Abstract
Chronic respiratory diseases (CRD), which include Chronic Obstructive Pulmonary Disease (COPD) and asthma, are significant global health issues, with air quality playing a vital role in exacerbating these conditions. This systematic review explores how monitoring indoor air quality (IAQ) can help manage and reduce respiratory exacerbations in CRD patients. A search of the Web of Science database, yielding 301 articles, was conducted following PRISMA guidelines. Of these, 60 met the inclusion criteria, and after screening, 21 articles were analyzed. The review identified substantial gaps in current research: the lack of standardization in IAQ monitoring; the need for considering geographic variability and for long-term longitudinal studies; and the importance of linking monitored air quality data with respiratory health indicators. It also stressed the importance of considering the heterogeneity of patients in the methodological study design, as well as the convenience of introducing recommendation systems to assess the true impact of corrective measures on indoor air quality in the homes of chronic respiratory patients. The integration of home-based IAQ monitoring with machine learning techniques to enhance our understanding of the relationship between IAQ and respiratory health is emerging as a key area for future research. Addressing all these challenges has the potential to mitigate the impact of CRD and improve the quality of life for patients.
1. Introduction
Chronic respiratory diseases (CRD) pose significant global health challenges, affecting millions and contributing substantially to morbidity and mortality rates worldwide. According to the World Health Organization (WHO), CRD account for approximately 7% of all annual deaths globally, underscoring their critical impact on public health and the urgent need for effective management and prevention strategies [1].
CRD are a major category of non-communicable diseases, with Chronic Obstructive Pulmonary Disease (COPD) and asthma being among the most prevalent and possessing a higher economic burden [2,3]. COPD is characterized by persistent airflow limitation and is one of the leading causes of death, with a prevalence projection of 600 million cases worldwide by 2050 [4]. Asthma, a chronic inflammatory disease that affects the airways, affects over 300 million individuals worldwide and poses a severe overall burden on healthcare systems, due to its high prevalence among children and young adults and associated morbidity [5,6]. Both diseases significantly reduce patients’ quality of life and impose a substantial economic burden due to frequent hospitalizations, medical visits, and loss of productivity.
Exacerbation, acute episodes of worsening respiratory symptoms, are critical events in CRD. COPD exacerbations account for most hospitalizations and are a leading cause of death among patients. Similarly, asthma exacerbations can lead to hospitalization and to a decline in lung function over time. Effective management and preventive strategies are crucial for reducing the frequency and severity of exacerbations, which can improve patient’s quality of life and reduce the associated economic burden [7,8,9,10].
The prevalence of CRD has increased in recent years, with an estimated rise of approximately 40% over the past three decades, largely due to pollution [2]. Despite the significant burden that CRD poses, they have received less public attention and research funding compared to other diseases such as cardiovascular disease, cancer, stroke, diabetes, and Alzheimer’s disease. This highlights the need for enhanced research and resource allocation.
A recent body of research highlights the influence of air quality on respiratory health. Epidemiological studies have consistently demonstrated the relationship between exposure to atmospheric pollutants and increased incidence and severity of respiratory diseases. In studying the triggers of exacerbations, some of which are well known, such as allergens or respiratory infections, causal relationships have been found with other factors, such as environmental pollutants [11]. Key outdoor pollutants, including fine particulate matter (PM2.5), nitrogen dioxide (NO2), and ozone (O3), have been linked to the development and exacerbation of diseases such as COPD and asthma [11,12,13,14,15,16]. These pollutants primarily originate from anthropogenic outdoor sources such as vehicular traffic, industry, and fossil fuel combustion, emphasizing the importance of public policies to improve air quality.
Indoor air quality (IAQ) is equally important, as individuals spend approximately 90% of their time indoors. Indoor pollutants, including particulate matter, volatile organic compounds (VOCs), carbon dioxide (CO2), and carbon monoxide (CO), may affect respiratory health [17,18].
