3.1. Thermogravimetric Analysis
TGA is an effective method for examining the thermal decomposition behavior of materials during temperature elevation. Differential thermogravimetry (DTG), derived from TGA curve differentiation, enables a more precise analysis of material mass changes that are difficult to detect by a TGA analysis through differential curve variations. For this investigation, both TGA and DTG analyses were employed to examine the thermal decomposition behavior of kenaf under various stabilization conditions.
Figure 2a,b present the TGA and DTG curves of kenaf, respectively, according to the stabilization conditions. Furthermore,
Table 1 presents the stabilization, carbonization, and total (stabilization yield × carbonization yield) yields of kenaf calculated from
Figure 2.
Based on the mass reduction rate changes with an increase in temperature, the thermal decomposition behavior of the stabilized kenaf, as shown in
Figure 2, was analyzed for two distinct phases at 400 °C. Step I (T < 400 °C) corresponds to the mass reduction due to the thermal decomposition of the crystallite components (cellulose, hemicellulose, and lignin) of the kenaf.
Figure 2b reveals a decreasing DTG peak area and intensity with an increasing chemical stabilization temperature. Notably, the DTG peak becomes undetectable at stabilization temperatures above 200 °C. In addition,
Table 1 indicates a steady decrease in the stabilization yield of the kenaf, from 93.3% to 43.1%, with an increasing stabilization temperature. Biomass materials such as kenaf contain cellulose, hemicellulose, and lignin, which undergo thermal decomposition at 240–350, 200–260, and 280–500 °C, respectively [
26,
27]. Phosphorous compounds induce crystallite decomposition and crosslinking reactions in biomass materials such as kenaf, with their crosslinking density increasing at higher stabilization temperatures [
16,
28]. These results suggest that some of the cellulose, hemicellulose, and lignin that make up kenaf are decomposed during chemical stabilization processes, and some are transformed into a crystallite structure that is not pyrolysis through crosslinking reactions.
Step II (T > 400 °C) represents the carbonization phase of the kenaf crystallite.
Figure 2b demonstrates the increasing DTG peak intensity and area with a rising stabilization temperature. In addition,
Table 1 shows that the carbonization yield of the kenaf increased from 17.6% to 56.3% as the stabilization temperature increased. Upon dissolution, the diammonium hydrogen phosphate used in this study dissociated into 2NH
4+ and HPO
42− (Equation (4)), with the HPO
42− partially converted into H
2PO
4− in water (Equation (5)) [
29]. The resulting H
2PO
4− interacted with the OH groups in the OH cellulose crystallite, leading to cellulose crosslinking through dehydration reactions [
30,
31]. As previously mentioned, the crosslinking reaction rate intensified with higher stabilization temperatures. The cellulose component of the kenaf crystallite underwent crosslinking, through the H
2PO
4− formed as a result of the diammonium hydrogen phosphate dissociation, and the accelerated crosslinking rates can be attributed to the elevated stabilization temperatures. In other words, the crystallite structure of the kenaf, which was changed through the crosslinking reaction in step 1, was carbonized as the temperature increased, and it is concluded that the crosslinking density of the kenaf increased as the stabilization temperature increased, resulting in an increase in the carbonization yield. In addition, since phosphate linkages formed in the kenaf crystallite that decomposed above 450 °C, the chemically stabilized DTG peak intensity and area of the kenaf increased with the stabilization temperature.
Consequently, both the decomposition rate and crosslinking density of the kenaf increased with the stabilization temperature. Among the chemically stabilized kenaf samples, K-AP-20 demonstrated a superior total yield (stabilization yield × carbonization yield). Therefore, K-AP-20 was selected as the optimal stabilization process for producing butane adsorption AC for enhanced economic efficiency.
3.2. X-Ray Diffraction Analysis
An XRD analysis serves as an effective method for examining crystallite structure changes in isotropic carbon materials. In this study, XRD techniques were employed to evaluate the crystallite structure changes in the AK-AC as a function of activation time.
Figure 3a,b present the XRD patterns for the chemically stabilized kenaf and the change in the AK-AC XRD patterns during the activation process. In
Figure 3a, the XRD pattern of the as-received specimen exhibits typical cellulose XRD pattern features (cellulose (110) and (200) peaks). In contrast, the K-AP-20 displays a distinctly broad C(002) peak at a diffraction angle of approximately 23°, deviating from the biomass XRD patterns. In
Section 3.1, the TGA analysis confirmed that the crystallite structure of kenaf undergoes both decomposition and crosslinking reactions during chemical stabilization. This process appears to transform the crystallite structure of kenaf from its original biomass form into an isotropic carbon material configuration through stabilization reactions (decomposition and crosslinking).
