Agent-Based Modeling of Epidemics: Approaches, Applications, and Future Directions
Abstract
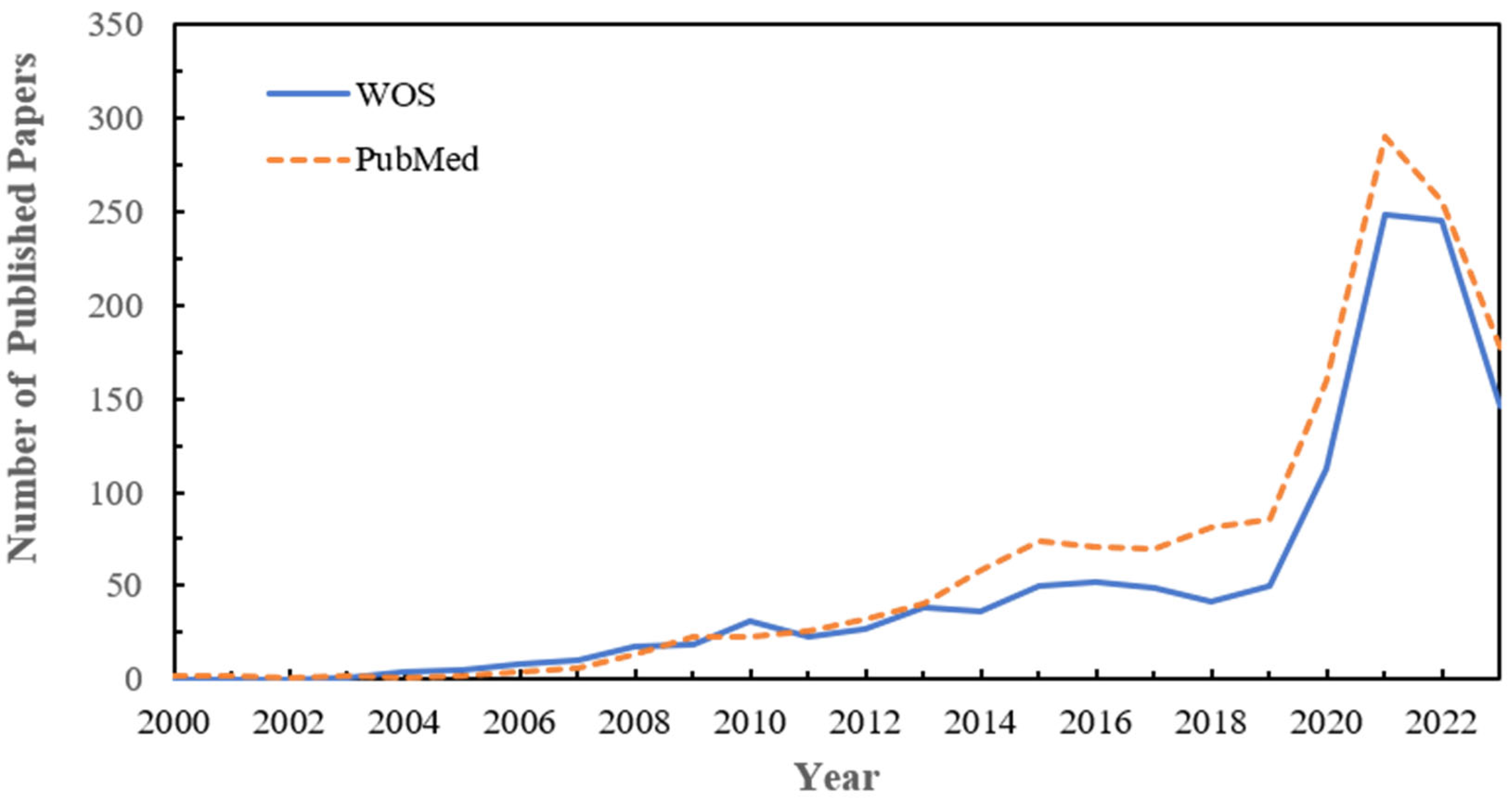
1. Introduction
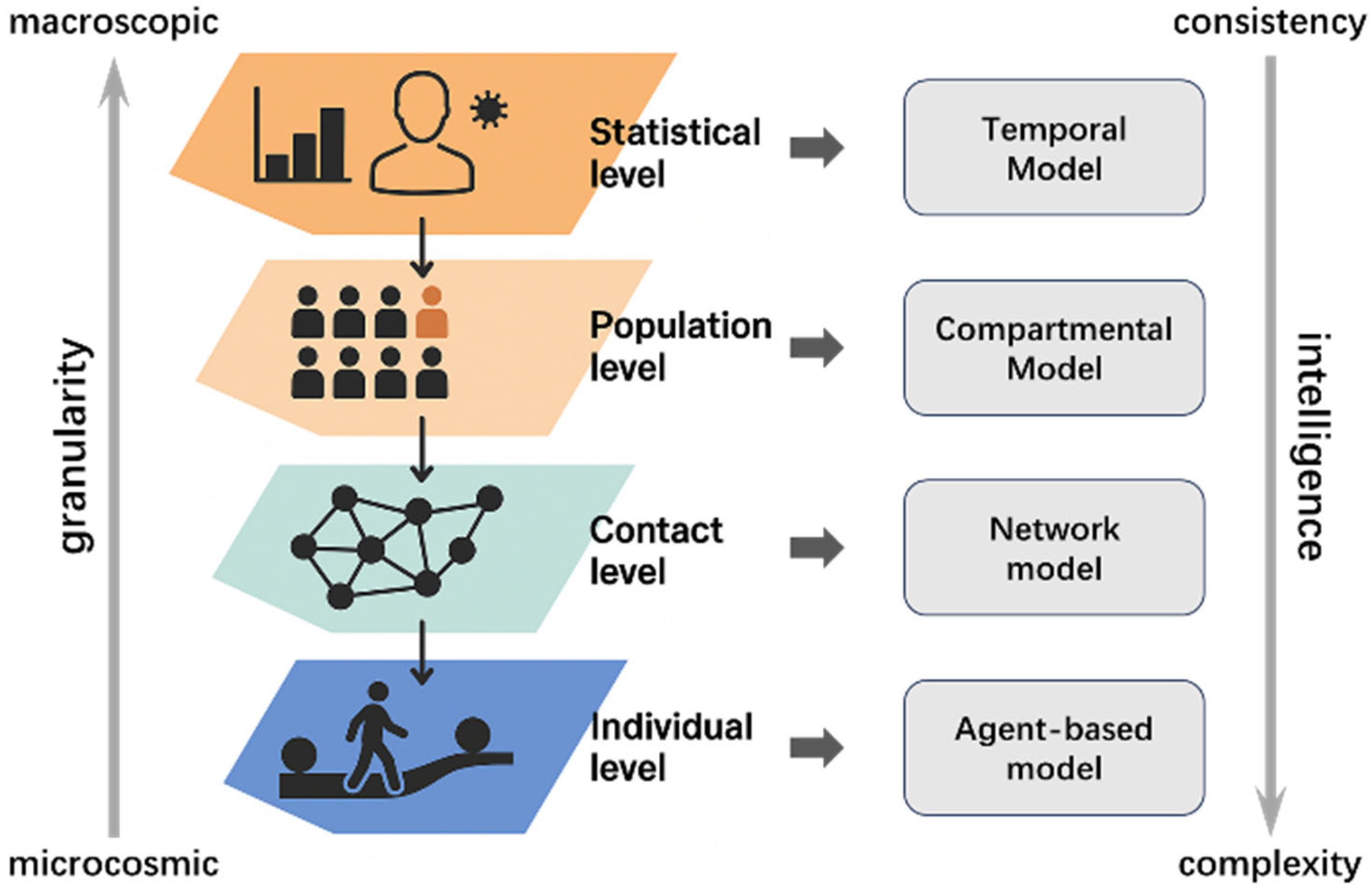
2. Related Works on Infectious Disease Modeling
2.1. Compartmental Model
2.2. Network Model
2.3. Temporal Models
3. Agent-Based Model and Characteristic Analysis
3.1. Current Status and Evolution
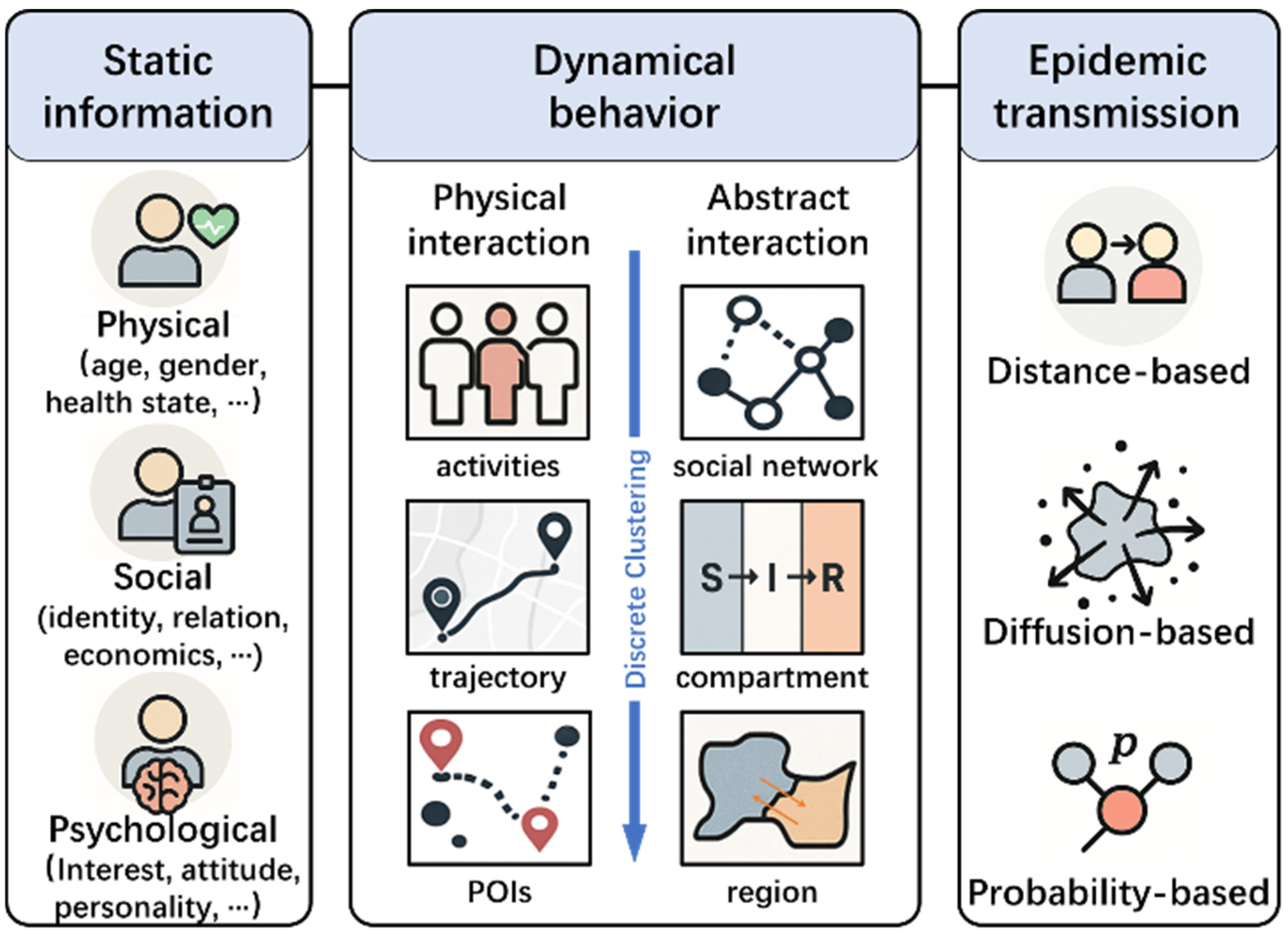
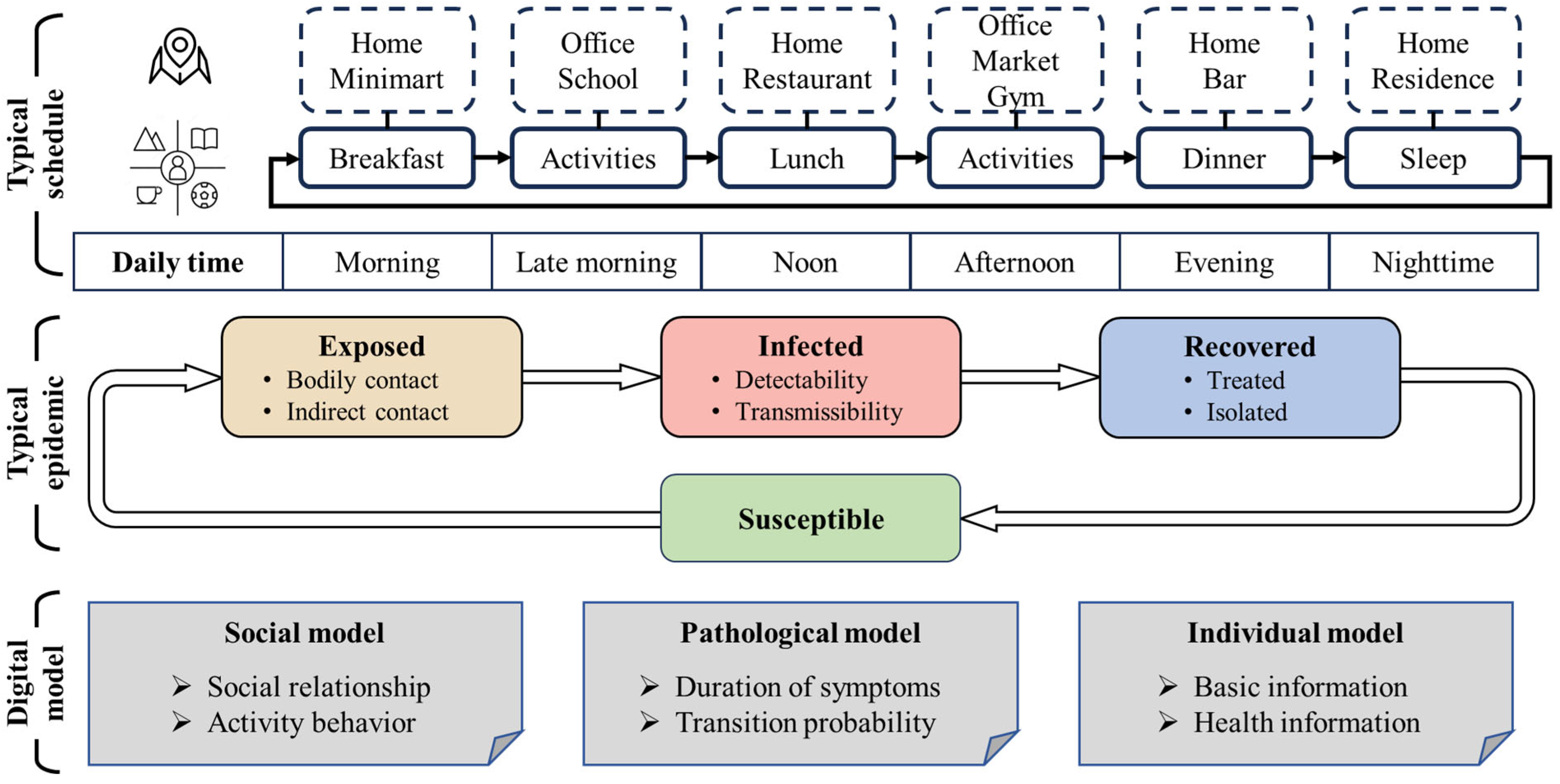
3.2. Agent Modeling Framework and Characteristics
4. Intervention Measures Evaluation Based on ABM
4.1. Revealing Transmission Chains and Sites of Disease Outbreak
4.2. Prospective Assessment of Vaccine Efficacy
4.3. Assessment of Nucleic Acid Testing and Mixed Intervention Measures
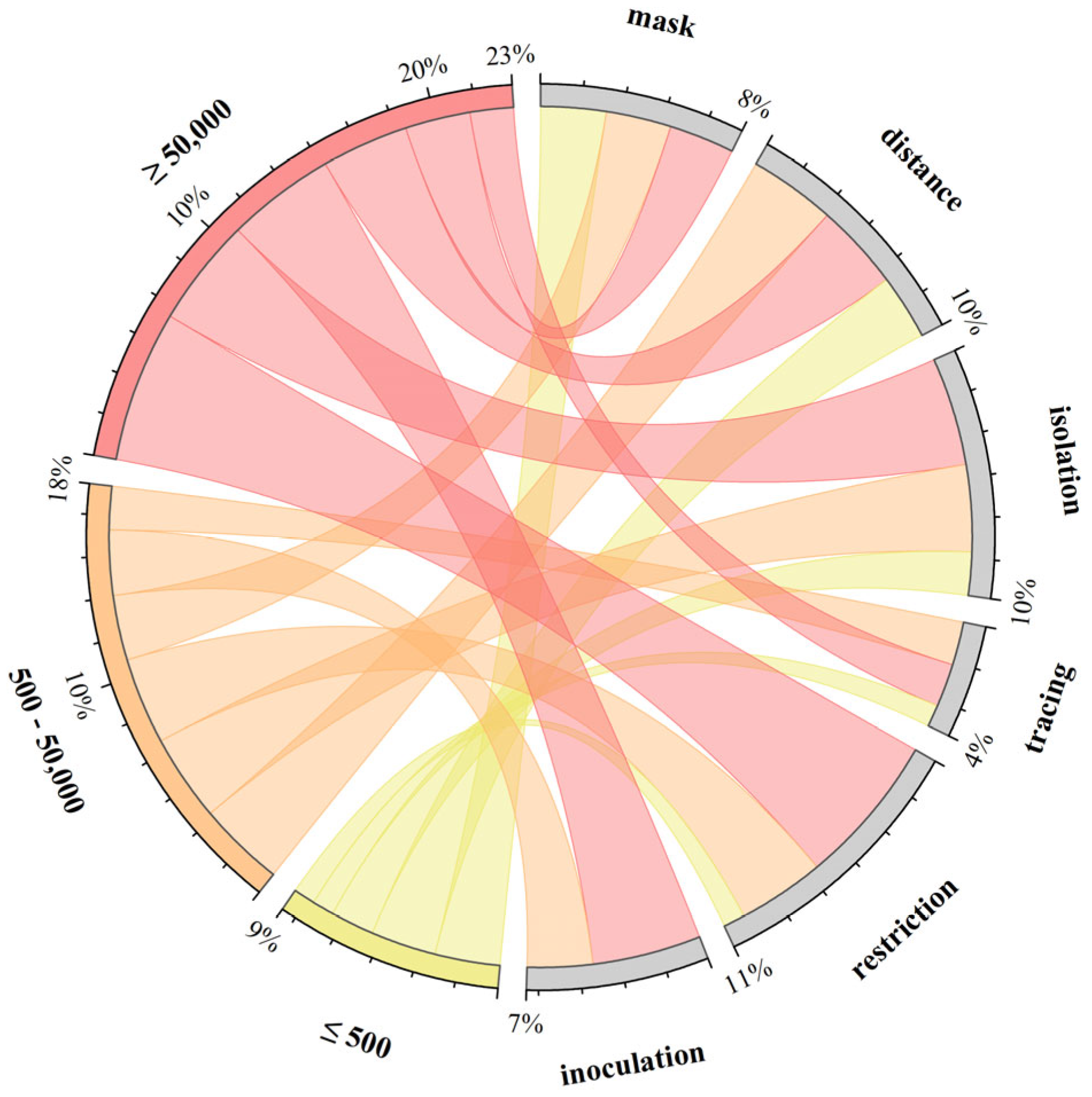
