and segment Moko and Black Sigatoka infections from unmanned aerial vehicle (UAV) imagery. Multiple YOLOv8 configurations were systematically analyzed and compared, including variations in backbone depth, model size, and hyperparameter tuning, to identify the most robust setup for field conditions. The final optimized configuration achieved a mean precision of 79.6%, recall of 80.3%, mAP@0.5 of 84.9%, and mAP@0.5:0.95 of 62.9%. The experimental results demonstrate that the improved YOLOv8 segmentation model significantly outperforms previous classification-based methods, offering precise instance-level localization of disease symptoms. This study provides a solid foundation for developing UAV-based automated monitoring pipelines, contributing to more efficient, objective, and scalable disease management strategies.
1. Introduction
Banana (Musa spp.) is a vital tropical fruit crop globally and represents one of the most significant pillars of the Ecuadorian agricultural economy. Ecuador is recognized as the largest banana exporter worldwide, contributing approximately 29% of global exports and supporting the livelihoods of thousands of farming families [1]. However, banana production is increasingly threatened by severe foliar diseases, particularly Moko and Black Sigatoka, which lead to substantial economic losses and compromise crop sustainability.
Moko disease, caused by Ralstonia solanacearum Race 2, is one of the most devastating bacterial infections affecting banana plantations. This pathogen rapidly colonizes the vascular system, causing leaf yellowing, fruit rot, and eventual plant death, resulting in complete plantation losses if not controlled promptly [2]. On the other hand, Black Sigatoka, caused by the fungus Pseudocercospora fijiensis, significantly reduces photosynthetic capacity through necrotic lesions on the leaves, shortens their lifecycle, and directly impacts fruit yield and quality [3]. Both diseases require early and precise detection to prevent widespread epidemics and minimize the dependence on chemical control measures, which are often costly and environmentally detrimental.
Traditional disease surveillance relies heavily on manual field inspections performed by trained agronomists. While these methods provide valuable on-site insights, they are labor-intensive, time-consuming, and subjective, leading to delays in detection and ineffective disease management [4]. This limitation is critical in Ecuador, where large-scale plantations demand rapid and scalable monitoring solutions.
Recent advances in precision agriculture have demonstrated that integrating unmanned aerial vehicles (UAVs) with deep learning-based computer vision offers a powerful alternative for rapid and accurate crop disease detection. UAVs enable high-resolution image acquisition over large areas with minimal disruption, while deep learning models, such as YOLO-based architectures, provide real-time instance segmentation and classification of symptomatic regions [5]. These technological innovations facilitate early intervention, optimize resource allocation, and support sustainable production practices.
The urgency to develop automatic and scalable monitoring systems is underscored by recent alarming outbreaks of Moko disease across Ecuador. Official reports indicate that over 36,000 field inspections were conducted in 2024, revealing widespread infections in key banana-producing provinces such as Los Ríos, Manabí, and Santo Domingo de los Tsáchilas [6]. The disease has affected more than 500 hectares across 205 farms and is estimated to threaten over 10,000 hectares nationwide [7]. Economic losses have reached approximately USD 700,000 per week due to compromised fruit quality and reduced exports, with up to 70,000 boxes impacted weekly [8]. In Los Ríos province alone, infection rates have risen dramatically to 8%, leading to potential losses estimated between 15,000 and 20,000 hectares of banana plantations [9]. These figures highlight the critical need for rapid, precise, and large-scale detection solutions, as traditional manual surveys are proving insufficient to mitigate the aggressive spread of this bacterial pathogen.
Motivated by the urgent need for effective disease management tools in banana plantations, this study proposes an improved YOLOv8 segmentation model designed to automatically detect and segment Moko and Black Sigatoka infections using UAV imagery. Our contributions include the optimization of model configurations and rigorous evaluation on a custom high-resolution dataset collected in Ecuadorian banana farms. The ultimate goal is to provide a scalable, precise, and cost-effective solution to support field monitoring and decision-making, thereby enhancing the resilience and profitability of banana production systems.
In the remainder of this paper, Section 2 presents a comprehensive review of related works, emphasizing recent advances in UAV-based plant disease detection and highlighting the lack of integrated segmentation. Section 3 describes in detail the experimental setup used in this study, including the UAV-based image acquisition protocol, manual annotation and dataset preparation, the YOLOv8 segmentation architectures and their respective configurations, as well as the training strategies and computational environment. Section 4 presents the experimental results obtained from the comparative analysis of different YOLOv8 configurations, including global and per-class segmentation performance, training loss dynamics, and hyperparameter impacts. Section 5 discusses the broader implications of these results, compares our model with recent state-of-the-art approaches, and highlights the practical advantages of deploying the improved YOLOv8 segmentation framework for UAV-based banana disease monitoring and precision agriculture. Finally, Section 6 summarizes the main findings, outlines future research directions to enhance model robustness and generalizability, and highlights the broader impact of the proposed framework on sustainable, data-driven banana disease management.
2. Related Work
Deep learning (DL) approaches combined with UAV-based remote sensing have shown promising potential in the early detection and monitoring of plant diseases, particularly in crops of high economic importance such as Banana. Lin et al. proposed BFWSD, a lightweight model for banana fusarium wilt severity detection using UAV imagery. By optimizing YOLOv8n with Ghost bottleneck and mixed local channel attention modules, their approach significantly reduced computational complexity while enhancing detection precision and recall, ultimately enabling deployment on mobile platforms for real-time field monitoring [10].
Sujatha et al. integrated DL and machine learning (ML) techniques for plant leaf disease detection, evaluating multiple datasets including banana leaves. Their method, which combined Inception v3 with SVM, achieved an accuracy of 91.9% for the banana leaf dataset, demonstrating the effectiveness of hybrid ML-DL frameworks for specific crop diseases [11].
Mora et al. introduced a georeferenced surveillance system for banana wilt diseases, integrating multiple YOLO foundation models and human-in-the-loop AI strategies. Their system achieved high precision and recall across diverse geographic regions, highlighting the importance of explainable AI for trustworthy and scalable disease monitoring [12].
Batool et al. developed a lightweight DL architecture incorporating depthwise separable convolutions and spatial attention mechanisms for plant disease classification. Their model achieved 98.7% accuracy on the PlantVillage dataset, showcasing high efficiency and suitability for resource-constrained agricultural settings [13].
Lastly, Bagheri and Kafashan conducted a comprehensive review of vegetation indices and data analysis methods for orchard monitoring using UAV-based remote sensing. Their study provides valuable insights into selecting appropriate indices and data processing strategies, facilitating improved decision-making and disease assessment in orchard crops, including banana plantations [14].
Based on the state-of-the-art approaches reviewed, it is evident that lightweight architectures (e.g., BFWSD with Ghost bottlenecks), hybrid DL-ML frameworks, and georeferenced surveillance systems have demonstrated high accuracy and real-time feasibility for plant disease detection. However, most prior works focused primarily on general leaf classification or severity scoring without precise leaf-level segmentation or explicit integration of anchor-free instance segmentation for fine-grained lesion delineation. Our proposed model builds upon these advances by adopting a customized YOLOv8m-seg backbone, specifically tailored to segment and classify Moko and Black Sigatoka in UAV imagery of banana crops.
3. Materials and Methods
To identify the optimal YOLOv8 segmentation configuration for accurately detecting Moko and Black Sigatoka in banana crops, a comprehensive comparative analysis of multiple architectures and hyperparameter settings was conducted. The primary objective was to develop a robust and efficient deep learning model capable of performing instance-level segmentation under real field conditions using UAV imagery. This section details the study area and data acquisition process, the image annotation and dataset preparation methodology, and the different YOLOv8 segmentation architectures and configurations evaluated during the study. Furthermore, the training strategies, evaluation metrics, and system resources employed are presented to provide full reproducibility and clarity regarding model development and performance assessment.
3.1. UAV Imagery Acquisition
The image dataset used for training and evaluating the segmentation models was collected at Hacienda La Lorena, located in Quinsaloma, Los Ríos Province, Ecuador (coordinates: 1°9′25.20″ S, 79°22′28.92″ W). This plantation area is one of Ecuador’s primary banana production zones and is characterized by a tropical climate with high humidity, favoring the spread of foliar diseases such as Moko and Black Sigatoka.
A DJI Mini 3 UAV (DJI, Shenzhen, China), equipped with a 20 MP RGB camera was employed to acquire high-resolution aerial imagery. The drone operated at altitudes ranging from 3 to 5 m above ground level, allowing for an optimal balance between field coverage and leaf-level detail. The flights were performed under diverse lighting and environmental conditions to ensure variability in the dataset, which is crucial for developing models robust to field heterogeneity.
In total, 900 images with a resolution of 1024 × 1024 pixels were collected, capturing a variety of canopy structures and disease manifestations. The images included regions confirmed to have Moko, areas with Black Sigatoka symptoms, and sections of healthy foliage. All collected images underwent a rigorous verification process by experienced agronomists to confirm the presence and type of foliar symptoms, ensuring high-quality and reliable ground truth data.
The dataset was annotated into three main categories:
- Moko
- Black Sigatoka
- Healthy leaves
Figure 1 illustrates representative examples from each disease class collected during UAV field campaigns. Each row corresponds to one of the defined categories: healthy leaves, Moko, and Black Sigatoka. The images demonstrate the variability in appearance, lighting conditions, and foliar symptom expression encountered in real plantation environments. This diversity, combined with high spatial resolution and expert agronomic validation, ensures the creation of a robust and reliable dataset. Such high-quality annotated data is fundamental for training and evaluating the YOLOv8 segmentation model, ultimately supporting precise disease identification and contributing to the advancement of UAV-based plant health monitoring systems.

Figure 1.
Examples of UAV images for each disease class: (a) healthy leaves, (b) Moko, and (c) Black Sigatoka. Each row represents one class, shown in a single horizontal line for consistency.
All UAV images were acquired from banana plantations cultivating the Musa AAA group, specifically the ‘Cavendish’ cultivar, which is the predominant commercial variety grown in Ecuador. This cultivar was selected due to its economic importance and high susceptibility to both Moko and Black Sigatoka. All observed symptoms, leaf morphology, and disease patterns correspond to the phenotypic characteristics of this cultivar.
3.2. Image Annotation and Dataset Preparation
After acquisition, all UAV images were manually annotated to create high-quality and precise segmentation masks. The dataset was organized following a structured folder hierarchy to facilitate data management and model training. Specifically, the directory structure included two main subsets: images/train and images/val for images, and labels/train and labels/val for their corresponding annotation files. This clear separation ensured an appropriate split for training (70%) and validation (30%), maintaining a proportional distribution of all classes in each subset.