Therefore, monitoring and controlling indoor environments is essential to prevent exposure to harmful contaminants. Monitoring technologies, including environmental sensors, play a pivotal role in assessing air quality, both indoors and outdoors [19,20,21,22]. These technologies enable the continuous measurement of various parameters such as PM1, PM2.5, PM10, NO2, O3, and sulfur dioxide (SO2) outdoors, as well as VOCs, CO2, and CO indoors. By providing online data, these sensors support the identification of pollution levels and enable an assessment of their impact on respiratory health, thus facilitating timely interventions.
The integration of AI in healthcare is revolutionizing the management of chronic diseases, including CRD. AI-based predictive models are being developed to forecast exacerbations of COPD and asthma. These models utilize clinical, environmental, and patient behavior data to identify patterns and predict acute episodes. This proactive approach allows for early and personalized interventions that have the potential to improve health outcomes and reduce healthcare costs [5,23]. AI technologies also play a critical role in evaluating the relationship between air quality and respiratory health.
AI-based predictive models which use data from environmental sensors and clinical patient data have shown promising results in predicting the impact of air pollution levels on the incidence and severity of respiratory exacerbations [17,19,21]. This information is crucial for developing effective mitigation strategies and public policies to protect the health of vulnerable populations.
Considering all the above-mentioned, using air quality monitoring technologies and AI in managing CRD offers promising avenues for improving patient outcomes and reducing the economic burden associated with these diseases. Nevertheless, there are gaps in research in this field that are hindering the development of predictive tools to improve the health management of these chronic respiratory patients. These identified gaps include the following:
- (a)
- The lack of methodological standardization in studies.
- (b)
- The need for long-term longitudinal trials that consider the spatial variability, individualized measures of exposure to pollutants, and the heterogeneity in demographic and health characteristics of patients.
- (c)
- The need for linking monitored air quality measurements with respiratory health markers collected from sensors, mobile applications, and the patient’s medical history.
- (d)
- The limited research studies focused on how the combination of indoor pollutants and the confounding factors beyond the isolated effects of each pollutant separately affect respiratory health, thus contributing to pro-inflammatory states and genetic and epigenetic alterations.
- (e)
- The lack of personalized early warning and recommendations systems that combine environmental monitoring with artificial intelligence techniques.
This study aimed to build and describe the current perspective on IAQ monitoring in the context of environmental health risks, and to provide a comprehensive guide to current technologies and their potential applications in the field of respiratory health. A multidisciplinary approach combining environmental monitoring with advanced technological solutions is advocated to address the challenges posed by asthma and COPD, the two most prevalent CRD.
This study reviewed scientific literature and identified the extent and gaps available to date on IAQ and its relationship to asthma and COPD progression. Three objectives were drawn up to achieve this task, as follows:
- (a)
- To determine peer-reviewed scientific studies that have been conducted on IAQ monitoring and its effect on the health of patients with asthma or COPD, and to create a synopsis of the empirical knowledge on this field to date.
- (b)
- To provide a focused understanding of the possibilities of monitoring IAQ to predict exacerbations in asthma and COPD and improve respiratory environmental health.
- (c)
- Identify action gaps in indoor monitoring air quality studies and possible actions to mitigate them.
2. Methods
2.1. Search Strategy and Inclusion and Exclusion Criteria
We undertook a systematic review to assess the evidence on the relationship between IAQ, COPD, and asthma. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used in this systematic review as a basis for reporting the findings [24]. The search aimed to identify studies with COPD and asthma patients in which IAQ parameters were monitored and linked to disease outcomes, such as acute exacerbation, using artificial intelligence or advanced statistics techniques.
Inclusion criteria covered English-language publications in peer-reviewed journals. Regularly issued conference proceedings, communications abstracts, and non-original articles (e.g., editorials and review papers) were excluded. Nevertheless, the reference list of the reviewed manuscripts was also screened to find potential eligible studies.
A systematic search in the Web of Science (SCI) database was undertaken. The search aimed to identify the evidence from the literature on experiences of IAQ monitoring applied to COPD and asthma. The search was performed on 14 May 2024, and the following search strings were used: (((ALL = (“COPD” OR “ASTHMA”)) AND ALL = (“indoor”)) AND ALL = (“air quality”)). Initially, searches that are more specific were performed, but finally, keywords that are more general were used to ensure the inclusion of potentially relevant studies.