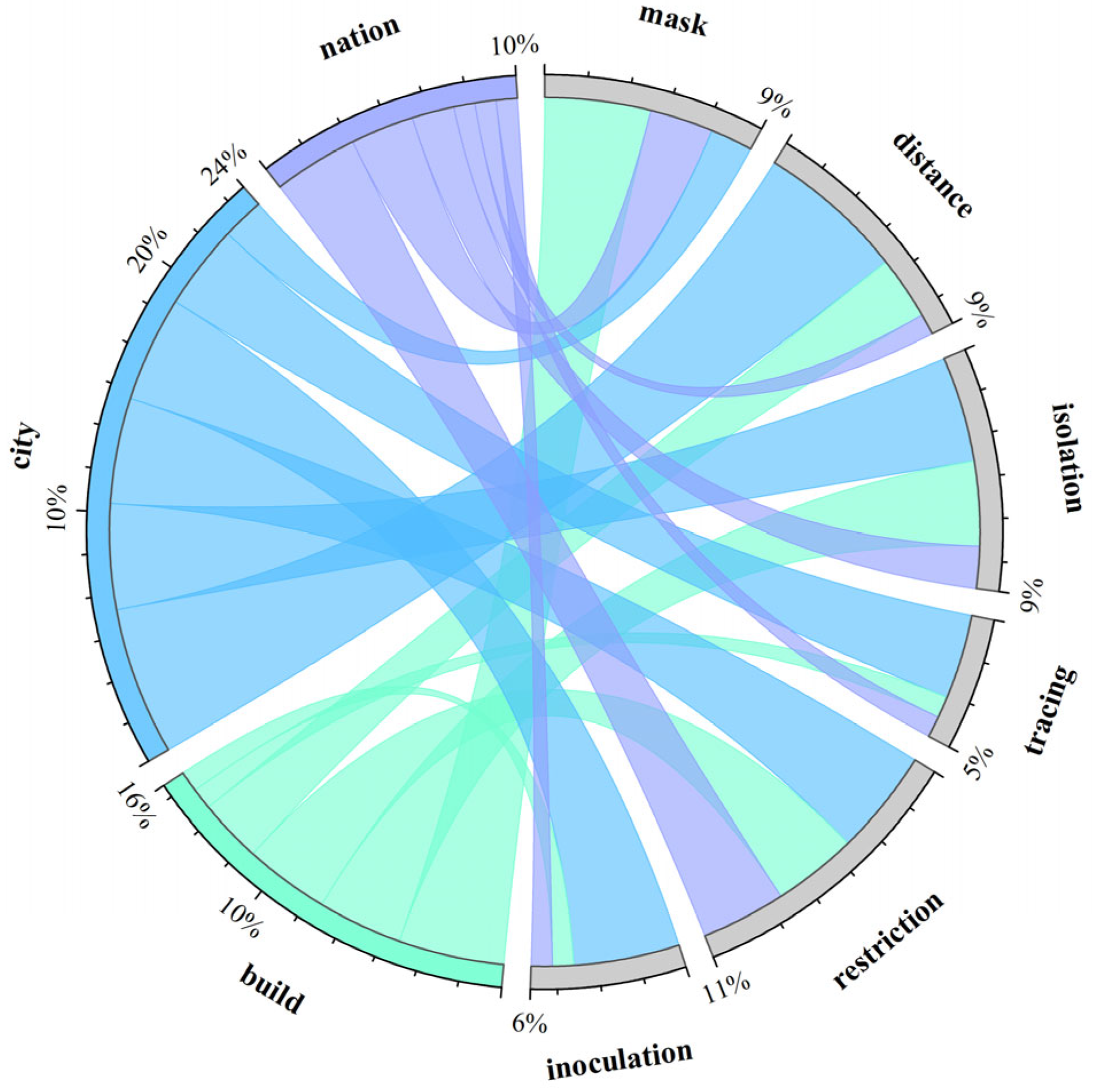
4.4. Cluster and Visual Analysis of Intervention Strategies
- Modeling of infectious diseases transmitted through modes such as animals, water sources, or transmission via injection, sexual activity, etc., was not included temporarily, with the focus primarily on diseases spread through respiratory and physical contact.
- Modeling of transmission in specific environments (e.g., prisons, refugee camps, hospitals) with unique characteristics was not included for comparison due to the special considerations necessitated by these environments.
- The term “build(s)” denotes small social units formed by a group of people within a specific geographical area, such as a floor, a building, or adjacent buildings within a region.
- The term “risk” refers to parameters not directly mentioned in the corresponding paper that denote specific infection quantities or approximate meanings, such as infection risk or probability of illness.
- Although nucleic acid testing is considered a significant measure, it was not explicitly listed here as it does not fall under direct intervention measures.
- Measures such as ventilation, disinfection, and improving sanitation, while present in a small number of studies, were not directly quantified in the corresponding literature.
- For the purpose of clustering and summarization, measures related to movement restrictions mentioned in the article, such as sheltering in place, staggered travel, population grouping management, etc., are collectively referred to as travel restriction measures.