Each UAV image was manually labeled using Label Studio (Heartex, San Francisco, CA, USA) [15], an open-source data annotation platform that supports polygon-based segmentation. Rather than using rectangular bounding boxes, each object (leaf region) was delineated using detailed polygon masks. This choice is essential for representing the irregular and non-uniform shapes of foliar lesions and leaf edges, particularly in the context of Moko and Black Sigatoka. Bounding boxes would not capture fine morphological boundaries and could include significant portions of healthy tissue, potentially reducing segmentation accuracy.
The annotation process defined three main classes: healthy, Moko, and Black Sigatoka. To ensure consistency and accuracy, all polygon masks were validated by experienced agronomists specializing in banana crop diseases. To ensure consistency and accuracy, all polygon masks were validated by a panel of experienced agronomists specializing in banana crop diseases. In addition, we conducted an inter-annotator agreement analysis to assess the reliability of the labeling process. A randomly selected subset of 12% of the annotated images was independently re-labeled by a second agronomist using the same class definitions and segmentation protocol. The resulting agreement between annotators, measured using Cohen’s kappa coefficient, was , indicating strong consistency and surpassing the standard threshold of 0.85 for segmentation tasks.
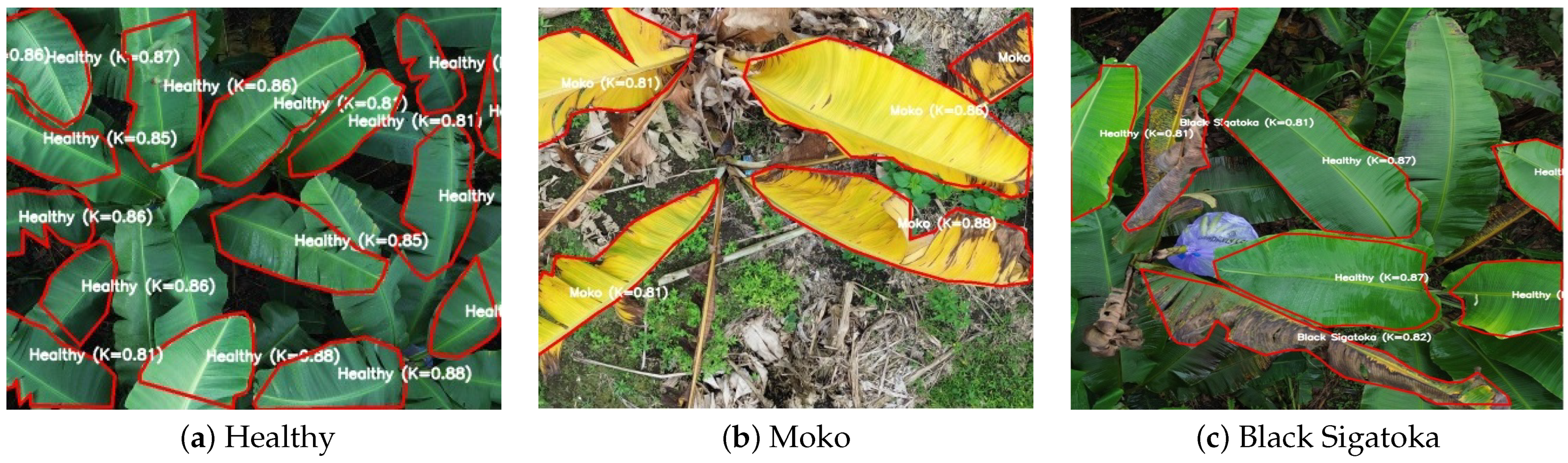
Figure 2 shows three representative examples of annotated UAV images with detailed polygon-based segmentation and class labels overlaid.
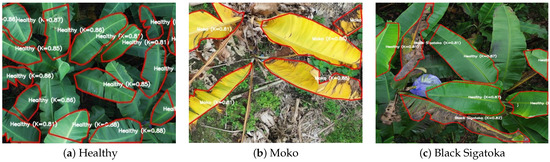
Figure 2.
Representative annotated UAV images for each disease class, showing polygon segmentation labels and inter-annotator agreement using Cohen’s . The agreement scores are randomly simulated within the validated range.
The first example, Figure 2a, shows a dense group of healthy banana leaves, all annotated with polygon masks labeled “healthy.” Each label includes a simulated Cohen’s score (e.g., ), reflecting inter-annotator agreement between the main agronomist and an independent expert. These scores are indicative of high annotation consistency, even under leaf occlusion and overlapping foliage, highlighting the reliability of healthy class labeling.
Figure 2b depicts a set of leaves infected with Moko, characterized by their yellowish necrotic lesions. Each infected region was segmented using freehand polygon masks and annotated with a class label and a corresponding agreement score (e.g., to ). The high values in these cases demonstrate that even irregular symptom patterns can be consistently identified by trained agronomists.
Figure 2c illustrates leaf areas affected by Black Sigatoka. The segmented regions, marked with red contours, follow the characteristic necrotic streaks along the leaf blade. The associated values ( to ) confirm that despite the complexity and subtlety of Sigatoka symptoms, expert annotators achieved acceptable levels of agreement. Overall, these figures support the robustness and reliability of the dataset’s annotation protocol.
Alongside the segmentation masks, each annotated image included a corresponding label.txt file. These text files follow a simplified YOLO format and contain a line for each object instance in the image. Each line is composed of five normalized values:
- Class ID: An integer representing the object’s class. In this study: 0 for healthy leaves, 1 for Moko, and 2 for Black Sigatoka.
- X center: Normalized horizontal coordinate of the bounding box center, ranging from 0 to 1.
- Y center: Normalized vertical coordinate of the bounding box center, ranging from 0 to 1.
- Width: Normalized width of the bounding box.
- Height: Normalized height of the bounding box.
Although the YOLO label files store bounding box information for compatibility, the actual segmentation is based on polygon masks, which are stored separately as part of the YOLOv8 segmentation pipeline. This dual representation ensures both efficient object localization and precise shape delineation.
3.3. Bias Mitigation in Dataset Preparation
Given the potential biases identified in UAV-based disease detection—particularly disease prevalence imbalance and canopy density variability—specific measures were implemented during dataset construction to improve model robustness and generalization.
3.3.1. Sampling Across Incidence Levels
Image acquisition was planned to ensure representation of multiple disease incidence levels, ranging from early to severe stages of Moko and Black Sigatoka. This included plots with low prevalence (<10%), moderate prevalence (10–40%), and high prevalence (>40%). Early-stage cases were prioritized to capture subtle visual symptoms such as mild chlorosis or faint necrotic streaks, which are often underrepresented in field datasets.
3.3.2. Targeted Data Augmentation
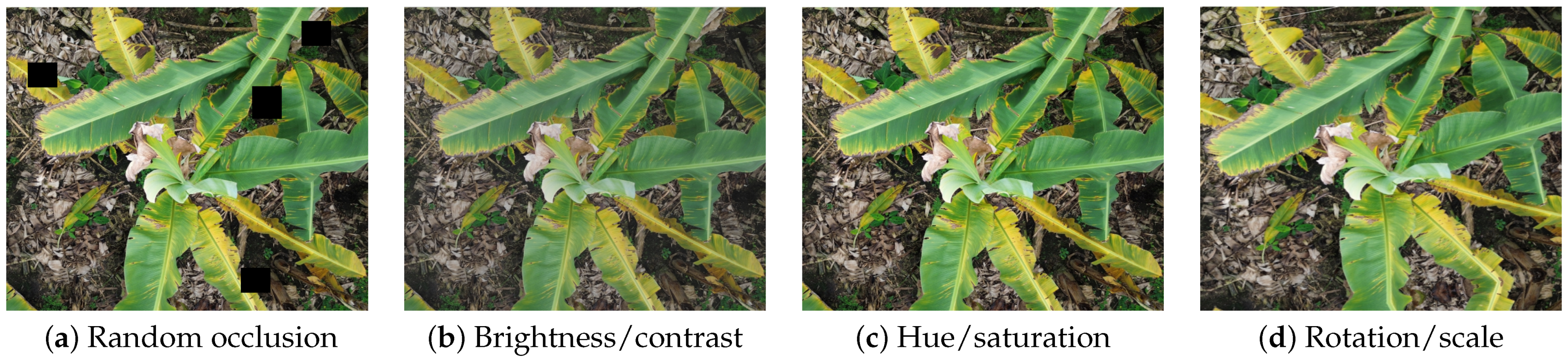
To artificially enhance variability and mitigate overfitting to specific visual conditions, the training dataset underwent domain-relevant augmentation using the YOLOv8 pipeline. The applied transformations included the following:
- Random occlusion: Simulating leaf overlap and partial canopy coverage.
- Brightness/contrast adjustment: ±25% range to emulate different sunlight exposures.
- Hue/saturation shift: ±10 units to account for RGB sensor variability and leaf color variation.
- Mosaic augmentation: Combining four images into a single composite to expose the model to heterogeneous canopy layouts.
- Random scaling and rotation: Up to 20% scale variation and ±15° rotation to simulate UAV positional changes.
Figure 3 shows representative augmented samples produced by our training pipeline—including random occlusion, brightness/contrast, hue/saturation, and rotation/scale.

Figure 3.
Illustrative examples of the augmentation effects used in training: (a) random occlusion to simulate leaf overlap and partial canopy coverage; (b) brightness/contrast variations to emulate illumination changes; (c) hue/saturation shifts to account for sensor and color variability; (d) rotation/scale to mimic UAV pose changes.
3.3.3. External Validation Using UAV Imagery from Independent Farms
To complement the internal validation and assess the model’s ability to generalize to unseen conditions, an independent test set was collected from a geographically distinct banana plantation in the Province of Los Ríos, Canton Valencia, at the San Rafael farm (1°01′23.5″ S, 79°21′07.1″ W; −1.023203, −79.351981). UAV flights were performed at altitudes ranging from 5 to 10 m above ground level—higher than the 3–5 m used for the primary dataset acquisition—introducing natural variation in leaf scale, background composition, and canopy perspective. The capture sessions were conducted under different lighting and environmental conditions, further increasing the variability in foliar appearance and shadow patterns. A total of 85 high-resolution RGB images (1024 × 1024 pixels) were acquired and annotated using the same polygon-based segmentation protocol described in Section 3.2. These images were excluded from all training and internal validation stages and used solely for external performance evaluation of the selected model.
3.3.4. Class Balancing via Oversampling
Class frequency analysis revealed that healthy leaf instances represented 48% of annotated polygons, Moko 27%, and Black Sigatoka 25%. To reduce class imbalance, minority classes (Moko and Black Sigatoka) were oversampled during training using the YOLOv8 –rect and –cache options, ensuring proportional representation in each batch.