As shown in
Figure 3b, all the AK-AC samples exhibit XRD patterns characteristic of an isotropic carbon material, exhibiting both C(002) and C(101) peaks. These patterns demonstrate a decreasing intensity with an extended activation time. This indicates ongoing oxidation reactions in the crystallite structure throughout the activation period, thus explaining the progressive decrease in the XRD pattern intensity. In addition, the AK-AC reveals a shift in the C(002) peak diffraction angle, from 23.2° to 21.9°, as the activation time increases. Previous TGA analyses have reported that phosphate linkages formed during the chemical stabilization of kenaf decompose above 450 °C [
16,
29]. In other words, the AK-AC is considered to have the weak bond energy of a crystal structure. Consequently, as the activation time increases, these weakly bound crystallite edges undergo continuous oxidation by the physical activating agent (H
2O), leading to increased interplanar spacing and a reduced C(002) peak central diffraction angle. Notably, all the AK-AC samples show exclusively isotropic carbon material XRD patterns, indicating an ash content below 1% for all the samples.
Figure 4a,b illustrate the evolution of the structural parameters (Lc: crystal height; La: crystal size) and interplanar distance (d
002, d
10l) in the AK-AC, respectively, with respect to the activation time. The graphitic crystal structure exhibits strong hybridized sp
2 bonds in the C(002) plane, whereas weak π bonds characterize the perpendicular direction. Physical activation mechanisms create pores through the oxidation of both the amorphous regions and crystallite edges in isotropic carbon materials [
15,
18]. Given that the AK-AC underwent steam activation, oxidation reactions occurred predominantly in the amorphous regions and crystallite edges, leading to more pronounced changes in d
002 relative to d
10l.
Figure 4a reveals that during the first 60 min, the AK-AC exhibited increases in the Lc and La of 9.87–11.33 and 31.90–43.02 Å, respectively. However, beyond 60 min, these values decreased to 10.28 and 33.80 Å, respectively. An XRD analysis provides statistical data for the crystallite structure changes in porous carbon materials. As previously noted, physical activation induces oxidation reactions in the amorphous regions and crystallite edges [
15,
18]. The observed increases in the Lc and La reflect relative increases due to the amorphous region’s oxidation rather than crystallite growth, whereas the La reduction indicates a crystallite size decrease due to the crystallite edge oxidation. This suggests that the AK-AC predominantly underwent oxidation in the amorphous regions during the first 40 min, followed by crystallite edge oxidation, causing the L
a to decrease with extended activation time.
Figure 4b demonstrates that the d
002 of the AK-AC increased from 3.84 to 3.94 Å during the first 80 min and then decreased to 3.82 Å over time, whereas d
10l remained relatively stable throughout. As shown in
Figure 3a, the kenaf precursor underwent crystallite reorganization during chemical stabilization. The phosphate linkages formed during this process decomposed during carbonization. The chemical stabilization process likely induced the formation of the AK-AC’s crystallite edge, characterized by weak bond energy due to the low crosslinking density. Notably, the expanded crystallite, resulting from the phosphate linkage decomposition, enhanced the activating agent’s (H
2O) penetration, promoting the oxidation of the amorphous regions and the weakly bound crystallite edge. This mechanism explains the increasing interplanar distance up to 80 min, followed by a rapid d
002 decrease due to the complete oxidation of the mono- or di-layer structures during the extended activation time.