5. Challenges and Prospects
5.1. The Current Challenges
5.2. Fusion Innovation in Interdisciplinary Studies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duan, W.; Fan, Z.C.; Zhang, P.; Guo, G.; Qiu, X.G. Mathematical and computational approaches to epidemic modeling: A comprehensive review. Front. Comput. Sci. 2015, 9, 806–826. [Google Scholar] [CrossRef]
- Dietz, K.; Heesterbeek, J. Daniel Bernoulli’s epidemiological model revisited. Math. Biosci. 2002, 180, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Godio, A.; Pace, F.; Vergnano, A. SEIR modeling of the Italian epidemic of SARS-CoV-2 using computational swarm intelligence. Int. J. Environ. Res. 2020, 17, 3535. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, Z.; Wang, K.; Wong, S.S.; Liang, W.; Zanin, M.; Liu, P.; Cao, X.; Gao, Z.; Mai, Z.; et al. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J. Thorac. Dis. 2020, 12, 165. [Google Scholar] [CrossRef]
- Chinazzi, M.; Davis, J.T.; Ajelli, M.; Gioannini, C.; Litvinova, M.; Merler, S.; Pastore y Piontti, A.; Mu, K.; Rossi, L.; Sun, K. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 2020, 53, 467–472. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Quintero, Y.; Ardila, D.; Camargo, E.; Rivas, F.; Aguilar, J. Machine learning models for the prediction of the SEIRD variables for the COVID-19 pandemic based on a deep dependence analysis of variables. Comput. Biol. Med. 2021, 134, 104500. [Google Scholar] [CrossRef]
- Cao, Q.; Heydari, B. Micro-level social structures and the success of covid-19 national policies. Nat. Comput. Sci. 2022, 2, 595–604. [Google Scholar] [CrossRef]
- Kong, L.C.; Duan, M.W.; Shi, J.; Hong, J.; Chang, Z.R.; Zhang, Z.J. Compartmental structures used in modeling COVID-19: A scoping review. Infect. Dis. Poverty 2022, 11, 9. [Google Scholar] [CrossRef]
- Geng, X.L.; Katul, G.G.; Gerges, F.; Bou-Zeid, E.; Nassif, H.; Boufadel, M.C. A kernel-modulated SIR model for Covid-19 contagious spread from county to continent. Proc. Natl. Acad. Sci. USA 2021, 118, 9. [Google Scholar] [CrossRef]
- Eden, M.; Castonguay, R.; Munkhbat, B.; Balasubramanian, H.; Gopalappa, C. Agent-based evolving network modeling: A new simulation method for modeling low prevalence infectious diseases. Health Care Manag. Sci. 2021, 24, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Steijvers, L.C.; Brinkhues, S.; Hoebe, C.J.; Van Tilburg, T.G.; Claessen, V.; Bouwmeester-Vincken, N.; Hamers, F.; Vranken, P.; Dukers-Muijrers, N.H. Social networks and infectious diseases prevention behavior: A cross-sectional study in people aged 40 years and older. PLoS ONE 2021, 16, e0251862. [Google Scholar] [CrossRef] [PubMed]
- Read, J.M.; Bridgen, J.R.; Cummings, D.A.; Ho, A.; Jewell, C.P. Novel coronavirus 2019-nCoV (COVID-19): Early estimation of epidemiological parameters and epidemic size estimates. Philos. Trans. R. Soc. B 2021, 376, 20200265. [Google Scholar] [CrossRef]
- Lee, H.; Noh, E.; Jeon, H.; Nam, E.W. Association between traffic inflow and COVID-19 prevalence at the provincial level in South Korea. Int. J. Infect. Dis. 2021, 108, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Khosrawipour, V.; Kocbach, P.; Mikolajczyk, A.; Ichii, H.; Zacharski, M.; Bania, J.; Khosrawipour, T. The association between international and domestic air traffic and the coronavirus (COVID-19) outbreak. J. Microbiol. Immunol. Infect. 2020, 53, 467–472. [Google Scholar] [CrossRef]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef]
- Cui, Y.; Ni, S.; Shen, S. A network-based model to explore the role of testing in the epidemiological control of the COVID-19 pandemic. BMC Infect. Dis. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Kiskowski, M.; Chowell, G. Modeling household and community transmission of Ebola virus disease: Epidemic growth, spatial dynamics and insights for epidemic control. Virulence 2016, 7, 163–173. [Google Scholar] [CrossRef]
- Sood, M.; Sridhar, A.; Eletreby, R.; Wu, C.W.; Levin, S.A.; Yagan, O.; Poor, H.V. Spreading processes with mutations over multilayer networks. Proc. Natl. Acad. Sci. USA 2023, 120, 12. [Google Scholar] [CrossRef]
- Luo, T.; Cao, Z.; Wang, Y.; Zeng, D.; Zhang, Q. Role of Asymptomatic COVID-19 Cases in Viral Transmission: Findings From a Hierarchical Community Contact Network Model. IEEE Trans. Autom. Sci. Eng. 2022, 19, 576–585. [Google Scholar] [CrossRef]
- Gwizdalla, T. Viral disease spreading in grouped population. Comput. Methods Programs Biomed. 2020, 197, 10. [Google Scholar] [CrossRef] [PubMed]
- Sujath, R.A.A.; Chatterjee, J.M.; Hassanien, A.E. A machine learning forecasting model for COVID-19 pandemic in India. Stoch. Environ. Res. Risk Assess. 2020, 34, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Pinter, G.; Felde, I.; Mosavi, A.; Ghamisi, P.; Gloaguen, R. COVID-19 pandemic prediction for Hungary; a hybrid machine learning approach. Mathematics 2020, 8, 890. [Google Scholar] [CrossRef]
- Zivkovic, M.; Bacanin, N.; Venkatachalam, K.; Nayyar, A.; Djordjevic, A.; Strumberger, I.; Al-Turjman, F. COVID-19 cases prediction by using hybrid machine learning and beetle antennae search approach. Sustain. Cities Soc. 2021, 66, 102669. [Google Scholar] [CrossRef]
- Lalmuanawma, S.; Hussain, J.; Chhakchhuak, L. Applications of machine learning and artificial intelligence for Covid-19 (SARS-CoV-2) pandemic: A review. Chaos Solitons Fractals 2020, 139, 110059. [Google Scholar] [CrossRef]
- Sun, Z.L.; Bai, R.H.; Bai, Z.G. The application of simulation methods during the COVID-19 pandemic: A scoping review. J. Biomed. Inform. 2023, 148, 10. [Google Scholar] [CrossRef]
- Lukens, S.; DePasse, J.; Rosenfeld, R.; Ghedin, E.; Mochan, E.; Brown, S.T.; Grefenstette, J.; Burke, D.S.; Swigon, D.; Clermont, G. A large-scale immuno-epidemiological simulation of influenza A epidemics. BMC Public Health 2014, 14, 15. [Google Scholar] [CrossRef]
- De-Leon, H.; Aran, D. MAM: Flexible Monte-Carlo Agent based model for modelling COVID-19 spread. J. Biomed. Inform. 2023, 141, 7. [Google Scholar] [CrossRef]
- Abar, S.; Theodoropoulos, G.K.; Lemarinier, P.; O’Hare, G.M.P. Agent Based Modelling and Simulation tools: A review of the state-of-art software. Comput. Sci. Rev. 2017, 24, 13–33. [Google Scholar] [CrossRef]
- Waleed, M.; Um, T.W.; Kamal, T.; Khan, A.; Zahid, Z.U. SIM-D: An Agent-Based Simulator for Modeling Contagion in Population. Appl. Sci.-Basel 2020, 10, 14. [Google Scholar] [CrossRef]
- Lee, B.Y.; Wong, K.F.; Bartsch, S.M.; Yilmaz, S.L.; Avery, T.R.; Brown, S.T.; Song, Y.; Singh, A.; Kim, D.S.; Huang, S.S. The Regional Healthcare Ecosystem Analyst (RHEA): A simulation modeling tool to assist infectious disease control in a health system. J. Am. Med. Inf. Assoc. 2013, 20, E139–E146. [Google Scholar] [CrossRef]
- Hinch, R.; Probert, W.J.M.; Nurtay, A.; Kendall, M.; Wymant, C.; Hall, M.; Lythgoe, K.; Bulas Cruz, A.; Zhao, L.; Stewart, A. OpenABM-Covid19—An agent-based model for non-pharmaceutical interventions against COVID-19 including contact tracing. PLoS Comput. Biol. 2021, 17, e1009146. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.C.; Stuart, R.M.; Mistry, D.; Abeysuriya, R.G.; Rosenfeld, K.; Hart, G.R.; Núñez, R.C.; Cohen, J.A.; Selvaraj, P.; Hagedorn, B. Covasim: An agent-based model of COVID-19 dynamics and interventions. PLoS Comput. Biol. 2021, 17, e1009149. [Google Scholar] [CrossRef]