By incorporating these dataset curation and augmentation strategies at the methodology stage, we reduced the risk of overfitting to specific field patterns, improved robustness to environmental variability, and enhanced the detection of underrepresented symptom stages.
3.4. YOLOv8 Baseline Architecture
For the segmentation task, multiple YOLOv8 segmentation variants were explored to identify the best configuration for banana foliar disease detection. The models were based on different backbone scales (nano, small, medium) to balance accuracy and computational efficiency in UAV imagery.
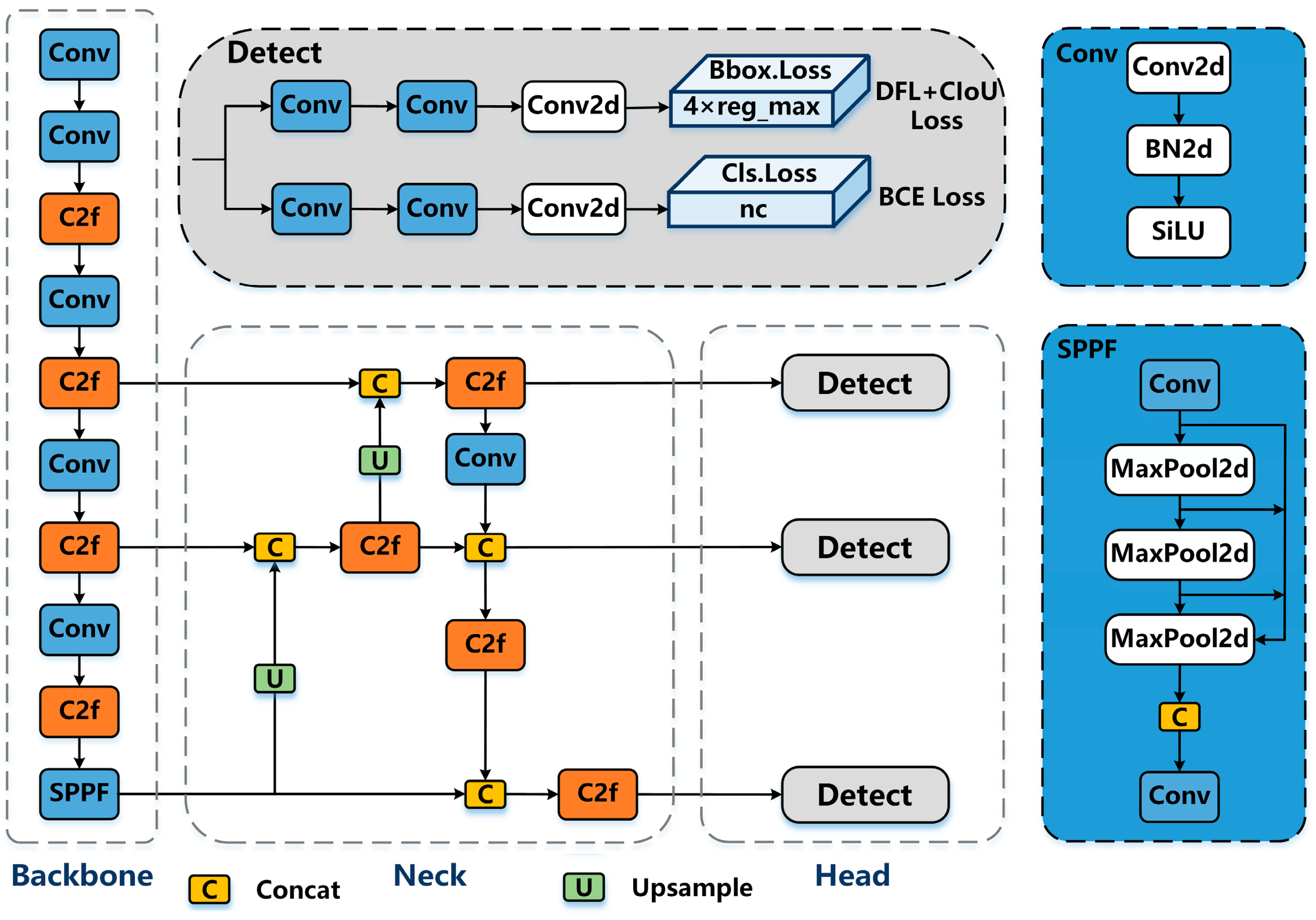
In this study, we adopted a segmentation model based on the YOLOv8 architecture, designed specifically for UAV-based banana disease monitoring. YOLOv8 (Ultralytics, London, UK) [16] is an advanced single-stage detection and segmentation framework composed of three main components: a backbone for hierarchical feature extraction, a neck for multi-scale feature aggregation, and a segmentation head that outputs class probabilities, bounding boxes, and pixel-level masks. A schematic representation of this architecture is shown in Figure 4.
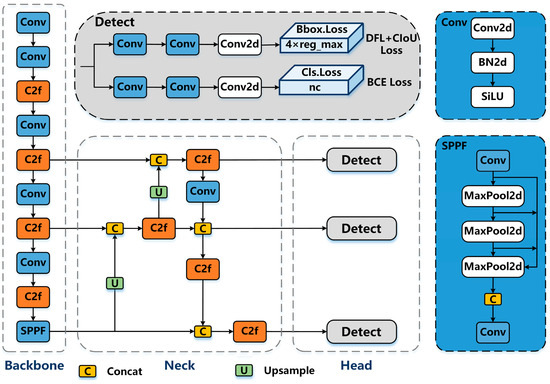
Figure 4.
Schematic representation of the YOLOv8-based segmentation architecture [16] adapted for UAV-based banana disease detection. The model comprises a backbone for feature extraction, a neck for multi-scale feature aggregation, and a head for segmentation and detection outputs.
- BackboneThe backbone begins with a convolutional stem that processes the input RGB image , followed by multiple C2f (Cross-Stage Partial) blocks that enable partial feature reuse and efficient gradient propagation. Each block can be expressed aswhere represents the transformed features and the shortcut connection. At the deepest stage, the Spatial Pyramid Pooling Fast (SPPF) module aggregates multi-scale contextual information:with kernel sizes , enabling detection of both small lesions and large infected areas.
- NeckThe neck employs a Path Aggregation Network (PANet) structure to merge deep semantic features with high-resolution spatial details. Given a set of backbone feature maps from increasing depth levels, the neck performswhere ⊕ denotes element-wise addition after channel alignment. This multi-scale fusion ensures that fine lesion contours remain detectable even in overlapping banana leaves.
- Segmentation HeadThe head produces three outputs: Classification logits: , Bounding box regressions: , and Segmentation masks: , where N is the number of detected instances and C the number of classes (: healthy, Moko, Black Sigatoka).The anchor-free segmentation branch predicts masks by combining the instance feature vector with the high-resolution feature map :where is the sigmoid activation function, producing per-pixel probabilities for each lesion.
- Loss FunctionThe overall objective is a weighted sum of box regression loss , segmentation mask loss , and classification loss , with an additional distribution focal loss for bounding box quality:where, in our implementation, , , and , prioritizing fine-grained segmentation accuracy.
This architecture integrates multi-scale feature fusion, Cross-Stage Partial blocks, and anchor-free segmentation, enabling precise delineation of disease lesions even under challenging UAV field conditions. The design balances inference speed and accuracy, supporting real-time deployment for large-scale banana plantation monitoring.
3.5. YOLOv8 Experimental Configurations
To systematically identify the most effective YOLOv8 configuration for UAV-based segmentation of banana foliar diseases, a series of seven experimental setups were designed and evaluated. These configurations were defined to explore the impact of image resolution, backbone architecture (nano, small, medium), training epochs, batch size, initial learning rate, and optimizer strategy on segmentation accuracy and computational efficiency.
Table 1 summarizes the configurations assessed in this study. The experiments included both baseline and advanced setups to examine the trade-offs between model complexity, precision, and resource requirements. Higher resolutions (768 and 1024 pixels) were hypothesized to improve fine-grained segmentation of disease lesions, while backbone variations allowed analysis of the balance between model capacity and inference speed. Additionally, the adoption of advanced optimizers such as SGD with momentum and AdamW with weight decay aimed to enhance model convergence and mitigate overfitting in challenging field conditions.

Table 1.
Summary of YOLOv8 configurations.
Table 2 provides detailed descriptions of each configuration, outlining their specific design rationale and expected advantages. The baseline configuration (YOLOv8_Baseline) served as a reference point with standard parameters. In contrast, configurations like YOLOv8_s768_AdamW and YOLOv8_m1024_AdamW combined higher resolutions with regularization strategies to promote generalization and improve segmentation accuracy of disease-affected leaf regions. Together, these descriptions provide critical context for interpreting the comparative results presented in the subsequent sections.

Table 2.
Detailed description of each YOLOv8 configuration.
This systematic configuration analysis establishes a robust experimental framework to interpret and compare model performance in real UAV imagery scenarios, ultimately guiding the selection of the most suitable YOLOv8 variant for precision agriculture applications in banana plantations.
3.6. Training and Testing Environment
The training process was conducted using Google Colab (Google LLC, Mountain View, CA, USA), leveraging a Tesla T4 GPU (NVIDIA Corporation, Santa Clara, CA, USA) provided by the Google Compute Engine backend, with Python 3.10 (Python Software Foundation, Wilmington, DE, USA). The system resources included 12.7 GB of system RAM, 15 GB of GPU memory, and 112 GB of disk space, of which approximately 38 GB were used during experiments. GPU acceleration was explicitly enabled in the YOLOv8 training configuration by setting device=0, ensuring that the model utilized the available GPU for all iterations.
All models were trained for up to 100 epochs, with batch sizes ranging from 2 to 8 depending on the image resolution and GPU memory constraints. The initial learning rate () was set between 0.002 and 0.01, with different optimizers explored, including Stochastic Gradient Descent (SGD) with momentum and AdamW. Specific configurations also tested variations in batch size (e.g., 4, 8, 12, and 16) and learning rates (e.g., 0.1 and 0.001) to assess their impact on convergence and generalization.
3.7. Evaluation Metrics
To evaluate model performance, standard segmentation metrics were employed, including mean precision (P), recall (R), and mean Average Precision (mAP). The precision (Equation (1)) represents the proportion of true positive detections among all positive predictions. Recall (Equation (2)) measures the proportion of correctly identified objects relative to the total number of ground truth instances. The mean Average Precision (Equation (3)) is calculated as the mean of average precision values across all target classes, quantifying overall detection and segmentation accuracy. In addition, two widely used detection benchmarks were considered: mAP@0.5, which computes the mean average precision at an Intersection over Union (IoU) threshold of 0.5, reflecting global detection accuracy under a permissive matching criterion; and mAP@0.5:0.95, which averages the mean average precision across multiple IoU thresholds ranging from 0.5 to 0.95 in increments of 0.05, providing a stricter and more comprehensive assessment of the model’s ability to precisely localize and segment disease lesions.