3.3. Raman Spectroscopy Analysis
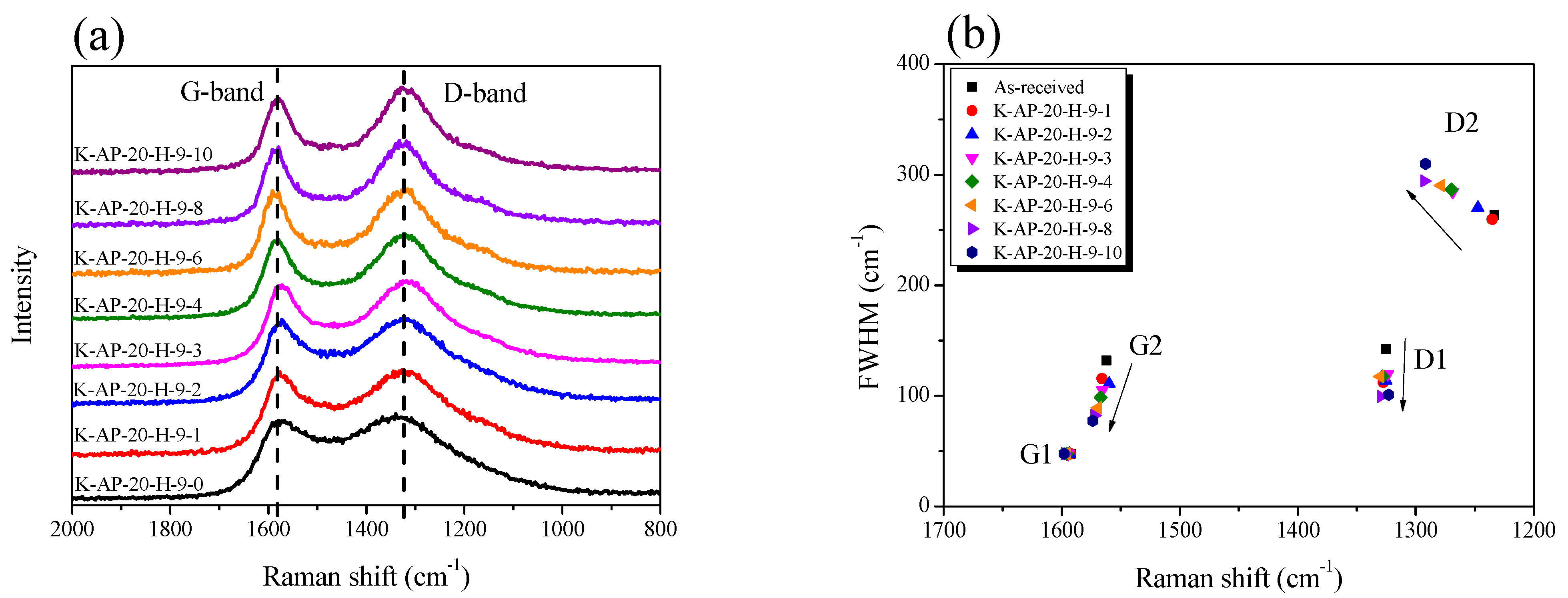
Together with XRD, Raman spectroscopy serves as a valuable analytical method for examining crystallite changes in porous carbon materials. As opposed to an XRD analysis, Raman spectroscopy is a useful method for analyzing the crystallite changes in carbon materials by providing information regarding the atomic bonding of different aspects of the microstructures of carbon materials. This study employed Raman spectroscopy to conduct a detailed analysis of the AK-AC evolution in terms of its carbon atomic bonding, structural defects, and crystallite changes throughout the activation period.
Figure 5a presents the Raman spectra of the AK-AC at various activation times, revealing two characteristic peaks: a G-band at 1580 cm
−1 (sp
2 hybridized graphitic structure) and D-band at 1360 cm
−1 (disordered graphitic lattice) [
23]. Furthermore, the valley between these bands exhibits an increasing depth with extended activation time, indicating continuous oxidation reactions in the amorphous region and crystallite structure, which aligns with the XRD results in
Section 3.2.
The Raman spectra analysis of the AK-AC employed Gaussian equation fitting for the four peaks (G1, G2, D1, and D2), with
Figure 5a illustrating the evolution of the FWHM for each peak during activation. The Raman spectrum components represent distinct structural features: G1 corresponds to sp
2 hybridized bonding (C–C bond) in the carbon crystallite basal plane, G2 indicates amorphous carbon and small crystallites, D1 represents crystallite edges, and D2 signifies a disordered graphitic lattice [
32]. First, in
Figure 5b, the FWHM of G1 shows no significant changes. As mentioned earlier, physical activation occurs through oxidation reactions in the amorphous regions of the porous carbon and at the edges of the crystallite. In addition, it must be taken into consideration that the basal plane of the sp² hybridized bonding (C-C bond) cannot be oxidized by the physical activation method [
32]. Therefore, the FWHM and intensity of the G1 peak does not change.
Second, both G2 and D1 show a progressive FWHM decrease during activation, with G2 exhibiting a more pronounced reduction. In the earlier XRD analysis, the oxidation reaction of AC-AC predominantly occurred in the amorphous region, followed by crystallite edge oxidation. The more rapid decrease in the FWHM of G2 compared to that of D1 reflects the faster oxidation rate of the amorphous regions versus the crystallite edges under physical activation conditions. Lastly, in contrast to G2 and D1, D2 displays a distinct peak, showing a continuous FWHM increase with activation time. As shown in
Figure 4b, AK-AC exhibits an increased d
002 as the crystallite edge with weak bond energy oxidizes. Thus, as shown in
Figure 6b, AK-AC exhibits an increasingly disordered graphite lattice due to the oxidation reactions of the crystallite edge with weak bond energy, resulting in an increased D2 peak FWHM with activation time.