- Wiratsudakul, A.; Suparit, P.; Modchang, C. Dynamics of Zika virus outbreaks: An overview of mathematical modeling approaches. PeerJ 2018, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.A.; Paltra, S.; Rehmann, J.; Nagel, K.; Conrad, T.O.F. Explicit modeling of antibody levels for infectious disease simulations in the context of SARS-CoV-2. iScience 2023, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- Sanstead, E.; Kenyon, C.; Rowley, S.; Enns, E.; Miller, C.; Ehresmann, K.; Kulasingam, S. Understanding Trends in Pertussis Incidence: An Agent-Based Model Approach. Am. J. Public Health 2015, 105, E42–E47. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Glass, K.; Lau, C.L.; Geard, N.; Graves, P.; Clements, A. A Synthetic Population for Modelling the Dynamics of Infectious Disease Transmission in American Samoa. Sci. Rep. 2017, 7, 9. [Google Scholar] [CrossRef]
- Kang, J.Y.; Aldstadt, J. Using multiple scale spatio-temporal patterns for validating spatially explicit agent-based models. Int. J. Geogr. Inf. Sci. 2019, 33, 193–213. [Google Scholar] [CrossRef]
- Koh, K.; Tang, K.C.; Axhausen, K.; Loo, B.P.Y. A metropolitan-scale, three-dimensional agent-based model to assess the effectiveness of the COVID-19 Omicron wave interventions in a hyperdense city: A case study of Hong Kong. Int. J. Infect. Dis. 2022, 122, 534–536. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, N.; Niu, B.D.; Wang, X.; Xiao, S.L.; Du, X.J.; Chen, T.; Yang, L.; Wang, D.Y.; Cowling, B.; et al. Effect of Rapid Urbanization in Mainland China on the Seasonal Influenza Epidemic: Spatiotemporal Analysis of Surveillance Data From 2010 to 2017. JMIR Public Health Surveill. 2023, 9, 12. [Google Scholar] [CrossRef]
- Alderton, S.; Macleod, E.T.; Anderson, N.E.; Machila, N.; Simuunza, M.; Welburn, S.C.; Atkinson, P.M. Exploring the effect of human and animal population growth on vector-borne disease transmission with an agent-based model of Rhodesian human African trypanosomiasis in eastern province, Zambia. PLoS Neglect. Trop. Dis. 2018, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Perkins, T.A.; Garcia, A.J.; Paz-Soldán, V.A.; Stoddard, S.T.; Reiner, R.C.; Vazquez-Prokopec, G.; Bisanzio, D.; Morrison, A.C.; Halsey, E.S.; Kochel, T.J.; et al. Theory and data for simulating fine-scale human movement in an urban environment. J. R. Soc. Interface 2014, 11, 12. [Google Scholar] [CrossRef]
- Augustijn, E.W.; Doldersum, T.; Useya, J.; Augustijn, D. Agent-based modelling of cholera diffusion. Stoch. Environ. Res. Risk Assess. 2016, 30, 2079–2095. [Google Scholar] [CrossRef]
- Mei, S.; Zhu, Y.F.; Qiu, X.G.; Zhou, X.; Zu, Z.H.; Boukhanovsky, A.V.; Sloot, P.M.A. Individual Decision Making Can Drive Epidemics: A Fuzzy Cognitive Map Study. IEEE Trans. Fuzzy Syst. 2014, 22, 264–273. [Google Scholar] [CrossRef]
- Kontorovsky, N.L.; Ferrari, C.G.; Pinasco, J.P.; Saintier, N. Kinetic modeling of coupled epidemic and behavior dynamics: The social impact of public policies. Math. Models Meth. Appl. Sci. 2022, 32, 2037–2076. [Google Scholar] [CrossRef]
- Yuan, H.; Long, Q.Y.; Huang, G.; Huang, L.Q.; Luo, S.Y. Different roles of interpersonal trust and institutional trust in COVID-19 pandemic control. Soc. Sci. Med. 2022, 293, 10. [Google Scholar] [CrossRef]
- Truscott, J.; Ferguson, N.M. Evaluating the Adequacy of Gravity Models as a Description of Human Mobility for Epidemic Modelling. PLoS Comput. Biol. 2012, 8, 12. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, D.; Liu, J.; Jian, I.Y. Assessing personal exposure to COVID-19 transmission in public indoor spaces based on fine-grained trajectory data: A simulation study. Build. Environ. 2022, 218, 109153. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, M.; Zhu, Z.; Yang, H.; Zhang, L.; Ghader, S. Modeling indoor-level non-pharmaceutical interventions during the COVID-19 pandemic: A pedestrian dynamics-based microscopic simulation approach. Transp. Policy 2021, 109, 12–23. [Google Scholar] [CrossRef]
- Bi, K.M.; Chen, Y.Y.; Zhao, S.N.; Ben-Arieh, D.; Wu, C.H. Modeling learning and forgetting processes with the corresponding impacts on human behaviors in infectious disease epidemics. Comput. Ind. Eng. 2019, 129, 563–577. [Google Scholar] [CrossRef]
- Abdulkareem, S.A.; Augustijn, E.W.; Mustafa, Y.T.; Filatova, T. Intelligent judgements over health risks in a spatial agent-based model. Int. J. Health Geogr. 2018, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Dorso, C.O.; Medus, A.; Balenzuela, P. Vaccination and public trust: A model for the dissemination of vaccination behaviour with external intervention. Phys. A Stat. Mech. Its Appl. 2017, 482, 433–443. [Google Scholar] [CrossRef]
- Nunner, H.; Buskens, V.; Corten, R.; Kaandorp, C.; Kretzschmar, M. Disease avoidance threatens social cohesion in a large-scale social networking experiment. Sci. Rep. 2023, 13, 15. [Google Scholar] [CrossRef]
- Cuevas, E. An agent-based model to evaluate the COVID-19 transmission risks in facilities. Comput. Biol. Med. 2020, 121, 103827. [Google Scholar] [CrossRef] [PubMed]
- Bedson, J.; Skrip, L.A.; Pedi, D.; Abramowitz, S.; Carter, S.; Jalloh, M.F.; Funk, S.; Gobat, N.; Giles-Vernick, T.; Chowell, G.; et al. A review and agenda for integrated disease models including social and behavioural factors. Nat. Hum. Behav. 2021, 5, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Ma, S.R.; Gao, C.Z.; Shao, E.Z.; Xie, Y.L.; Zhang, Y.; Geng, L.; Li, Y. Disease Simulation in Airport Scenario Based on Individual Mobility Model. ACM Trans. Intell. Syst. Technol. 2023, 14, 24. [Google Scholar] [CrossRef]
- Liao, C.Y.; Chen, X.; Zhuo, L.; Liu, Y.; Tao, H.Y.; Burton, C.G. Reopen schools safely: Simulating COVID-19 transmission on campus with a contact network agent-based model. Int. J. Digit. Earth 2022, 15, 381–396. [Google Scholar] [CrossRef]
- Luo, W. Visual analytics of geo-social interaction patterns for epidemic control. Int. J. Health Geogr. 2016, 15, 16. [Google Scholar] [CrossRef]
- Sadeghi, N.; Gerami-Seresht, N. Improving occupational safety in office spaces in the post-pandemic era. Sustain. Cities Soc. 2023, 98, 19. [Google Scholar] [CrossRef]
- Lasser, J.; Hell, T.; Garcia, D. Assessment of the Effectiveness of Omicron Transmission Mitigation Strategies for European Universities Using an Agent-Based Network Model. Clin. Infect. Dis. 2022, 75, 2097–2103. [Google Scholar] [CrossRef]
- Barker, A.K.; Alagoz, O.; Safdar, N. Interventions to Reduce the Incidence of Hospital-Onset Clostridium difficile Infection: An Agent-Based Modeling Approach to Evaluate Clinical Effectiveness in Adult Acute Care Hospitals. Clin. Infect. Dis. 2018, 66, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; Wong, K.F.; Mueller, L.E.; Gussin, G.M.; McKinnell, J.A.; Tjoa, T.; Wedlock, P.T.; He, J.Y.; Chang, J.S.; Gohil, S.K.; et al. Modeling Interventions to Reduce the Spread of Multidrug-Resistant Organisms Between Health Care Facilities in a Region. JAMA Netw. Open 2021, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.; Wang, J.; Zhong, Z. An agent-based study on the airborne transmission risk of infectious disease in a fever clinic during COVID-19 pandemic. Build. Environ. 2022, 218, 109118. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, T.; Naylor, D.; Segre, A.M.; Thomas, G.; Herman, T.; Polgreen, P.M. Using Sensor Networks to Study the Effect of Peripatetic Healthcare Workers on the Spread of Hospital-Associated Infections. J. Infect. Dis. 2012, 206, 1549–1557. [Google Scholar] [CrossRef]