As a first step, the title and abstract of each study were screened for eligibility. The full text of the screened manuscripts was then assessed for eligibility. Two authors conducted the screening, and disagreements were solved by consensus. The resulting eligible articles were included in the review.
2.2. Data Extraction and Quality Assessment
Two reviewers (P.C.-M., D.S.-L.) extracted the data necessary to complete each table, each one-half of the papers.
An agreed data extraction form was used. In case of doubts or discrepancies, a second reviewer was consulted. If discrepancies persisted, they were resolved with the participation of a third reviewer (D.S.-M.). The data items extracted included the following: year of publication, authors, target population (patients with asthma or COPD), study time and sample size, age of patients, clinical data used in the study, a brief description of the objectives and results of the research, pollutants that were monitored, sensors used to gather indoor IAQ data, information about calibration of sensors, data communications, sampling rate, details of data post-processing, and information on the development of predictive models and the application of conventional statistics and machine learning techniques.
Two reviewers assessed all included articles for quality following the Mixed Method Appraisal Tool (MMAT) [25]. The MMAT is an effective tool for quality assessment in mixed-method review studies. MMAT consists of four criteria to assess quantitative (descriptive, randomized, and non-randomized) and qualitative studies. Each study design was evaluated according to its methodological domain (qualitative, quantitative randomized controlled, quantitative non-randomized, quantitative descriptive, fixed methods). The MMAT metric was estimated by assigning 25% to each criterion that was fully addressed. The metric ranged from 0 to 100%. Disagreements were handled with the participation of a third reviewer.