3.5. N2/77K Isotherm Adsorption–Desorption Analysis
An N
2/77K isotherm adsorption–desorption analysis is a highly useful analytical method for evaluating the pore characteristics of AC. This study employed N
2/77K isotherm adsorption–desorption curves to examine the changes in the pore characteristics of the AK-AC across the activation period. According to the IUPAC standards, these curves can be categorized into eight distinct types, with each curve pattern allowing for the analysis of the AC pore characteristics [
35].
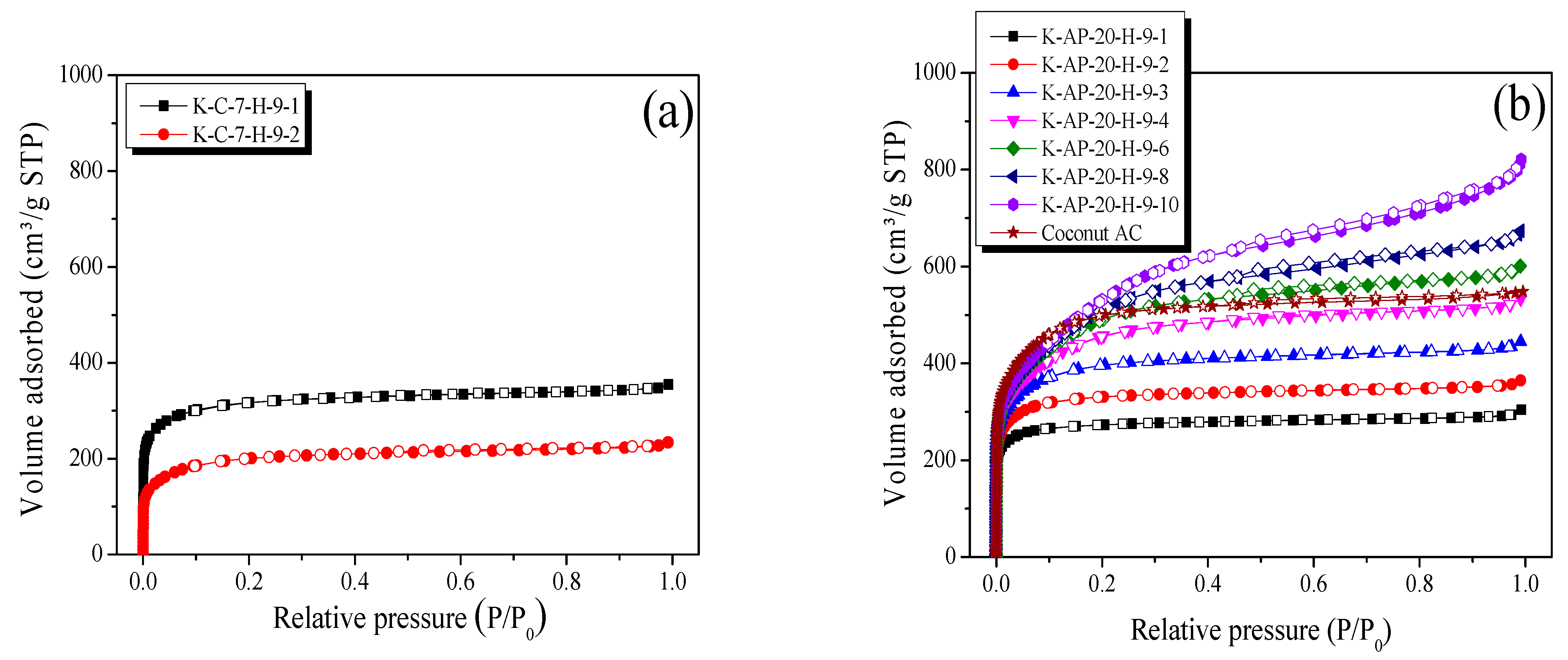
Figure 7 illustrates the N
2/77K isotherm adsorption–desorption behavior of both the CK-AC and AK-AC in relation to the activation time.