- Gilman, R.T.; Mahroof-Shaffi, S.; Harkensee, C.; Chamberlain, A.T. Modelling interventions to control COVID-19 outbreaks in a refugee camp. BMJ Glob. Health 2020, 5, 9. [Google Scholar] [CrossRef]
- Crooks, A.T.; Hailegiorgis, A.B. An agent-based modeling approach applied to the spread of cholera. Environ. Model. Softw. 2014, 62, 164–177. [Google Scholar] [CrossRef]
- Seresht, N.G. Enhancing resilience in construction against infectious diseases using stochastic multi-agent approach. Autom. Constr. 2022, 140, 13. [Google Scholar] [CrossRef]
- Qiao, Q.Y.; Cheung, C.; Yunusa-Kaltungo, A.; Manu, P.; Cao, R.F.; Yuan, Z.Y. An interactive agent-based modelling framework for assessing COVID-19 transmission risk on construction site. Saf. Sci. 2023, 168, 26. [Google Scholar] [CrossRef]
- Macal, C.M.; North, M.J.; Collier, N.; Dukic, V.M.; Wegener, D.T.; David, M.Z.; Daum, R.S.; Schumm, P.; Evans, J.A.; Wilder, J.R.; et al. Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: A dynamic agent-based simulation. J. Transl. Med. 2014, 12, 12. [Google Scholar] [CrossRef]
- Kasaie, P.; Andrews, J.R.; Kelton, W.D.; Dowdy, D.W. Timing of Tuberculosis Transmission and the Impact of Household Contact Tracing An Agent-based Simulation Model. Am. J. Respir. Crit. Care Med. 2014, 189, 845–852. [Google Scholar] [CrossRef]
- O’Neil, C.A.; Sattenspiel, L. Agent-based modeling of the spread of the 1918–1919 flu in three Canadian fur trading communities. Am. J. Hum. Biol. 2010, 22, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nikolaev, A.; Bian, L.; Li, L.; Li, L. Investigating transmission dynamics of influenza in a public indoor venue: An agent-based modeling approach. Comput. Ind. Eng. 2021, 157, 12. [Google Scholar] [CrossRef]
- Goyal, R.; Hotchkiss, J.; Schooley, R.T.; De Gruttola, V.; Martin, N.K. Evaluation of Severe Acute Respiratory Syndrome Coronavirus 2 Transmission Mitigation Strategies on a University Campus Using an Agent-Based Network Model. Clin. Infect. Dis. 2021, 73, 1735–1741. [Google Scholar] [CrossRef]
- D’Orazio, M.; Bernardini, G.; Quagliarini, E. Sustainable and resilient strategies for touristic cities against COVID-19: An agent-based approach. Saf. Sci. 2021, 142, 16. [Google Scholar] [CrossRef]
- Kahn, R.; Holmdahl, I.; Reddy, S.; Jernigan, J.; Mina, M.J.; Slayton, R.B. Mathematical Modeling to Inform Vaccination Strategies and Testing Approaches for Coronavirus Disease 2019 (COVID-19) in Nursing Homes. Clin. Infect. Dis. 2022, 74, 597–603. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Cummings, D.A.T.; Fraser, C.; Cajka, J.C.; Cooley, P.C.; Burke, D.S. Strategies for mitigating an influenza pandemic. Nature 2006, 442, 448–452. [Google Scholar] [CrossRef]
- Lanzas, C.; Chen, S. Complex system modelling for veterinary epidemiology. Prev. Vet. Med. 2015, 118, 207–214. [Google Scholar] [CrossRef]
- Pais, C.M.; Godano, M.I.; Juarez, E.; del Prado, A.; Manresa, J.B.; Rufiner, H.L. City-scale model for COVID-19 epidemiology with mobility and social activities represented by a set of hidden Markov models. Comput. Biol. Med. 2023, 160, 29. [Google Scholar] [CrossRef] [PubMed]
- España, G.; Grefenstette, J.; Perkins, A.; Torres, C.; Carey, A.C.; Diaz, H.; de la Hoz, F.; Burke, D.S.; van Panhuis, W.G. Exploring scenarios of chikungunya mitigation with a data-driven agent-based model of the 2014–2016 outbreak in Colombia. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef]
- Lo Iacono, G.; Cunningham, A.A.; Fichet-Calvet, E.; Garry, R.F.; Grant, D.S.; Leach, M.; Moses, L.M.; Nichols, G.; Schieffelin, J.S.; Shaffer, J.G.; et al. A Unified Framework for the Infection Dynamics of Zoonotic Spillover and Spread. PLoS Neglect. Trop. Dis. 2016, 10, 24. [Google Scholar] [CrossRef]
- Athreya, S.; Menon, G.I.; Sundaresan, R. COVID-19 Modeling for India and a Roadmap for the Future. Commun. ACM 2022, 65, 82–87. [Google Scholar] [CrossRef]
- Chowell, G.; Sattenspiel, L.; Bansal, S.; Viboud, C. Mathematical models to characterize early epidemic growth: A review. Phys. Life Rev. 2016, 18, 66–97. [Google Scholar] [CrossRef] [PubMed]
- Syga, S.; David-Rus, D.; Schälte, Y.; Hatzikirou, H.; Deutsch, A. Inferring the effect of interventions on COVID-19 transmission networks. Sci. Rep. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Cliff, O.M.; Harding, N.; Piraveenan, M.; Erten, E.Y.; Gambhir, M.; Prokopenko, M. Investigating spatiotemporal dynamics and synchrony of influenza epidemics in Australia: An agent-based modelling approach. Simul. Model. Pract. Theory 2018, 87, 412–431. [Google Scholar] [CrossRef]
- Ajelli, M.; Merler, S.; Fumanelli, L.; Piontti, A.P.Y.; Dean, N.E.; Longini, I.M.; Halloran, M.E.; Vespignani, A. Spatiotemporal dynamics of the Ebola epidemic in Guinea and implications for vaccination and disease elimination: A computational modeling analysis. BMC Med. 2016, 14, 10. [Google Scholar] [CrossRef]
- López, J.A.M.; García, B.A.; Bentkowski, P.; Bioglio, L.; Pinotti, F.; Boëlle, P.Y.; Barrat, A.; Colizza, V.; Poletto, C. Anatomy of digital contact tracing: Role of age, transmission setting, adoption, and case detection. Sci. Adv. 2021, 7, 12. [Google Scholar] [CrossRef]
- Shamil, M.S.; Farheen, F.; Ibtehaz, N.; Khan, I.M.; Rahman, M.S. An agent-based modeling of COVID-19: Validation, analysis, and recommendations. Cogn. Comput. 2024, 16, 1723–1734. [Google Scholar] [CrossRef]
- Aleta, A.; Martín-Corral, D.; Piontti, A.P.Y.; Ajelli, M.; Litvinova, M.; Chinazzi, M.; Dean, N.E.; Halloran, M.E.; Longini, I.M.; Merler, S.; et al. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 2020, 4, 964–971. [Google Scholar] [CrossRef]
- Kustudic, M.; Niu, B.; Liu, Q.Y. Agent-based analysis of contagion events according to sourcing locations. Sci. Rep. 2021, 11, 12. [Google Scholar] [CrossRef]
- Burke, D.S.; Epstein, J.M.; Cummings, D.A.T.; Parker, J.I.; Cline, K.C.; Singa, R.M.; Chakravarty, S. Individual-based computational modeling of smallpox epidemic control strategies. Acad. Emerg. Med. 2006, 13, 1142–1149. [Google Scholar] [CrossRef]
- Merler, S.; Ajelli, M.; Fumanelli, L.; Vespignani, A. Containing the accidental laboratory escape of potential pandemic influenza viruses. BMC Med. 2013, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Cooley, P.; Brown, S.; Cajka, J.; Chasteen, B.; Ganapathi, L.; Grefenstette, J.; Hollingsworth, C.R.; Lee, B.Y.; Levine, B.; Wheaton, W.D.; et al. The Role of Subway Travel in an Influenza Epidemic: A New York City Simulation. J. Urban Health 2011, 88, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Pilesjö, P.; Shirzadi, M.R.; Fadaei, R.; Mansourian, A. A spatially explicit agent-based modeling approach for the spread of Cutaneous Leishmaniasis disease in central Iran, Isfahan. Environ. Model. Softw. 2016, 82, 330–346. [Google Scholar] [CrossRef]
- Tabasi, M.; Alesheikh, A.A.; Sofizadeh, A.; Saeidian, B.; Pradhan, B.; AlAmri, A. A spatio-temporal agent-based approach for modeling the spread of zoonotic cutaneous leishmaniasis in northeast Iran. Parasites Vectors 2020, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shea, K.; Ferrari, M. Logistical constraints lead to an intermediate optimum in outbreak response vaccination. PLoS Comput. Biol. 2018, 14, 20. [Google Scholar] [CrossRef]
- Lee, B.Y.; Brown, S.T.; Cooley, P.C.; Zimmerman, R.K.; Wheaton, W.D.; Zimmer, S.M.; Grefenstette, J.J.; Assi, T.M.; Furphy, T.J.; Wagener, D.K.; et al. A Computer Simulation of Employee Vaccination to Mitigate an Influenza Epidemic. Am. J. Prev. Med. 2010, 38, 247–257. [Google Scholar] [CrossRef]