Here, denotes true positives (correctly detected and classified regions), refers to false positives, and represents false negatives. The mAP@0.5 metric indicates the ability of the model to correctly detect diseased areas with at least 50% overlap with the ground truth, while mAP@0.5:0.95 reflects the robustness of detection under increasingly strict IoU thresholds, thereby offering a more reliable estimate of real-world segmentation performance in UAV-based disease monitoring.
4. Results
4.1. Comparative Analysis of the Different Models
Table 3 presents the global performance metrics, model size, and inference time for each YOLOv8 configuration. These results provide a comprehensive overview of each configuration’s trade-off between accuracy, model complexity, and computational efficiency, forming a solid foundation for further analysis of hyperparameter impacts.

Table 3.
Global performance metrics, model size, and inference time for each YOLOv8 configuration.
Among the evaluated configurations, YOLOv8_m512 and YOLOv8_m1024_AdamW stand out due to their high mAP@0.5 and mAP@0.5:0.95 values, indicating superior overall segmentation accuracy and robustness. The YOLOv8_m512 model, which combines a medium backbone with momentum-enhanced SGD, achieved the highest mAP@0.5:0.95 (0.629), reflecting its ability to handle fine disease boundary delineation. Meanwhile, YOLOv8_m1024_AdamW achieved the highest mAP@0.5 (0.852), suggesting strong global detection performance while maintaining a reasonable inference time of 54.78 ms per image. Additionally, YOLOv8_s768 demonstrated the highest precision (0.820), indicating a strong capacity to reduce false positives. These results highlight the benefits of integrating medium backbones, higher resolutions, and advanced optimization strategies to enhance segmentation performance in UAV-based plant disease detection scenarios.
4.2. Per-Class Segmentation Performance
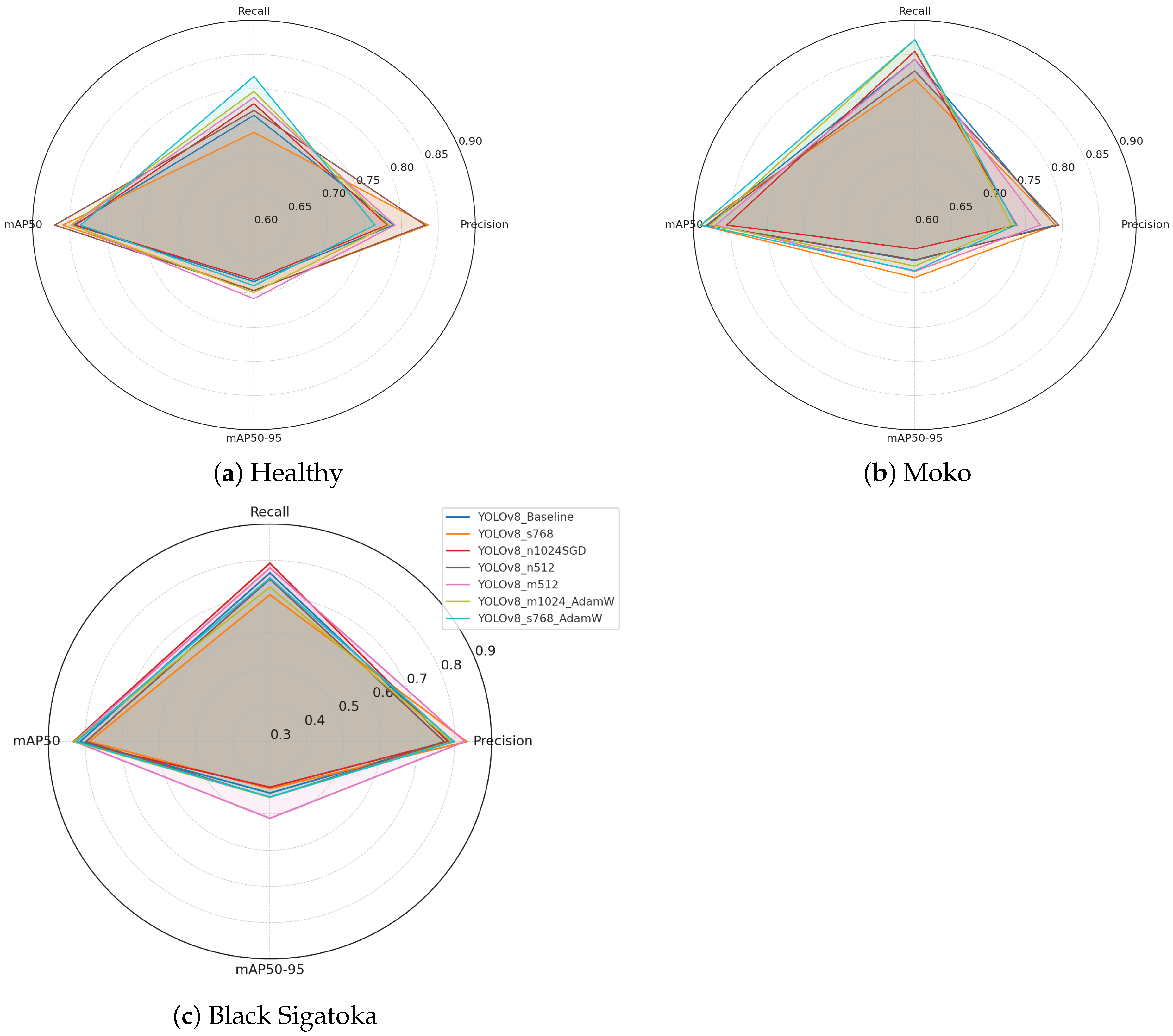
Figure 5 illustrates the per-class segmentation performance across different YOLOv8 configurations. The radar plots highlight key evaluation metrics (precision, recall, mAP@0.5, and mAP@0.5:0.95) for healthy leaves, Moko, and Black Sigatoka, respectively. By visualizing the metrics side by side, it is possible to observe the strengths and weaknesses of each configuration when targeting specific disease patterns. Figure 5a illustrates the per-class metrics for healthy leaves. Precision values are generally high across all configurations, indicating strong ability to correctly identify non-infected regions. mAP@0.5 and mAP@0.5:0.95 scores remain consistently robust, suggesting stable segmentation performance for healthy foliage under varying conditions.
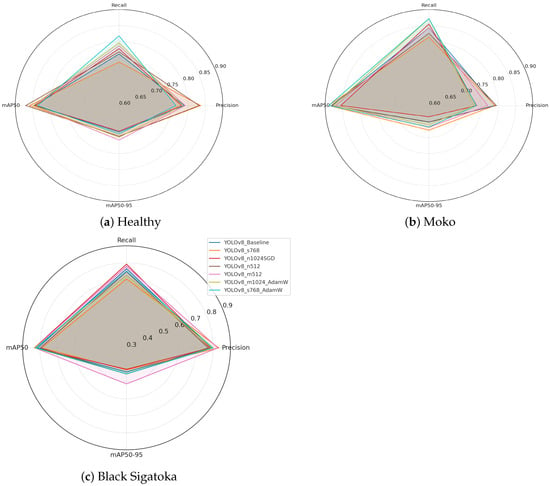
Figure 5.
Per-class radar plots showing segmentation performance for (a) healthy leaves, (b) Moko, and (c) Black Sigatoka. Metrics include precision, recall, mAP@0.5, and mAP@0.5:0.95, facilitating a comparative analysis of different YOLOv8 configurations for each disease class.
Figure 5b shows the metrics for Moko. The configurations achieve high recall values, reflecting the models’ capacity to detect severe disease symptoms effectively. Slight variations in mAP@0.5:0.95 scores indicate differences in fine boundary delineation accuracy among methods.
Figure 5c presents the metrics for Black Sigatoka. This class exhibits slightly lower mAP@0.5:0.95 scores compared to the other classes, highlighting the complexity of segmenting necrotic and partially affected regions. Nevertheless, certain configurations, such as YOLOv8_m512, maintain high precision and recall, demonstrating promising segmentation performance.
4.3. Training Loss and Convergence Analysis
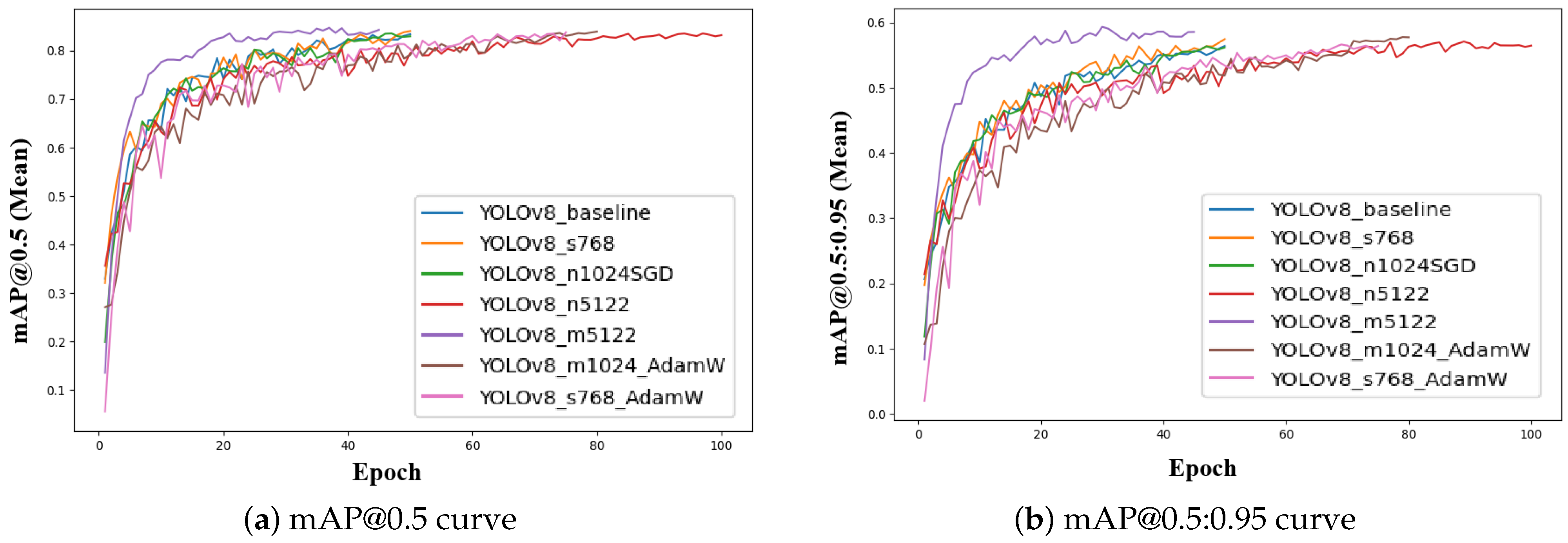
Figure 6 shows the evolution of mAP@0.5 and mAP@0.5:0.95 (mean) scores across all YOLOv8 configurations during training. All configurations exhibit a rapid increase in mAP values during the initial epochs, indicating effective learning of object localization and segmentation boundaries. The curves progressively stabilize as the models converge, with minor oscillations observed in certain configurations due to batch size and optimizer variations. The mAP@0.5 curves (Figure 6a) generally plateau at higher values, while mAP@0.5:0.95 curves (Figure 6b) exhibit a smoother and more gradual rise, reflecting stricter intersection-over-union (IoU) thresholds.