Figure 7a reveals that the CK-AC consistently exhibited Type I (a) behavior, as defined by the International Union of Pure and Applied Chemistry (IUPAC) standards, independent of the activation period. In particular, the nitrogen adsorption amount of the CK-AC decreased as the activation time increased. Type I (a) adsorption–desorption curves manifest when mono-layer adsorption takes place through strong Van der Waals force interactions between the pore wall of an AC and an adsorbate (N
2), mostly signifying ACs with microporous characteristics. In other words, the specific surface area and pores characteristic of CK-AC did not develop despite an extended activation time, and the CK-AC is considered to have a microporous structure. In contrast, the AK-AC demonstrated three distinct N
2/77K isotherm adsorption–desorption patterns across the different activation periods. Initially, the AK-AC exhibited IUPAC Type I (a) characteristics during the first 30 min, transitioned to Type I (b) behavior up through 60 min, and ultimately exhibited Type IV characteristics beyond 60 min. IUPAC Type I (b) patterns indicate that ACs possess a wider range of pore diameters than Type I (a), encompassing both micropores and narrow mesopores [
35]. IUPAC Type IV patterns can be observed in ACs with well-developed mesoporous structures [
35]. These observations indicate that the AK-AC differed from the CK-AC and underwent progressive mesopore development, with the pore characteristics transitioning from microporous to micro-mesoporous to mesoporous as the activation time increased. The N
2/77K isotherm adsorption–desorption analysis of the Coconut AC revealed IUPAC Type I (b) patterns. Therefore, the Coconut AC is considered to have micropores and narrow mesopores.
The hysteresis phenomenon in N
2/77K isotherm adsorption-desorption curves encompasses six distinct types under the IUPAC classification, with the pore morphology of each AC being differentiated based on the hysteresis loop shape [
35]. Hysteresis is known to occur due to capillary condensation at pressures below saturation pressure after single- or multi-layer adsorption by mesopore walls [
35].
Figure 7a reveals that the CK-AC exhibited minimal hysteresis with regard to the activation duration. In a previous study on the crystallite changes in kenaf during chemical stabilization, crystallite transformation from a cellulose crystallite to an isotropic carbon material crystallite structure was demonstrated. The CK-AC developed wedge-shaped pores in its cellulose structure without structural transformation, and the absence of capillary condensation in these pores explains the lack of hysteresis in its adsorption–desorption curves [
35]. In contrast, as shown in
Figure 7b, the AK-AC exhibited IUPAC H4-type hysteresis from 40 min, with a progressively increasing hysteresis area over longer activation periods. H4-type hysteresis is typically observed in ACs with slit-shaped pore characteristics [
35]. Moreover,
Figure 4a shows crystallite edge oxidation after 40 min. Thus, although the AK-AC initially formed wedge-shaped pores due to the amorphous oxidation of the surface during early activation, as the activation time increased, the oxidation reaction of the crystallite proceeded from the exterior to the interior, and the oxidation of the crystallite edges progressed, leading to its transformation into a slit-shaped pore structure. Therefore, K-AP-20-H-9-10, with the longest activation time, is considered to have the largest hysteresis. In addition, the Coconut AC was observed to have IUPAC H4-type hysteresis, as shown in
Figure 7b. Therefore, this indicates that the Coconut AC concurrently developed micropores and narrow mesopores with slit-shaped pore characteristics.