Finally, the narrative synthesis methodology was applied due to the heterogeneity of the findings on study approaches, settings, and intervention factors [26].
2.3. Risk-of-Bias Assessment
Two potential sources of individual studies’ bias were identified in this study. First, the eventual omission of systematic reviews of recently completed clinical studies, or other studies not published in journals. Second, the publication’s language for studies included in this review was limited to English, which is a potential source of bias that must be considered [27].
3. Results
3.1. Study Selection
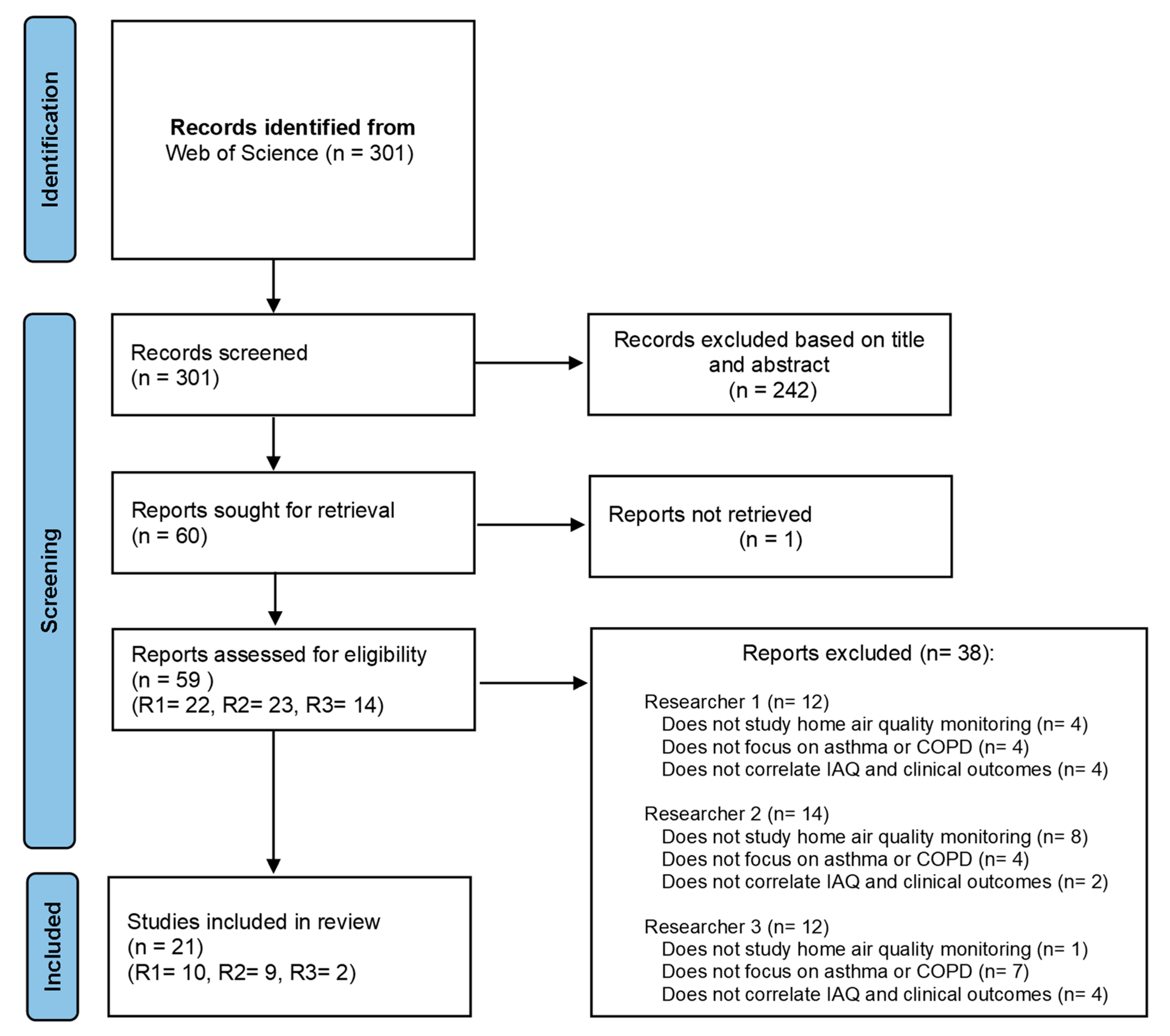
The database search revealed 301 records. The title, abstract, and keywords were reviewed and 242 articles that did not meet inclusion criteria were excluded. One article could not be retrieved, and the full text of the remaining 59 potentially relevant articles was assessed. Thirty-seven articles were excluded. Reasons for exclusion included the following: not involving a study on home air quality monitoring (N = 13); not focusing on asthma or COPD (N = 15); and not correlating IAQ and clinical outcomes (N = 10). Finally, 21 articles were included, all published in English. The PRISMA flow diagram illustrated in Figure 1 describes the process followed in this review.
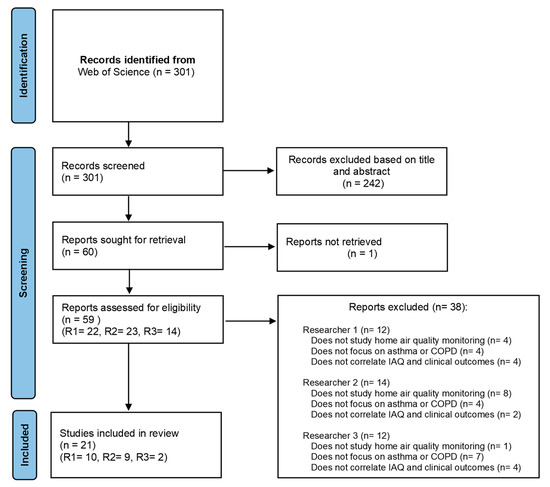
Figure 1.
Flow Diagram for study selection following the preferred reporting items for systematic reviews and the meta-analyses (PRISMA) statement.