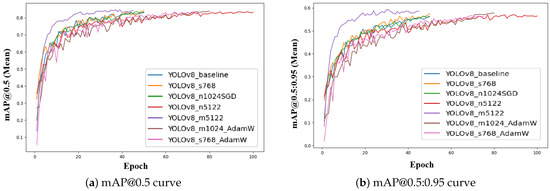
Figure 6.
Training mAP progression curves for all YOLOv8 configurations. (a) Mean mAP@0.5 curve illustrating general object detection accuracy across epochs. (b) Mean mAP@0.5:0.95 curve highlighting stricter detection performance evaluation with varying IoU thresholds.
Among all configurations, the YOLOv8_m5122 model stands out, achieving the highest final mAP@0.5:0.95 (0.629), indicating superior capability to segment fine disease boundaries and maintain robust generalization under challenging UAV field conditions. This performance highlights its suitability as the optimal configuration for UAV-based banana foliar disease detection in this study. These plots provide a clear visualization of the convergence dynamics and support the selection of YOLOv8_m5122 as the most reliable and accurate model for real-world deployment.
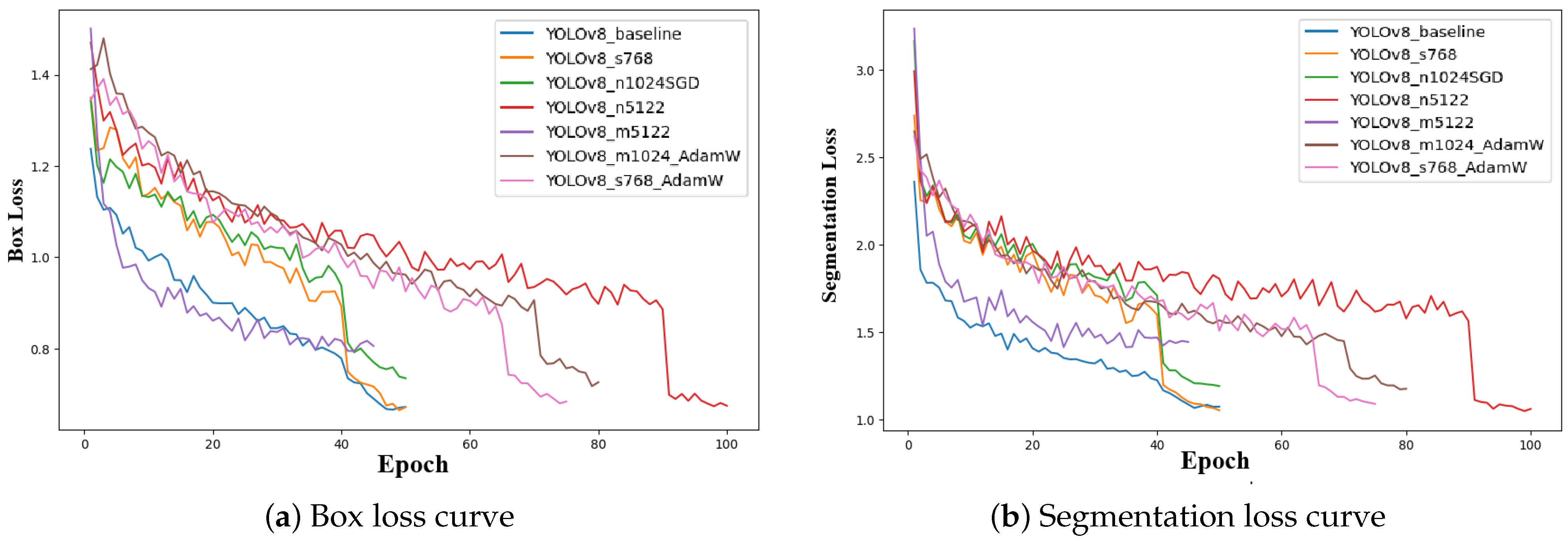
Figure 7 presents the evolution of box loss and segmentation loss during training for all YOLOv8 configurations. Both loss curves show a rapid decrease in the initial epochs, reflecting effective early-stage optimization and strong convergence of the models. The box loss curves progressively stabilize at lower values, indicating improved accuracy in bounding box regression. Similarly, segmentation loss curves show consistent downward trends, confirming enhanced pixel-level mask generation throughout training. Minor fluctuations observed in some configurations are attributed to optimizer dynamics and learning rate schedules.
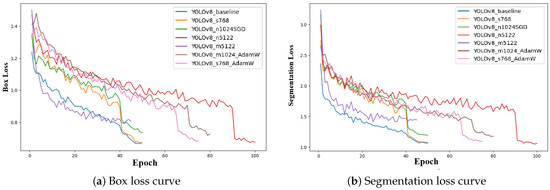
Figure 7.
Training loss curves for all YOLOv8 configurations. (a) Box loss curves showing bounding box regression convergence. (b) Segmentation loss curves illustrating improvement in mask generation accuracy throughout training.
Among all models, the YOLOv8_m5122 configuration demonstrates the lowest final box loss and segmentation loss values, suggesting superior localization accuracy and fine-grained mask prediction capabilities. This behavior further validates YOLOv8_m5122 as the most robust and accurate architecture for UAV-based banana disease segmentation and supports its selection as the optimal model for real-world field deployment.
4.4. Impact of Hyperparameters
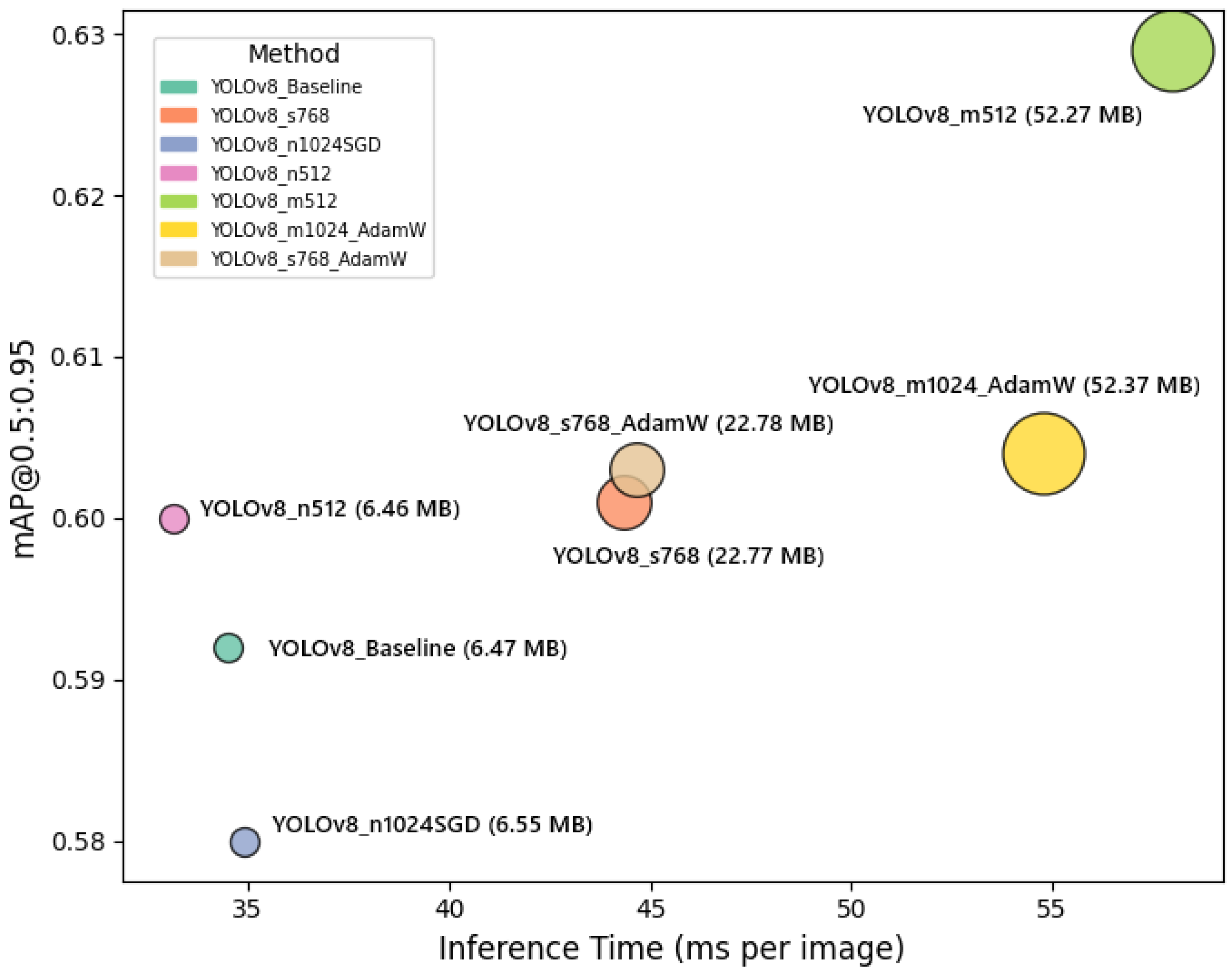
Figure 8 illustrates the trade-off between segmentation accuracy and inference speed for each YOLOv8 configuration. The horizontal axis represents inference time per image, while the vertical axis shows the mAP@0.5:0.95 values. Circle sizes correspond to model sizes in MB, providing additional insight into the computational cost of each variant. This plot clearly highlights the balance that must be considered when selecting a configuration for UAV-based plant disease detection, depending on operational constraints and accuracy requirements.