Table 2 presents the evolution of the textural properties of the CK-AC and AK-AC across the activation period. Since ACs produced by the physical activation method have a pore development mechanism that is activated through the oxidation reactions of the crystallite structure (amorphous or crystallite edge) via an activating agent (H
2O), the crystallite and pore characteristics are closely correlated [
15,
18]. In addition, previous research has confirmed that pore development behavior in ACs by the physical activation method proceeds through micropore development from amorphous oxidation and mesopore development from crystallite edge oxidation [
15]. Therefore, in this study, the textural properties of the AK-AC were analyzed based on the previous analysis of crystallite structure changes as a function of the activation time. According to
Table 2, both materials exhibited declining activation yields, 34.7% to 4.3% for the CK-AC and 27.7% to 3.0% for the AK-AC, with an extended activation time and through ongoing crystallite structure oxidation. The CK-AC exhibited a decrease in the specific surface area and mesopore volume, from 1200 to 760 m
2/g and 0.08 to 0.07 cm
3/g, respectively. Note that the pore characteristics of K-C-H-9-3 could not be analyzed due to the complete oxidation of the carbon crystallite that left only ash residue. Conversely, compared to the CK-AC, the AK-AC demonstrated superior pore characteristics, achieving specific surface area and mesopore volume increases, from 1080 to 1940 m
2/g and 0.05 to 0.63 cm
3/g, respectively. In particular, K-AP-20-H-9-6, K-AP-20-H-9-8, and K-AP-20-H-9-10 surpassed the commercial Coconut AC in both specific surface area and mesopore ratio. As evidenced by
Figure 3a, the kenaf underwent a structural transformation from cellulose to an isotropic carbon material during stabilization. Previous research has reported that biomass-derived AC produced without a stabilization process underwent activation reactions primarily at the crystallite edges, resulting in no micropore development, even with an increased activation time [
36]. Therefore, in this study, since the CK-AC was produced through a physical activation process without any particular stabilization, the activation reactions occurred primarily at the crystallite edges, resulting in no development of a specific surface area, despite the increased activation time. In contrast to the CK-AC, the AK-AC underwent crystallite decomposition, dehydration, and crosslinking reactions through a chemical stabilization process, leading to crystallite establishment. Although derived from the same precursor as the CK-AC, the AK-AC developed distinct pore characteristics due to different chemical stabilization processes. Therefore, the pore development behavior of the AK-AC was divided into two stages based on the crystallite structure oxidation reactions. First, the initial activation reaction (initial 30 min) showed oxidation predominantly in the amorphous regions (
Figure 4a). Subsequently, during the later activation reaction (after 40 min), the AK-AC exhibited oxidation reactions predominantly at the crystallite edges (
Figure 4a). Therefore, the AK-AC underwent amorphous oxidation in the first 30 min of the activation period, resulting in a significant increase in the micropore volume, from 0.42 to 0.58 cm
3/g. In addition, after 40 min, the AK-AC exhibited a greater increase in mesopore volume (0.24 to 0.63 cm
3/g) compared to its micropore volume (0.58 to 0.63 cm