- Lee, B.Y.; Brown, S.T.; Cooley, P.; Grefenstette, J.J.; Zimmerman, R.K.; Zimmer, S.M.; Potter, M.A.; Rosenfeld, R.; Wheaton, W.D.; Wiringa, A.E.; et al. Vaccination Deep Into a Pandemic Wave Potential Mechanisms for a “Third Wave” and the Impact of Vaccination. Am. J. Prev. Med. 2010, 39, E21–E29. [Google Scholar] [CrossRef]
- Zachreson, C.; Chang, S.L.; Cliff, O.M.; Prokopenko, M. How will mass-vaccination change COVID-19 lockdown requirements in Australia? Lancet Reg. Health-West. Pac. 2021, 14, 12. [Google Scholar] [CrossRef]
- Faucher, B.; Assab, R.; Roux, J.; Levy-Bruhl, D.; Kiem, C.T.; Cauchemez, S.; Zanetti, L.; Colizza, V.; Boëlle, P.Y.; Poletto, C. Agent-based modelling of reactive vaccination of workplaces and schools against COVID-19. Nat. Commun. 2022, 13, 11. [Google Scholar] [CrossRef]
- Liu, F.C.; Enanoria, W.T.A.; Zipprich, J.; Blumberg, S.; Harriman, K.; Ackley, S.F.; Wheaton, W.D.; Allpress, J.L.; Porco, T.C. The role of vaccination coverage, individual behaviors, and the public health response in the control of measles epidemics: An agent-based simulation for California. BMC Public Health 2015, 15, 16. [Google Scholar] [CrossRef]
- Laskowski, M.; Duvvuri, V.R.; Buckeridge, D.L.; Wu, G.; Wu, J.H.; Moghadas, S.M. Influenza H3N2 variant viruses with pandemic potential: Preventing catastrophe in remote and isolated Canadian communities. Prev. Med. 2013, 57, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Vitali, A.; Mazzoleni, M.; Previdi, F. An agent-based model to assess large-scale COVID-19 vaccination campaigns for the Italian territory: The case study of Lombardy region. Comput. Methods Programs Biomed. 2022, 224, 107029. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef]
- Abeysuriya, R.G.; Sacks-Davis, R.; Heath, K.; Delport, D.; Russell, F.M.; Danchin, M.; Hellard, M.; McVernon, J.; Scott, N. Keeping kids in school: Modelling school-based testing and quarantine strategies during the COVID-19 pandemic in Australia. Front. Public Health 2023, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Vilches, T.N.; Rafferty, E.; Wells, C.R.; Galvani, A.P.; Moghadas, S.M. Economic evaluation of COVID-19 rapid antigen screening programs in the workplace. BMC Med. 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Asgary, A.; Cojocaru, M.G.; Najafabadi, M.M.; Wu, J.H. Simulating preventative testing of SARS-CoV-2 in schools: Policy implications. BMC Public Health 2021, 21, 18. [Google Scholar] [CrossRef]
- Vilches, T.N.; Nourbakhsh, S.; Zhang, K.V.; Juden-Kelly, L.; Cipriano, L.E.; Langley, J.M.; Sah, P.; Galvani, A.P.; Moghadas, S.M. Multifaceted strategies for the control of COVID-19 outbreaks in long-term care facilities in Ontario, Canada. Prev. Med. 2021, 148, 7. [Google Scholar] [CrossRef]
- Huang, Q.R.; Sun, Y.X.; Jia, M.M.; Zhang, T.; Chen, F.Y.; Jiang, M.Y.; Wang, Q.; Feng, L.Z.; Yang, W.Z. Quantitative Analysis of the Effectiveness of Antigen- and Polymerase Chain Reaction-Based Combination Strategies for Containing COVID-19 Transmission in a Simulated Community. Engineering 2023, 28, 234–242. [Google Scholar] [CrossRef]
- Sah, P.; Fitzpatrick, M.C.; Zimmer, C.F.; Abdollahi, E.; Juden-Kelly, L.; Moghadas, S.M.; Singer, B.H.; Galvani, A.P. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc. Natl. Acad. Sci. USA 2021, 118, 12. [Google Scholar] [CrossRef]
- Nishi, A.; Dewey, G.; Endo, A.; Neman, S.; Iwamoto, S.K.; Ni, M.Y.; Tsugawa, Y.; Iosifidis, G.; Smith, J.D.; Young, S.D. Network interventions for managing the COVID-19 pandemic and sustaining economy. Proc. Natl. Acad. Sci. USA 2020, 117, 30285–30294. [Google Scholar] [CrossRef]
- Li, K.K.F.; Jarvis, S.A.; Minhas, F. Elementary effects analysis of factors controlling COVID-19 infections in computational simulation reveals the importance of social distancing and mask usage. Comput. Biol. Med. 2021, 134, 13. [Google Scholar] [CrossRef] [PubMed]
- Bagger, N.-C.F.; van der Hurk, E.; Hoogervorst, R.; Pisinger, D. Reducing disease spread through optimization: Limiting mixture of the population is more important than limiting group sizes. Comput. Oper. Res. 2022, 142, 105718. [Google Scholar] [CrossRef]
- Hoertel, N.; Blachier, M.; Blanco, C.; Olfson, M.; Massetti, M.; Rico, M.S.; Limosin, F.; Leleu, H. A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nat. Med. 2020, 26, 1417–1421. [Google Scholar] [CrossRef]
- Mao, L. Agent-based simulation for weekend-extension strategies to mitigate influenza outbreaks. BMC Public Health 2011, 11, 10. [Google Scholar] [CrossRef]
- Peak, C.M.; Childs, L.M.; Grad, Y.H.; Buckee, C.O. Comparing nonpharmaceutical interventions for containing emerging epidemics. Proc. Natl. Acad. Sci. USA 2017, 114, 4023–4028. [Google Scholar] [CrossRef]
- Silva, W.; Das, T.K.; Izurieta, R. Estimating disease burden of a potential A(H7N9) pandemic influenza outbreak in the United States. BMC Public Health 2017, 17, 13. [Google Scholar] [CrossRef]
- Mehra, A.H.A.; Shafieirad, M.; Zamani, I. Leader-following consensus and qualitative analysis of a new multi-agent-based epidemic model. IET Contr. Theory Appl. 2024, 18, 408–421. [Google Scholar] [CrossRef]
- Novakovic, A.; Marshall, A.H. The CP-ABM approach for modelling COVID-19 infection dynamics and quantifying the effects of non-pharmaceutical interventions. Pattern Recognit. 2022, 130, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Vilches, T.N.; Tariq, M.; Galvani, A.P.; Moghadas, S.M. The impact of mask-wearing and shelter-in-place on COVID-19 outbreaks in the United States. Int. J. Infect. Dis. 2020, 101, 334–341. [Google Scholar] [CrossRef]
- Borkowski, M.; Podaima, B.W.; McLeod, R.D. Epidemic modeling with discrete-space scheduled walkers: Extensions and research opportunities. BMC Public Health 2009, 9, 19. [Google Scholar] [CrossRef]
- Gomez, J.; Prieto, J.; Leon, E.; Rodríguez, A. INFEKTA—An agent-based model for transmission of infectious diseases: The COVID-19 case in Bogotá, Colombia. PLoS ONE 2021, 16, e0245787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, W.Z. Research on demand forecasting and distribution of emergency medical supplies using an agent-based model. Chaos Solitons Fractals 2023, 177, 14. [Google Scholar] [CrossRef]
- Pray, I.W.; Pizzitutti, F.; Bonnet, G.; Gonzales-Gustavson, E.; Wakeland, W.; Pan, W.K.; Lambert, W.E.; Gonzalez, A.E.; Garcia, H.H.; O’Neal, S.E.; et al. Validation of a spatial agent-based model for Taenia solium transmission (“CystiAgent”) against a large prospective trial of control strategies in northern Peru. PLoS Neglect. Trop. Dis. 2021, 15, 20. [Google Scholar] [CrossRef]
- Vilches, T.N.; Abdollahi, E.; Cipriano, L.E.; Haworth-Brockman, M.; Keynan, Y.; Sheffield, H.; Langley, J.M.; Moghadas, S.M. Impact of non-pharmaceutical interventions and vaccination on COVID-19 outbreaks in Nunavut, Canada: A Canadian Immunization Research Network (CIRN) study. BMC Public Health 2022, 22, 9. [Google Scholar] [CrossRef]
- Alagoz, O.; Sethi, A.K.; Patterson, B.W.; Churpek, M.; Safdar, N. Effect of Timing of and Adherence to Social Distancing Measures on COVID-19 Burden in the United States A Simulation Modeling Approach. Ann. Intern. Med. 2021, 174, 50–57. [Google Scholar] [CrossRef]