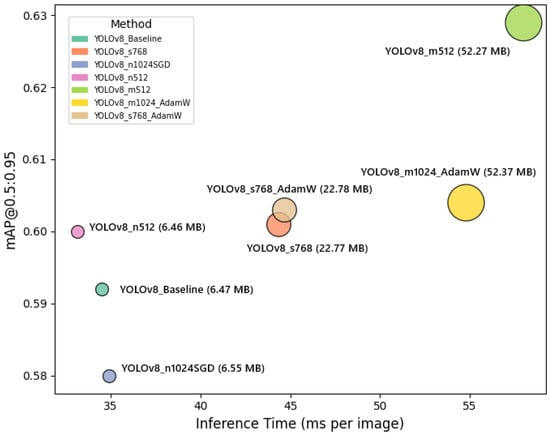
Figure 8.
Trade-off between segmentation accuracy and inference speed for different YOLOv8 configurations. The horizontal axis shows inference time per image (ms), and the vertical axis shows mAP@0.5:0.95. Circle sizes represent model sizes (MB), highlighting computational and performance trade-offs among configurations.
As shown in Figure 8, the YOLOv8 configurations exhibit a clear trade-off between segmentation accuracy, inference speed, and model complexity. The YOLOv8_m512 model achieves the highest mAP@0.5:0.95 (0.629), but it comes with a larger model size (52.27 MB) and moderate inference time (58.0 ms), indicating its suitability for scenarios where accuracy is prioritized over speed. Meanwhile, YOLOv8_n512 and YOLOv8_n1024SGD configurations demonstrate faster inference times (33.13 ms and 34.92 ms, respectively) with relatively smaller model sizes (around 6.5 MB), making them more appropriate for real-time or resource-constrained UAV deployments, albeit with slightly lower mAP@0.5:0.95 values. The YOLOv8_s768 variant shows a favorable balance, achieving a higher precision (0.820) and maintaining a competitive mAP@0.5:0.95 (0.601), with moderate model size (22.77 MB) and inference time (44.36 ms). These observations emphasize the necessity of selecting a model configuration that aligns with the specific operational constraints and performance requirements of UAV-based plant disease monitoring missions.
4.5. Architecture Adjustments for Banana Disease Detection
After analyzing the results from seven different YOLOv8 segmentation configurations, the banano_seg_v8_m5122 variant was selected as the optimal model for UAV-based detection of Moko and Black Sigatoka in banana crops. The chosen model is based on the YOLOv8m-seg architecture, comprising 191 layers and 27,241,385 parameters, with approximately 110.4 GFLOPs. This model achieved a precision of 0.796, recall of 0.803, mAP@0.5 of 0.849, and the highest mAP@0.5:0.95 of 0.629 among all tested configurations. These metrics reflect a robust ability to delineate disease boundaries precisely while minimizing false positives and false negatives.
The modifications aim to enhance fine lesion delineation, improve robustness under variable field conditions, and reduce false positives in overlapping foliage regions.
4.5.1. Input Resolution and Multi-Scale Context Enhancement
The input resolution was set to pixels to balance fine detail capture and inference speed, crucial for real-time UAV monitoring of banana crops. This resolution ensures sufficient granularity to detect small necrotic lesions characteristic of Black Sigatoka and the diffuse wilting patterns of Moko, while keeping computational demands practical for field deployment.
To further improve context awareness, the Spatial Pyramid Pooling Fast (SPPF) module was emphasized. This allows the model to integrate multi-scale contextual information, capturing both large-scale foliar patterns and fine-scale symptomatic areas.
Each with different kernel sizes enables detection of varying lesion sizes and shapes across different disease stages.
4.5.2. Custom Class Head for Banana Diseases
The head layer was reconfigured to explicitly predict three relevant classes: healthy leaves, Moko, and Black Sigatoka. This custom class design directly reflects the agronomic reality of banana plantations and supports targeted interventions.
where . Additionally, adopting an anchor-free segmentation approach improves fine-edge delineation of irregular and overlapping symptoms, which is essential for distinguishing complex disease zones often present in banana canopies.
4.5.3. Loss Function Weight Adjustments
The loss function was reweighted to emphasize accurate segmentation over pure detection, reflecting the agronomic need for precise spatial mapping of infected leaf regions rather than just bounding box localization.
with empirically defined weights:
By increasing , the model prioritizes high-quality mask prediction, which is crucial for guiding selective pruning, targeted fungicide application, and other precision agriculture actions.
4.5.4. Feature Reuse with Cross-Stage Partial Blocks
Cross-Stage Partial (C2f) blocks were strategically retained and extended to enhance feature reuse and preserve fine structural details. This is critical for accurately segmenting thin necrotic streaks and heterogeneous lesion edges, typical in Black Sigatoka progression.
Mathematically expressed as
Here, denotes transformed features and the shortcut, supporting robust gradient propagation and fine-grained texture preservation.
4.5.5. Upsampling and Multi-Level Fusion in Neck
Additional upsampling and multi-level concatenations were introduced in the neck to integrate deep semantic features with high-resolution spatial information. This multi-scale fusion is vital for segmenting irregular leaf patterns and mixed infection zones that often co-occur in banana fields.
where represents features from early backbone stages. This configuration enhances localization precision, even when leaves overlap or show partial symptom development.
4.5.6. Adjusted Depth and Width Multipliers
Depth and width multipliers were fine-tuned to achieve a trade-off between model complexity and field deployment efficiency.
with and . This adjustment reduces over-parameterization, improving generalization on banana foliage while maintaining sufficient capacity to capture complex lesion morphology in diverse environmental conditions.
4.6. Inference and Visual Results
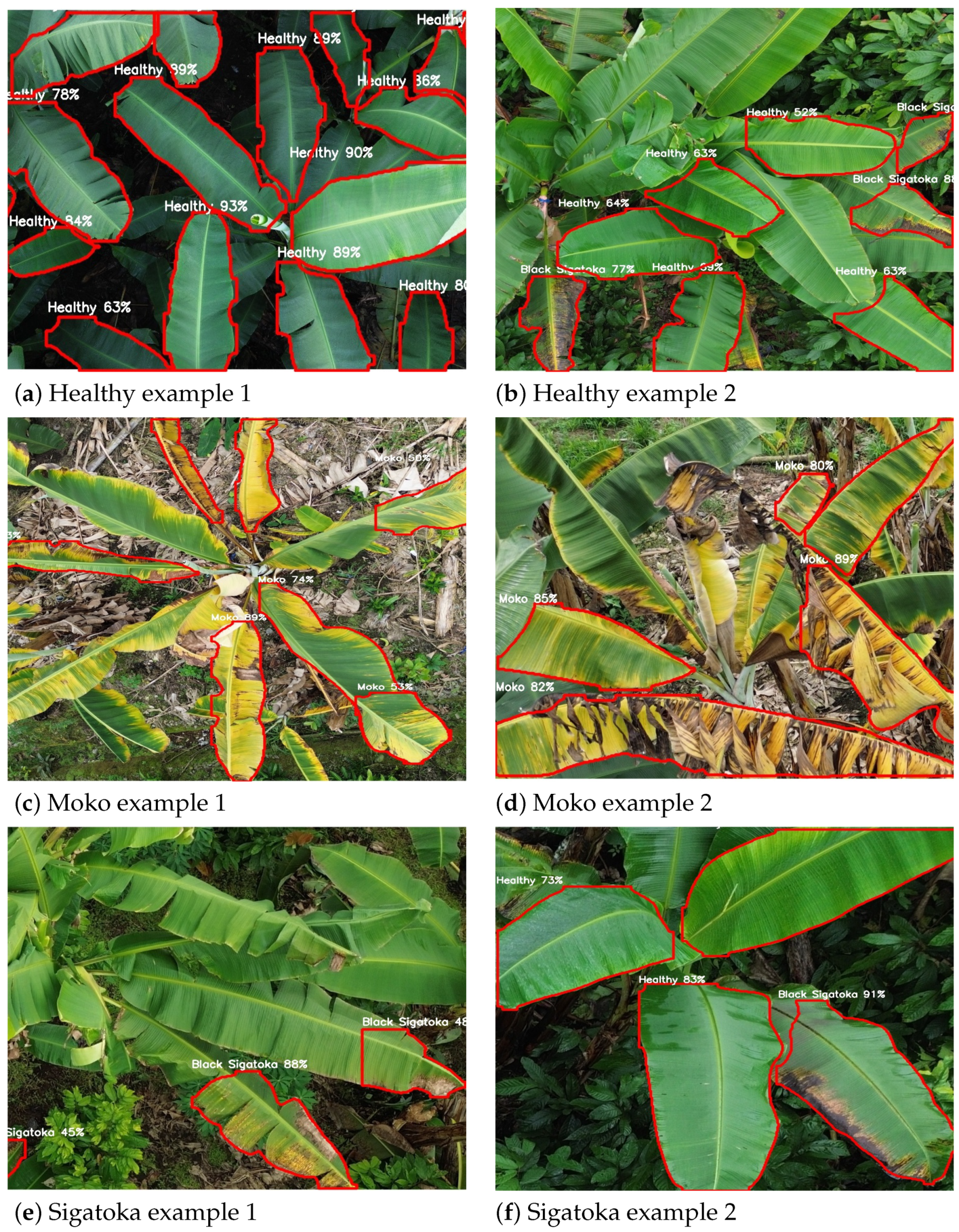
Figure 9 showcases representative qualitative results obtained from the best-performing configuration, YOLOv8_m512 (detailed in Section 4.5). These examples illustrate segmentation predictions across different classes, including healthy leaves, Moko, and Black Sigatoka. Each subfigure displays clear leaf boundaries delineated in red, with the class label and corresponding confidence score centrally annotated. The high confidence values reflect robust disease detection and precise leaf contour delineation, demonstrating the practical applicability of the model for UAV-based monitoring in banana crops.
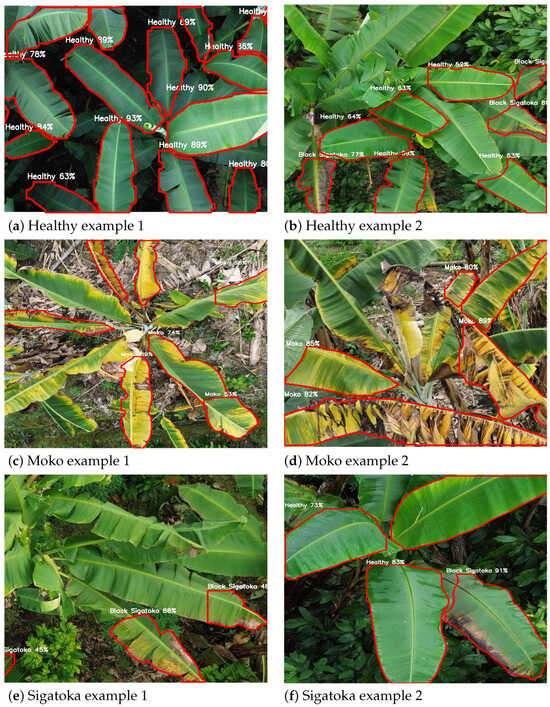
Figure 9.