3.2. Study Characteristics
3.2.1. Publication and Study Design Type
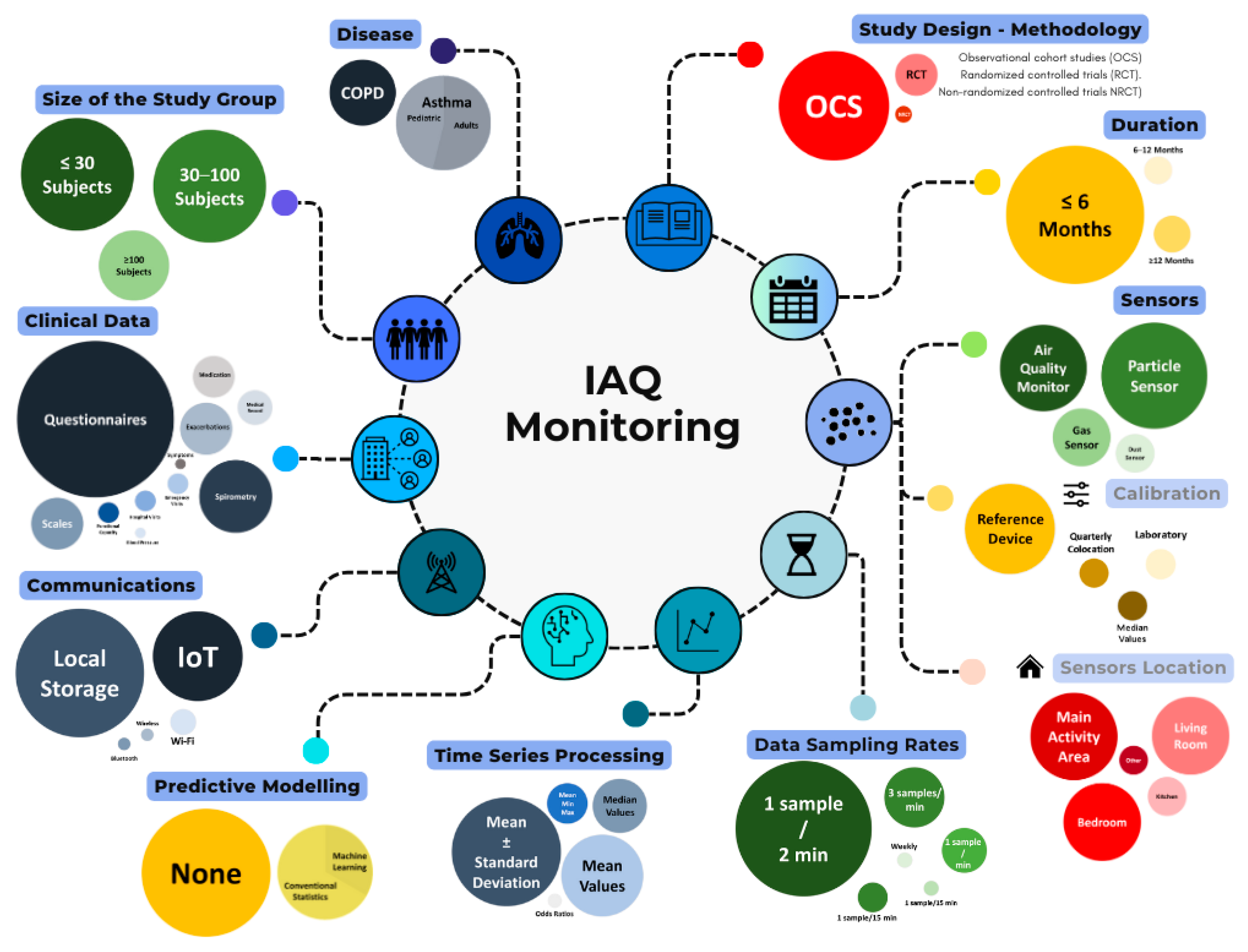
Table 1 presents the main characteristics of the selected studies, highlighting key initial information such as their targeted disease and the primary objective. The 21 papers included in this review featured an air quality study linked to outcomes in asthma and COPD. Figure 2 synthesizes the findings.
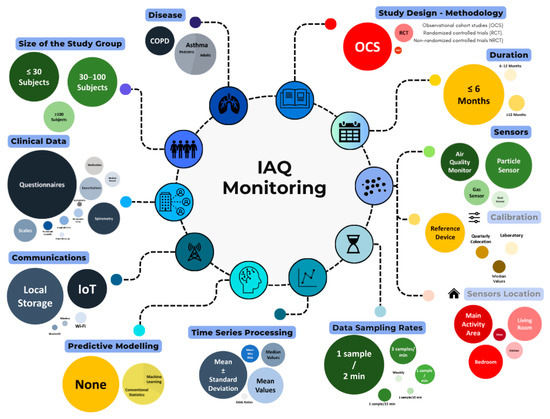
Figure 2.