- Scott, N.; Abeysuriya, R.G.; Delport, D.; Sacks-Davis, R.; Nolan, J.; West, D.; Sutton, B.; Wallace, E.M.; Hellard, M. COVID-19 epidemic modelling for policy decision support in Victoria, Australia 2020-2021. BMC Public Health 2023, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Mostaço-Guidolin, L.C.; Greer, A.L.; Wu, J.H.; Moghadas, S.M. The Impact of Demographic Variables on Disease Spread: Influenza in Remote Communities. Sci. Rep. 2011, 1, 7. [Google Scholar] [CrossRef]
- Tiwari, I.; Sarin, P.; Parmananda, P. Predictive modeling of disease propagation in a mobile, connected community using cellular automata. Chaos 2020, 30, 6. [Google Scholar] [CrossRef]
- Longini, I.M., Jr.; Nizam, A.; Xu, S.; Ungchusak, K.; Hanshaoworakul, W.; Cummings, D.A.; Halloran, M.E. Containing pandemic influenza at the source. Science 2005, 309, 1083–1087. [Google Scholar] [CrossRef]
- Scott, N.; Palmer, A.; Delport, D.; Abeysuriya, R.; Stuart, R.M.; Kerr, C.C.; Mistry, D.; Klein, D.J.; Sacks-Davis, R.; Heath, K.; et al. Modelling the impact of relaxing COVID-19 control measures during a period of low viral transmission. Med. J. Aust. 2021, 214, 79–83. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, Q.; Xu, B.Y.; Feng, R.; Li, C.C.; Xue, J.X.; Zhou, B.; Xu, M.L. Agent-Based Campus Novel Coronavirus Infection and Control Simulation. IEEE Trans. Comput. Soc. Syst. 2022, 9, 688–699. [Google Scholar] [CrossRef]
- Benneyan, J.; Gehrke, C.; Ilies, I.; Nehls, N. Community and Campus COVID-19 Risk Uncertainty Under University Reopening Scenarios: Model-Based Analysis. JMIR Public Health Surveill. 2021, 7, 18. [Google Scholar] [CrossRef]
- Tatapudi, H.; Das, T.K. Impact of school reopening on pandemic spread: A case study using an agent-based model for COVID-19. Infect. Dis. Model. 2021, 6, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Cao, Z.D.; Wang, Y.Z.; Zhu, B.; Zeng, D.; Wang, F.Y.; Qiu, X.G.; Song, H.B.; Wang, Y. An ACP Approach to Public Health Emergency Management: Using a Campus Outbreak of H1N1 Influenza as a Case Study. IEEE Trans. Syst. Man Cybern.-Syst. 2013, 43, 1028–1041. [Google Scholar] [CrossRef]
- Potter, M.A.; Brown, S.T.; Cooley, P.C.; Sweeney, P.M.; Hershey, T.B.; Gleason, S.M.; Lee, B.Y.; Keane, C.R.; Grefenstette, J.; Burke, D.S. School closure as an influenza mitigation strategy: How variations in legal authority and plan criteria can alter the impact. BMC Public Health 2012, 12, 11. [Google Scholar] [CrossRef]
- Kerr, C.C.; Mistry, D.; Stuart, R.M.; Rosenfeld, K.; Hart, G.R.; Núñez, R.C.; Cohen, J.A.; Selvaraj, P.; Abeysuriya, R.G.; Jastrzebski, M.; et al. Controlling COVID-19 via test-trace-quarantine. Nat. Commun. 2021, 12, 2993. [Google Scholar] [CrossRef]
- Rockett, R.J.; Arnott, A.; Lam, C.; Sadsad, R.; Timms, V.; Gray, K.A.; Eden, J.S.; Chang, S.; Gall, M.; Draper, J.; et al. Revealing COVID-19 transmission in Australia by SARS-CoV-2 genome sequencing and agent-based modeling. Nat. Med. 2020, 26, 1398–1404. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Ai, C.; Chen, H.L.; Chen, B.; Duan, W.; Qiu, X.G.; Lu, X.; He, M.; Zhao, Z.M.; Liu, Z. Understanding the Necessity and Economic Benefits of Lockdown Measures to Contain COVID-19. IEEE Trans. Comput. Soc. Syst. 2023, 10, 1888–1900. [Google Scholar] [CrossRef]
- Najmi, A.; Nazari, S.; Safarighouzhdi, F.; Miller, E.J.; MacIntyre, R.; Rashidi, T.H. Easing or tightening control strategies: Determination of COVID-19 parameters for an agent-based model. Transportation 2022, 49, 1265–1293. [Google Scholar] [CrossRef]
- Shekh, B.; de Doncker, E.; Prieto, D. Hybrid Multi-threaded Simulation of Agent-Based Pandemic Modeling using Multiple GPUs. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine-Medical Informatics and Decision Making, Washington, DC, USA, 9–12 November 2015; pp. 1478–1485. [Google Scholar]
- Edali, M. Using linear regression metamodels for evaluating interventions in an individual-based influenza epidemic model. Simul. Model. Pract. Theory 2023, 126, 15. [Google Scholar] [CrossRef]
- Bradhurst, R.A.; Roche, S.E.; East, I.J.; Kwan, P.; Garner, M.G. Improving the computational efficiency of an agent-based spatiotemporal model of livestock disease spread and control. Environ. Model. Softw. 2016, 77, 1–12. [Google Scholar] [CrossRef]
- Willem, L.; Stijven, S.; Tijskens, E.; Beutels, P.; Hens, N.; Broeckhove, J. Optimizing agent-based transmission models for infectious diseases. BMC Bioinform. 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.L.; Feng, Z.Z.; Thein, H.H. A Parallel Sliding Region Algorithm to Make Agent-Based Modeling Possible for a Large-Scale Simulation: Modeling Hepatitis C Epidemics in Canada. Ieee J. Biomed. Health Inform. 2016, 20, 1538–1544. [Google Scholar] [CrossRef]
- Fain, B.G.; Dobrovolny, H.M. GPU acceleration and data fitting: Agent-based models of viral infections can now be parameterized in hours. J. Comput. Sci. 2022, 61, 11. [Google Scholar] [CrossRef]
- Lombardo, G.; Pellegrino, M.; Tomaiuolo, M.; Cagnoni, S.; Mordonini, M.; Giacobini, M.; Poggi, A. Fine-grained agent-based modeling to predict covid-19 spreading and effect of policies in large-scale scenarios. IEEE J. Biomed. Health Inform. 2022, 26, 2052–2062. [Google Scholar] [CrossRef]
- Nardini, J.T.; Baker, R.E.; Simpson, M.J.; Flores, K.B. Learning differential equation models from stochastic agent-based model simulations. J. R. Soc. Interface 2021, 18, 23. [Google Scholar] [CrossRef]
- Perumal, R.; van Zyl, T.L. Surrogate-assisted strategies: The parameterisation of an infectious disease agent-based model. Neural Comput. Appl. 2025, 37, 627–638. [Google Scholar] [CrossRef]
- Huang, D.Q.; Dong, W.; Wang, Q. Spatial and temporal analysis of human infection with the avian influenza A (H7N9) virus in China and research on a risk assessment agent-based model. Int. J. Infect. Dis. 2021, 106, 386–394. [Google Scholar] [CrossRef]
- Pray, I.W.; Wakeland, W.; Pan, W.; Lambert, W.E.; Garcia, H.H.; Gonzalez, A.E.; Seth, E. O’Neal for the Cysticercosis Working Group in Peru. Understanding transmission and control of the pork tapeworm with CystiAgent: A spatially explicit agent-based model. Parasites Vectors 2020, 13, 13. [Google Scholar] [CrossRef]
- Siettos, C.I.; Russo, L. Mathematical modeling of infectious disease dynamics. Virulence 2013, 4, 295–306. [Google Scholar] [CrossRef]
- Du, E.H.; Chen, E.; Liu, J.; Zheng, C.M. How do social media and individual behaviors affect epidemic transmission and control? Sci. Total Environ. 2021, 761, 10. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, A.; Vilches, T.; Moghadas, S.M. Cost-effectiveness of Prophylactic Zika Virus Vaccine in the Americas. Emerg. Infect. Dis. 2019, 25, 2191–2196. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, J.; Yu, C.; Fei, J.; Luo, T.; Cao, Z. Agent-Based Modeling of Epidemics: Approaches, Applications, and Future Directions. Technologies 2025, 13, 272. https://doi.org/10.3390/technologies13070272
Zhang X, Wang J, Yu C, Fei J, Luo T, Cao Z. Agent-Based Modeling of Epidemics: Approaches, Applications, and Future Directions. Technologies. 2025; 13(7):272. https://doi.org/10.3390/technologies13070272
Chicago/Turabian StyleZhang, Xiangyu, Jiaojiao Wang, Chunmiao Yu, Jiaqiang Fei, Tianyi Luo, and Zhidong Cao. 2025. "Agent-Based Modeling of Epidemics: Approaches, Applications, and Future Directions" Technologies 13, no. 7: 272. https://doi.org/10.3390/technologies13070272
APA StyleZhang, X., Wang, J., Yu, C., Fei, J., Luo, T., & Cao, Z. (2025). Agent-Based Modeling of Epidemics: Approaches, Applications, and Future Directions. Technologies, 13(7), 272. https://doi.org/10.3390/technologies13070272