3/g), due to the crystallite edge oxidation reactions.
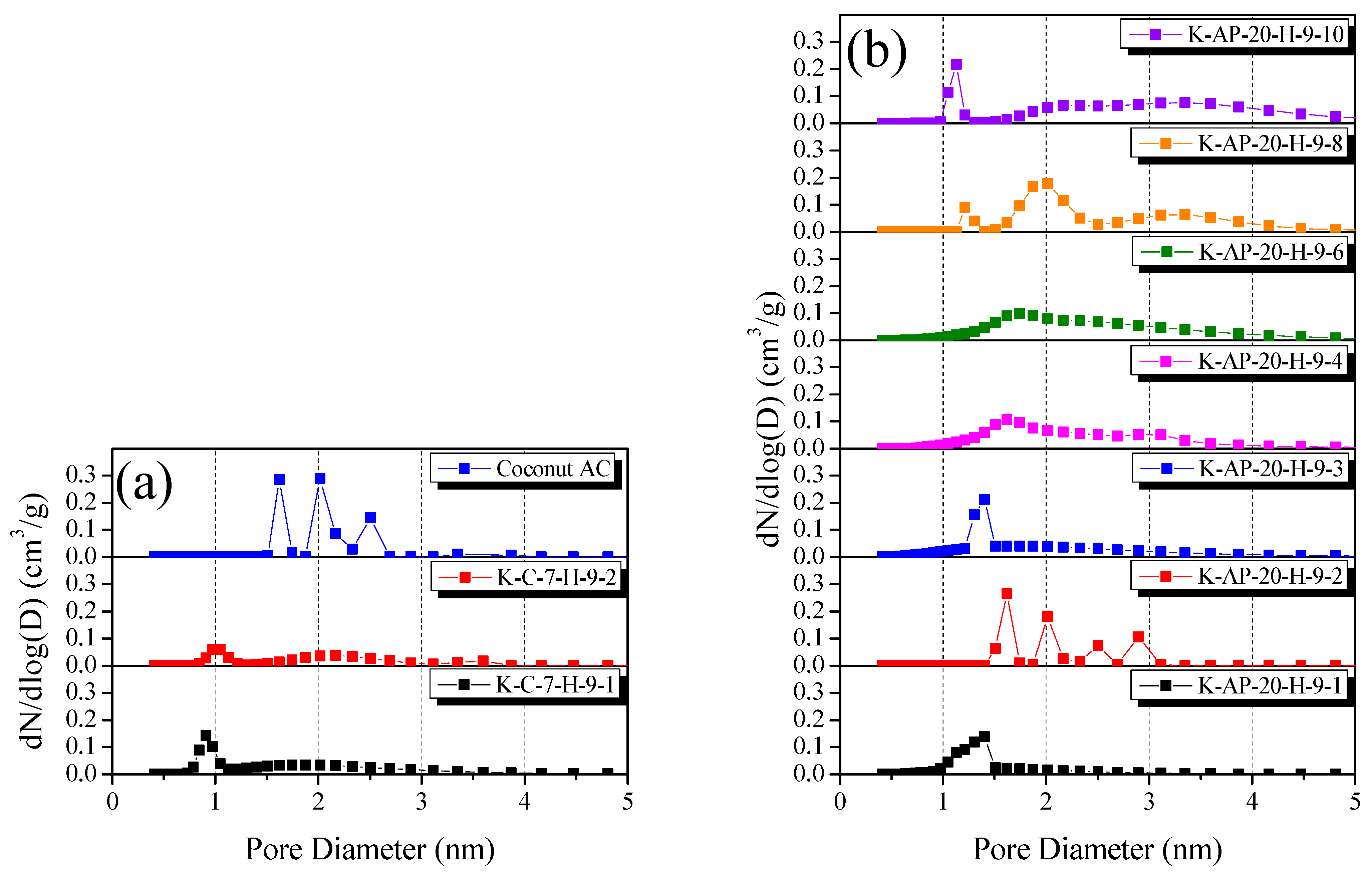
The NLDFT method provides the most effective approach for analyzing AC PSD.
Figure 8 illustrates the PSD curves for the CK-AC and AK-AC as a function of the activation time. The PSD curves exhibit distinct patterns corresponding to the changes in the texture properties.
Figure 8a reveals that all the CK-AC samples developed both micropores (less than 2 nm in diameter) and mesopores (less than 3 nm in diameter). The physical activation method generates pore development through two distinct mechanisms: pore deepening (maintaining pore diameter) and pore drilling (expanding pore diameter) [
37]. As previously noted,
Table 2 reveals that the crystallite of the CK-AC, derived from cellulose, underwent oxidation primarily at the crystallite edges, limiting the specific surface area development. Consequently, since the CK-AC developed pores through pore drilling due to the oxidation reaction of the crystallite edges, we determined that not only micropores but also mesopores developed for all activation times.
The AK-AC demonstrated distinct PSD curve evolution patterns. The initial activation phase (up to 30 min) produced sharp PSD curves characterized by micropores less than 2 nm in diameter. The subsequent activation phase (beyond 40 min) revealed the development of both micropores (less than 2 nm in diameter) and mesopores (exceeding 2 nm in diameter), with the PSD curves showing a significantly broader distribution than those of K-AP-20-H-9-1, K-AP-20-H-9-2, and K-AP-20-H-9-3. As evidenced by
Figure 4a, after 40 min of activation time, the crystallite oxidation reaction of the AK-AC was dominant at the crystallite edge. This indicates that although during early activation micropores developed through amorphous oxidation-induced pore deepening, from 40 min, mesopore development occurred through pore drilling by the crystallite edge oxidation reactions, resulting in broadened PSD curves.
Figure 8b reveals, from its PSD curves, that the Coconut AC developed micropores ranging from 1.5 to 2 nm and mesopores from 2 to 2.5 nm. According to
Table 2, although the Coconut AC achieved a comparable specific surface area (1820 m
2/g) to that of K-AP-20-H-9-6, it exhibited a lower mesopore volume ratio. Furthermore, although both materials utilized biomass precursors, the AK-AC underwent chemical stabilization processing, distinguishing it from the Coconut AC. Thus, the crystallite structure differences between the AK-AC (chemically stabilized kenaf) and Coconut AC (coconut shell) precursors caused the Coconut AC to exhibit a different PSD than that of K-AP-20-H-9-6, despite having similar specific surface areas. Consequently, the utilization of diammonium hydrogen phosphate is considered highly effective for the chemical stabilization process to develop specific surface area and mesopore volume in ACs.
3.6. Butane Breakthrough Analysis
The breakthrough curve technique is commonly utilized to evaluate the harmful gas adsorption characteristics of porous carbon. In this study, breakthrough curves were employed to evaluate the butane adsorption characteristics of the AC. The breakthrough point of the breakthrough curve was set at C/C0 = 0.1.
Figure 9a illustrates the adsorption and desorption behavior of butane (99.999%) for both the AK-AC and Coconut AC.
Figure 9b provides a magnified view of the breakthrough points on the breakthrough curves. The complete breakthrough curve results are summarized in
Table 3.