Graphical summary of evaluated aspects in IAQ monitoring.
These studies were categorized based on whether they addressed asthma or COPD. Of the 21 studies, 43% (N = 9) focused on COPD [11,12,21,22,28,29,30,31,32], while 57% (N = 12) focused on asthma [17,18,33,34,35,36,37,38,39,40,41,42].
Regarding the methodology, there were 14 observational cohort studies (OCS), with 9 focused on asthma [17,18,33,34,35,36,38,41,42], and 5 on COPD [11,12,21,22,28]. Four asthmas [37,38,40,41] and two COPD studies [29,30] encompassed randomized controlled trials (RCT). Two COPD studies [31,32] performed non-randomized controlled trials (NRCT).


Table 1.
Main characteristics of the included studies.
Table 1.
Main characteristics of the included studies.
| Year | Authors | Country | Disease | Study Design | Study Population | Objective |
|---|---|---|---|---|---|---|
| 2023 | J. Kang et al. [29] | South Korea | COPD | RCT | Adults | To assess the effectiveness of a behavioral intervention in reducing PM2.5 exposure and to improve clinical outcomes in patients with COPD. |
| 2023 | N. N. Hansel et al. [30] | USA | COPD | RCT | Adults | To determine whether placement of active portable HEPA cleaners can improve respiratory morbidity in former smokers. |
| 2023 | A. McCarron et al. [33]. | UK | Asthma | OCS | Adults | To investigate the acute effects of personal exposure to PM2.5 on self-reported asthma-related health. |
| 2023 | I. Kang et al. [35] | USA | Asthma | OCS | Adults | To examine the joint impacts of exposures to residential indoor and outdoor air pollutants and housing risk factors on asthma-related health outcomes. |
| 2022 | G. P. Bălă et al. [28] | Romania | COPD | OCS | Adults | To assess the degree of microparticulate pollution in the houses of COPD patients and to determine whether the values recorded correlate to COPD exacerbations. |
| 2022 | B. J. John et al. [22] | Germany | COPD | OCS | Adults | To identify the affected surroundings to take early steps to reduce the risk of COPD and other respiratory illnesses by introducing an AQI real-time device. |
| 2022 | U. Rabbani et al. [18] | Pakistan | Asthma | OCS | Adults | To assess the association of formaldehyde, CO, and PM2.5 with respiratory symptoms, asthma, and post-bronchodilator reversibility. |
| 2022 | J. Woo et al. [37] | South Korea | Asthma | RCT | Pediatric | To predict PEFR with a deep learning algorithm through RNNs and DNNs trained using 4 months of linked data of IAQ and PEFR. |
| 2022 | L. Nurhussien et al. [32] | USA | COPD | NRCT | Adults | To study how daily pollutant exposures impact lung function in COPD, considering the influence of eosinophil levels as indicators of airway inflammation and pollution susceptibility. |
| 2021 | H. Kim et al. [11] | South Korea | COPD | OCS | Adults | To assess if indoor particulate matter concentration is affected by a life behavior pattern, and if it can be reduced with appropriate lifestyle modification. |
| 2021 | C.T. Wu et al. [21] | Taiwan | COPD | OCS | Adults | To develop a prediction system using lifestyle data, environmental factors, and patient symptoms for the early detection of acute exacerbations of COPD in the upcoming 7 days. |
| 2021 | W.D. Bae et al. [17] | South Korea | Asthma | OCS | Adults | To compare ten machine-learning techniques, using imbalanced sampling to improve nine of them in predicting how IAQ affects patients’ PEFR. |
| 2021 | C.I. Prasasti et al. [34] | Indonesia | Asthma | OCS | Pediatric | To study how VOCs, PM2.5, and household environmental exposure relate to respiratory allergic symptoms in children in Surabaya, Indonesia. |
| 2021 | A.Y. Lim et al. [42] | South Korea | Asthma | OCS | Adults + Pediatric | To evaluate the IAQ level and daily health symptoms of adults and children living in EEHs compared to conventional buildings over one year. |