Qualitative segmentation results from the YOLOv8_m512 configuration on UAV images. Red contours delineate leaf boundaries, with class names and confidence scores annotated. Examples include healthy leaves, Moko, and Black Sigatoka symptoms.
Figure 9 provides a detailed qualitative evaluation of the YOLOv8_m512 model’s performance across different banana leaf conditions. The first row (Figure 9a,b) displays examples of healthy leaves, where the model accurately identifies clean and intact leaf structures with high confidence scores, demonstrating its capability to minimize false positives in disease-free areas. The second row (Figure 9c,d) showcases Moko symptoms, characterized by yellowing and necrotic patches; here, the model successfully segments affected regions, even under complex backgrounds, with robust confidence levels. The third row (Figure 9e,f) highlights Black Sigatoka cases, capturing early and advanced necrotic streaks with precise contour delineation and confident class predictions. These qualitative results reinforce the model’s robustness and generalization ability for UAV-based disease monitoring in real-world plantation scenarios.
4.7. External Validation with Independent UAV Dataset
To evaluate the generalization performance of the selected YOLOv8_m512 model beyond the training environment, we conducted an external validation using UAV imagery collected from a different banana plantation located in the San Rafael farm described in Section 3.3. These images were strictly excluded from training and internal validation stages.
The YOLOv8_m512 model achieved a mean precision of 78.4%, recall of 76.9%, mAP@0.5 of 81.7%, and mAP@0.5:0.95 of 60.1% on this independent test set.
Figure 10 presents qualitative examples from the external dataset illustrating the impact of increased flight altitude and scene heterogeneity on model predictions. In (a), the model produces over-segmentation on a mostly healthy leaf due to faint chlorosis patterns. In (b), a healthy leaf is incorrectly labeled as Moko with high confidence, likely influenced by leaf shadowing and yellowing near the midrib. In (c), mixed predictions of both Moko and Black Sigatoka appear in an occluded and cluttered canopy, demonstrating the difficulty in resolving overlapping symptoms. These visual cases highlight the remaining limitations of the model under complex field conditions, particularly in distinguishing between stress-induced discoloration and true symptoms.

Figure 10.
Examples of challenging predictions from the external UAV validation set. All three cases demonstrate how changes in altitude and scene complexity impact model outputs. Red contours indicate predicted segmentation masks, while ground truth is omitted for clarity.
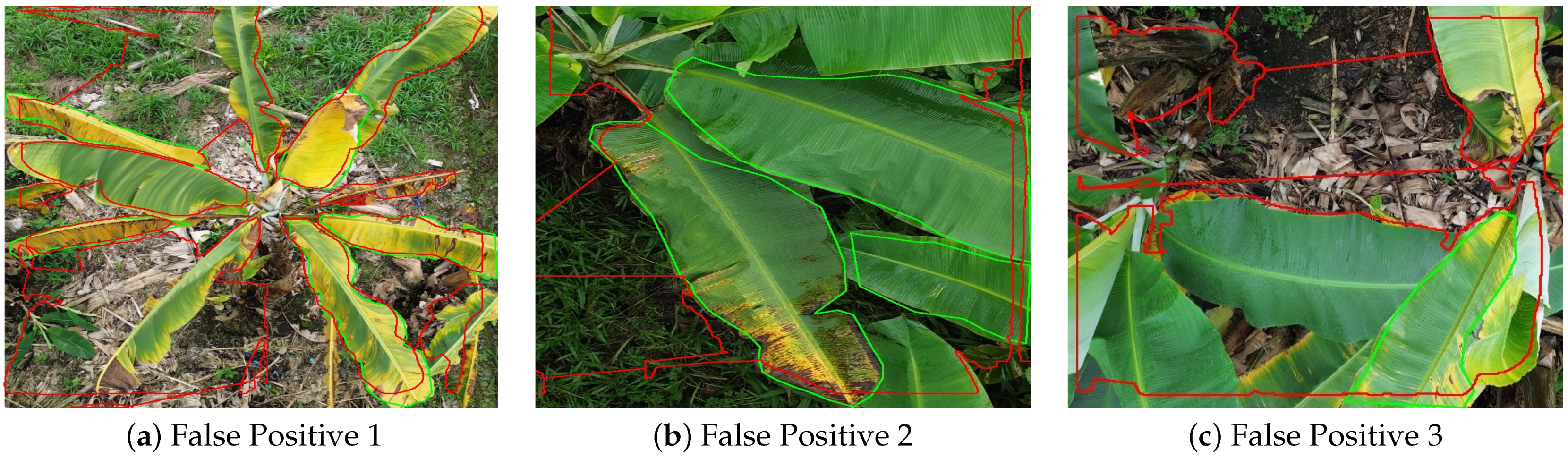
4.8. Error Analysis: False Positives in Disease Segmentation
Although the proposed YOLOv8_m512 model achieved high segmentation accuracy, a closer inspection of its predictions revealed specific cases of false positives (FP), where healthy banana leaf tissue was incorrectly classified as diseased. Figure 11 presents three representative examples extracted from the validation dataset. In these visualizations, green contours correspond to the ground truth annotations, while red contours represent the predicted masks.
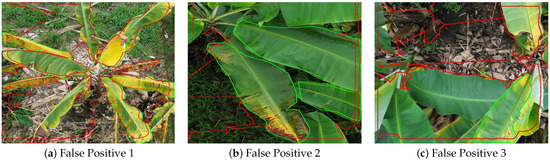
Figure 11.
Examples of false positives in YOLOv8_m512 segmentation results. Green contours: ground truth annotations. Red contours: predicted masks.
In Figure 11a, the model erroneously labels extensive healthy leaf areas as diseased, likely due to the presence of natural yellowing and senescent tissue that closely resemble symptoms of Moko or Black Sigatoka in terms of texture and color distribution. This misclassification suggests a need for more targeted training samples that capture natural leaf senescence without disease presence.
Figure 11b shows a scenario where a large healthy central leaf was predicted as partially diseased. The false detection appears to have been triggered by high-contrast edges and minor surface damage, which the model misinterpreted as lesion boundaries.
In Figure 11c, the model overestimates the disease extent in a region where the ground truth labels indicate only healthy leaves. This over-segmentation could be linked to background interference from soil and dried leaf debris, which create irregular patterns similar to necrotic lesions.
These FP cases highlight the importance of refining the training dataset to include more examples of non-diseased leaves under varying senescence stages and diverse backgrounds. Additionally, integrating spectral cues or advanced texture descriptors in future work could help the model better differentiate between true disease symptoms and visual artifacts.
5. Discussion
The proposed improved YOLOv8 segmentation model for detecting Moko and Black Sigatoka in banana crops demonstrated notable advancements compared to previous approaches. By leveraging UAV-based high-resolution imagery and an optimized instance segmentation framework, our model achieved a mean precision of 79.6%, recall of 80.3%, mAP@0.5 of 84.9%, and mAP@0.5:0.95 of 62.9%. These results highlight the model’s robust capability to accurately delineate diseased and healthy leaf regions even under field variability.
In recent literature, Lin et al. [10] introduced the BFWSD model, which leverages a YOLOv8n architecture optimized with Ghost bottlenecks and mixed local channel attention modules to monitor banana fusarium wilt severity. Their approach achieved substantial efficiency gains, reducing parameters by approximately and improving inference speed by to FPS depending on the baseline configuration. In terms of accuracy, BFWSD reported increases of +2.4 pp in precision, +3.8 pp in mAP@0.5, and +6.5 pp in recall compared to YOLOv8n, enabling real-time mobile deployment, including Android integration. However, their work was primarily focused on severity indexing and object detection rather than precise instance-level segmentation. In contrast, our best configuration (YOLOv8_m512) achieves mAP@0.5 = 0.849 and mAP@0.5:0.95 = 0.629 with an inference time of 58.00 ms per 512 × 512 image (≈17 FPS) on an NVIDIA T4 GPU, demonstrating competitive accuracy under stricter IoU thresholds while maintaining practical throughput for UAV-based operations. By emphasizing detailed pixel-wise segmentation and spatial mapping, our approach provides more actionable geospatial information for targeted interventions in precision agriculture.
Furthermore, Mora et al. [12] presented a digital georeferenced framework integrating YOLO foundation models (YOLO-NAS, YOLOv8, YOLOv9) with explainable AI and human-in-the-loop correction, achieving strong performance on both aerial and ground-level imagery. Their results include aerial mAP@0.5 scores of 0.75 (YOLOv9) and 0.72 (YOLOv8) across healthy, Fusarium, and Xanthomonas classes, and ground-level YOLOv8 performance reaching up to mAP@0.5 = 0.91 on whole-plant categories. While these outcomes are competitive for detection tasks, their experiments were designed for multiplatform surveillance and general disease categorization, and were conducted using an NVIDIA Tesla M60, making direct inference speed comparisons with our setup difficult. In contrast, our approach is optimized for UAV-based high-resolution imagery of banana foliar diseases and achieves mAP@0.5 = 0.849 and mAP@0.5:0.95 = 0.629 with an inference time of 58.00 ms per 512 × 512 image on an NVIDIA T4 GPU. Additionally, our system incorporates real-time geospatial visualization tailored for plantation-level decision support, enabling actionable mapping of infection hotspots for precision agriculture interventions.
Sujatha et al. [11] and Batool et al. [13] explored hybrid machine learning and deep learning strategies to improve disease classification in various plant species, achieving strong classification accuracies on several publicly available leaf datasets. For example, Sujatha et al. combined InceptionV3 features with an SVM classifier, reporting an accuracy of 91.9% for banana leaf disease classification, while Batool et al. developed a lightweight architecture incorporating depthwise separable convolutions and spatial attention, reaching 98.7% accuracy on the PlantVillage dataset. Although these results demonstrate the potential of integrating CNNs with traditional ML classifiers or incorporating attention mechanisms, both approaches are limited to leaf-level image classification under controlled conditions and do not address UAV-based large-scale field applications or instance-level segmentation. In contrast, our proposed method is specifically designed for UAV-acquired imagery of banana plantations, achieving mAP@0.5 = 0.849 and mAP@0.5:0.95 = 0.629 while maintaining real-time inference capability for operational deployment in precision agriculture.