The analysis of
Figure 9 and
Table 3 reveals an increasing trend in the breakthrough points and butane adsorption capacity in the following order: K-AP-20-H-9-1 (2.66 min, 0.31 g/g) < K-AP-20-H-9-2 (3.58 min, 0.42 g/g) < K-AP-H-20-9-3 (3.80 min, 0.45 g/g) < Coconut AC (4.38 min, 0.52 g/g) < K-AP-20-H-9-4 (4.77 min, 0.56 g/g) < K-AP-20-H-9-6 (5.52 min, 0.65 g/g) < K-AP-20-H-9-8 (6.43 min, 0.76 g/g) < K-AP-20-H-9-10 (6.68 min, 0.79 g/g). For the AK-AC, both the breakthrough point and capacity increase with longer activation times. Conversely, although
Table 2 shows that the Coconut AC has a specific surface area (1820 m
2/g) similar to that of K-AP-20-H-9-6 (1800 m
2/g), it exhibited a lower breakthrough point and capacitance values. In other words, it is concluded that the butane adsorption characteristics of the AC were significantly affected by the pore characteristics rather than the specific surface area.
In previous studies, it has been confirmed that the gas adsorption characteristics of porous carbon are affected by the pore diameter rather than the specific surface area [
38,
39,
40,
41,
42]. In other words, even if the specific surface area of porous carbon increases, it is difficult to improve its gas adsorption characteristics, but the gas adsorption characteristics of porous carbon improve as the pore volume of the pore diameter that can be involved with the adsorbed gas increases [
38,
39,
40,
41,
42]. Additionally, our previous research demonstrated that the butane adsorption characteristics of AC are predominantly influenced by the development of mesopores with diameters between 2.8 and 3.8 nm, rather than by the specific surface area [
16].
Figure 8 shows that as the activation time increases, the PSD curves of the AK-AC show the progressive development of both the micropores and mesopores. Notably, after 40 min, the AK-AC exhibits a significant development of mesopores, with diameters ranging from 2 to 4 nm. In contrast, the Coconut AC only shows the development of micropores and mesopores with diameters less than 3 nm. As a result, despite having a similar specific surface area to that of K-AP-20-H-9-6, the Coconut AC exhibits a lower breakthrough point and capacitance values, which can be attributed to its underdeveloped PSD in the 2.8–3.8 nm diameter pore range.
In
Figure 9, the butane desorption amount of the AK-AC increased with an increased activation time. Previous research has established that the butane desorption characteristics of AC improve with an increasing mesopore volume [
16].
Table 2 indicates that the mesopore volume ratio of the AK-AC increased from 10.6% to 50.0% according to the activation time. That is, the butane desorption characteristics of the AK-AC improved due to an increase in the mesopore volume. In particular, K-AP-20-H-9-10 was observed to have a similar butane desorption rate as the Coconut AC due to its having the highest mesopore volume ratio, despite having the highest butane adsorption capacity.
The previous breakthrough curve experiments revealed that the butane adsorption capacity of AC is primarily influenced by the PSD rather than by the specific surface area. Therefore, the correlation coefficient between the butane adsorption capacity and PSD was analyzed. First, in
Figure 8, the PSD curves of each activated carbon were divided into 0.5 nm increments over a pore diameter range of 0.0–5.0 nm. The sum of the pore volumes of each activated carbon over the range of pore diameters was then calculated and plotted with the butane adsorption capacity of each activated carbon (X-axis: pore volume according to pore diameter, Y-axis: butane adsorption capacity). Finally, the correlation between the pore volume and butane adsorption capacity as a function of the pore diameter for each calculated activated carbon is shown in
Figure 10 [
16]. The activated carbons (AK-AC and Coconut AC) demonstrate that the strongest correlation between the PSD and butane adsorption capacity occurs in a pore diameter range of 3.0–4.0 nm, with a correlation coefficient of 0.87. This indicates that the butane adsorption capacity is most significantly influenced by the development of mesopores within this specific diameter range. These findings align with those of the breakthrough curve results, as well as with previous research [
16].
In conclusion, the chemical stabilization process employing diammonium hydrogen phosphate successfully established an optimal crystallite in kenaf through decomposition and crosslinking reactions. Thus, kenaf was manufactured into porous carbon for butane adsorption. Notably, the fabricated AC demonstrated superior butane adsorption characteristics, achieving up to a 52% higher butane adsorption capacity than the Coconut AC. Therefore, the results suggest that AK-AC can potentially be utilized in automotive canisters rather than AC manufactured through chemical activation methods in the future.