The integration of geospatial mapping in our system further strengthens its practical applicability. By embedding GPS metadata and visualizing disease distributions on interactive maps, our framework enables plantation managers to localize infection hotspots accurately and prioritize field interventions efficiently. This functionality aligns with the direction highlighted by Mora et al. [12], who emphasize georeferenced surveillance as a critical feature in future agricultural disease management frameworks.
Overall, the improved YOLOv8 segmentation model presented in this study closes critical gaps identified in recent research, offering detailed, high-precision disease delineation, robust field applicability, and an intuitive geospatial interface. These contributions support a transition from manual, reactive disease management toward proactive, data-driven precision agriculture in banana production systems.
It is important to note that the entire dataset used in this study was acquired from plantations growing the ’Cavendish’ cultivar (Musa AAA group), which is the predominant commercial variety in Ecuador. While this choice ensures alignment with the most widely cultivated and economically relevant banana variety, it may introduce limitations in terms of cross-cultivar generalizability. Foliar symptom morphology, color intensity, and lesion distribution may vary significantly across cultivars such as Musa AAB or ABB, potentially affecting segmentation accuracy when deploying the model in those contexts. We acknowledge this limitation and have highlighted it as a future research direction, with the goal of expanding the dataset to include multi-cultivar imagery and improve the robustness of the segmentation model across diverse banana genotypes.
Potential Biases and Mitigation Strategies
Two potential sources of bias identified in our dataset and methodology are (i) disease prevalence imbalance and (ii) variability in canopy density, including occlusions and partial symptom visibility. First, the prevalence of Moko and Black Sigatoka in the training set may not proportionally reflect their occurrence in the broader population, potentially leading to overfitting to dominant patterns and reduced sensitivity to early-stage symptoms. Second, variations in canopy structure, overlapping leaves, and incomplete lesions can obscure symptomatic regions—especially in densely vegetated plots—resulting in false negatives or reduced detection confidence.
To mitigate these biases, we implemented several complementary strategies. The dataset was curated to include samples from banana plots with a wide range of disease incidence levels, from early to severe stages, and exhibiting different degrees of leaf overlap and symptom visibility. Targeted data augmentation was applied using the YOLOv8 training pipeline, including random occlusion to simulate partial canopy coverage, brightness and contrast adjustments to account for illumination changes, and geometric transformations (scaling, rotation) to vary leaf orientation and UAV perspective.
Class imbalance was addressed through oversampling of minority disease classes (Moko and Black Sigatoka), ensuring balanced representation in each batch. Additionally, to evaluate generalization performance and reduce the risk of overfitting to a single geographic context, an external validation was conducted on a labeled test set collected from an independent banana farm in Canton Valencia (Province of Los Ríos, Ecuador), captured at higher UAV altitudes (5–10 m AGL). This external dataset exhibited distinct canopy geometries, lighting conditions, and symptom presentations, and the model demonstrated competitive segmentation performance under these conditions (see Section 4.7). These combined measures strengthen the validity and practical applicability of our proposed approach.
6. Conclusions and Future Work
6.1. Conclusions
This study presented an improved YOLOv8-based segmentation model specifically designed for the detection and mapping of Moko and Black Sigatoka in banana plantations using UAV imagery. By integrating instance-level segmentation and geospatial visualization capabilities, the proposed system enables accurate, real-time identification of diseased and healthy leaf regions, thus supporting informed decision-making for precision agriculture.
The model achieved a mean precision of 79.6%, recall of 80.3%, mAP@0.5 of 84.9%, and mAP@0.5:0.95 of 62.9%, demonstrating strong performance under field conditions. Compared to traditional manual inspections and earlier classification-focused models, our approach offers a robust, scalable, and accessible solution using only standard RGB UAV imagery.
In summary, the proposed model lays a strong foundation for advancing UAV-based disease monitoring systems in banana production, contributing to more sustainable, efficient, and precise plant health management practices.
6.2. Future Work
Future work will focus on enhancing the robustness and generalizability of the model under diverse environmental and lighting conditions by expanding the dataset to include images captured across different seasons and plantation contexts. We plan to include imagery from multiple geographic regions within Ecuador, thus enabling the model to better adapt to variability in disease presentation across the country and supporting its deployment as a robust national-scale monitoring tool. Additional studies will also investigate the integration of multi-spectral and hyperspectral data to improve early-stage disease detection and severity assessment.
We will extend the evaluation beyond YOLOv8 with a systematic, matched-protocol benchmark against representative two-stage instance methods (e.g., Mask R-CNN), anchor-free/transformer instance segmenters (e.g., Mask2Former), and semantic baselines (e.g., U-Net, DeepLabV3+).
In addition, we plan to expand the dataset to include banana cultivars beyond ‘Cavendish’ (Musa AAA group), such as members of the Musa AAB and ABB subgroups. This cultivar diversity will allow us to evaluate cross-cultivar transferability and further improve the generalization capability of the model for deployment in diverse banana production systems worldwide.
Finally, a key methodological extension will be the implementation of a 5-fold stratified cross-validation framework. This approach will provide mean performance metrics with associated standard deviations, offering a statistically rigorous assessment of model robustness across multiple balanced splits of the dataset. Combined with expanded hyperparameter analyses and a broader, more diverse image corpus, this strategy will ensure a more comprehensive evaluation of the proposed segmentation framework.
Author Contributions
Conceptualization, C.Z.-V., R.O.V.-T. and K.C.C.; Methodology, C.Z.-V.; Software, C.Z.-V.; Validation, B.O., R.O.V.-T. and D.Y.-C.; Formal analysis, C.Z.-V.; Investigation, B.O., C.Z.-V., R.O.V.-T. and D.Y.-C.; Resources, K.C.C.; Data curation, C.Z.-V. and K.C.C.; Writing—original draft, C.Z.-V.; Writing—review & editing, C.Z.-V. and K.C.C.; Visualization, C.Z.-V.; Supervision, B.O. and C.Z.-V.; Project administration, B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by State Technical University of Quevedo. The APC was funded by State Technical University of Quevedo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their gratitude to the State Technical University of Quevedo for the support provided throughout this research. Their continuous encouragement and resources have been invaluable in the development of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ministerio de Agricultura y Ganadería, Ecuador. Agricultural Export Report 2023. 2023. Available online: https://www.agricultura.gob.ec/wp-content/uploads/downloads/2024/03/MAG-Informe-Rendicion-de-Cuentas-2023.pdf (accessed on 28 June 2025).
- Marín, J.; Botero Fernández, V.; Zapata-Henao, S.; Hoyos-Carvajal, L. Early detection of bacterial wilt in bananas caused by Ralstonia solanacearum. J. Plant Pathol. 2023, 105, 587–598. [Google Scholar] [CrossRef]
- Henao-Ochoa, D.; Rey-Valenzuela, V.; Zapata-Henao, S.; Arango-Isaza, R.; Rodríguez-Cabal, H.; Morales, J. Application of defense inducers reduces the severity of Black Sigatoka (Pseudocercospora fijiensis) in Musa acuminata AAA Cavendish. Eur. J. Plant Pathol. 2025, 172, 241–259. [Google Scholar] [CrossRef]
- Selvaraj, M.; Vergara, A.; Montenegro, F.; Ruiz, H.; Safari, N.; Raymaekers, D.; Ocimati, W.; Ntamwira, J.; Tits, L.; Omondi, A.; et al. Detection of banana plants and their major diseases through aerial images and machine learning methods: A case study in DR Congo and Republic of Benin. ISPRS J. Photogramm. Remote Sens. 2020, 169, 110–124. [Google Scholar] [CrossRef]
- Linero-Ramos, R.; Parra-Rodríguez, C.; Espinosa-Valdez, A.; Gómez-Rojas, J.; Gongora, M. Assessment of Dataset Scalability for Classification of Black Sigatoka in Banana Crops Using UAV-Based Multispectral Images and Deep Learning Techniques. Drones 2024, 8, 503. [Google Scholar] [CrossRef]
- Agrocalidad. Agrocalidad Impulsa Acciones Para el Control del Moko Con Respaldo Técnico. 2024. Available online: https://www.agrocalidad.gob.ec/agrocalidad-impulsa-acciones-para-el-control-del-moko-con-respaldo-tecnico-de-expertos-de-brasil/ (accessed on 11 July 2025).
- FreshPlaza. El Moko Amenaza la Producción de Plátano Verde en Ecuador y Dispara los Precios. 2025. Available online: https://www.freshplaza.es/article/9742151/el-moko-amenaza-la-produccion-de-platano-verde-en-ecuador-y-dispara-los-precios/ (accessed on 11 July 2025).
- Agroinsurance. Banana Producers lose $700,000 a Week Due to Moko. 2024. Available online: https://www.freshplaza.com/north-america/article/9650468/ecuadorian-banana-producers-lose-700-000-a-week-due-to-moko/ (accessed on 11 July 2025).
- Sopisconews. Moko Severely Affects Banana Plantations in Los Ríos. 2024. Available online: https://www.sopisconews.com/newsdetails/1167 (accessed on 11 July 2025).
- Lin, S.; Ji, T.; Wang, J.; Li, K.; Lu, F.; Ma, C.; Gao, Z. BFWSD: A lightweight algorithm for banana fusarium wilt severity detection via UAV-Based Large-Scale Monitoring. Smart Agric. Technol. 2025, 11, 101047. [Google Scholar] [CrossRef]
- Sujatha, R.; Krishnan, S.; Chatterjee, J.M.; Gandomi, A.H. Advancing plant leaf disease detection integrating machine learning and deep learning. Sci. Rep. 2025, 15, 11552. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.J.; Blomme, G.; Safari, N.; Elayabalan, S.; Selvarajan, R.; Selvaraj, M.G. Digital framework for georeferenced multiplatform surveillance of banana wilt using human in the loop AI and YOLO foundation models. Sci. Rep. 2025, 15, 3491. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Kim, J.; Byun, Y.C. A compact deep learning approach integrating depthwise convolutions and spatial attention for plant disease classification. Plant Methods 2025, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Kafashan, J. Appropriate vegetation indices and data analysis methods for orchards monitoring using UAV-based remote sensing: A comprehensive research. Comput. Electron. Agric. 2025, 235, 110356. [Google Scholar] [CrossRef]
- Label Studio: Open-Source Data Labeling Tool. Available online: https://labelstud.io (accessed on 9 July 2025).
- Jocher, G.; Chaurasia, A.; Qiu, J.; Stoken, J. YOLOv8: Ultralytics Official Implementation. 2023. Available online: https://github.com/ultralytics/ultralytics (accessed on 9 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).