| 2020 | SE. Guo et al. [31] | Taiwan | COPD | NRCT | Adults | The study explored how educating patients with COPD about particulate matter (PM) affects their knowledge of air pollution prevention, self-care, symptom management, and indoor PM levels. |
| 2020 | E.N. Schachter et al. [36] | USA | Asthma | OCS | Pediatric | To study how indoor pollutants and their seasonal changes affect asthma exacerbations in children with moderate to severe asthma. |
| 2020 | R. J. Khusial et al. [38] | Netherlands, UK, Greece, and Germany | Asthma | RCT + OCS | Adults | To assess the clinical effectiveness and technology acceptance of myAirCoach-supported self-management on top of usual care in patients with asthma using inhalation medication. |
| 2020 | G. H. Lee et al. [39] | South Korea | Asthma | RCT | Pediatric | To study air purifiers’ impact on indoor air pollutant levels and asthma control in children, using HEPA filters. |
| 2020 | S. Kim et al. [40] | South Korea | Asthma | RCT | Pediatric | To evaluate the effects of indoor PM2.5 on children’s PEFR, with a daily intervention of air purifiers with filter on, compared with groups with filter off. |
| 2019 | R. Chi et al. [12] | China | COPD | OCS | Adults | To compare the effects of outdoor- and indoor-originated PM2.5 on cardiopulmonary function in COPD patients and healthy elderly adults. |
| 2019 | S. Hussain et al. [41] | UK | Asthma | OCS | Adults | To assess if overnight particulate matter exposure affects daily symptoms, lung function, and inflammation in asthmatic patients sensitive to dust mites. |
3.2.2. Subjects, Study Sample and Follow-Up Months
Table 2 provides a more in-depth analysis of the obtained data, focusing on the collected medical information and the experimental duration of the trial. Additionally, the key findings from each study were highlighted. Along with the data from Table 1, the analysis further explores the selected studies, emphasizing the patient population under investigation, as well as sample size and study duration.
Regarding the types of patients studied, a total of six asthma studies [17,18,33,35,38,41] and 100% of studies on COPD [11,12,21,22,28,29,30,31,32] focused on an adult population. Five asthma studies targeted a pediatric population [34,36,37,39,40]. In addition, one asthma study included a mixed approach with both adult and pediatric populations [42]. The sample size of the studies reveals that as the sample size decreases, the number of asthma studies increases. In contrast, studies in COPD tended to have a larger sample size compared to asthma studies.
The duration of the studies varied, with an average length of 5.9 ± 4.4 months. Most of the studies on asthma (67%) [17,18,34,36,37,39,40,41] and COPD (67%) [11,21,22,30,31,32] had a duration of less than 6 months.


Table 2.
Clinical data, duration, study sample, and main results of the included studies.
Table 2.
Clinical data, duration, study sample, and main results of the included studies.
| Ref. | Clinical Data Gathered During the Study | Follow-Up | Study Sample (Subjects) | Results | MMAT Score |
|---|---|---|---|---|---|
| [29] | Questionnaires (SGRQ, CAT), exacerbations | 9 months | 106 | Good adherence to the intervention led to improved CAT scores and lower PM2.5 levels compared to poor adherence in the control group. Checking air quality forecasts regularly reduced CAT scores across all activities. | 100% |
| [30] | Questionnaires (SGRQ, CAT), scales (mMRC, BCSS), exacerbations, functional capacity (6 min walk test distance) | 6 months | 116 | The active filter group showed better SGRQ symptom scores, fewer respiratory symptoms, and lower rates of exacerbations and rescue medication use than the sham group. In the active group, a 30–40% reduction in NO2 and more than 40% in PM2.5 was observed. | 100% |
| [33] | Self-reported asthma symptoms, medication use | 12 months | 28 | PM2.5 levels varied by location. Higher PM2.5 exposure increased asthma symptom likelihood, but it did not affect reliever inhaler use. | 75% |
| [35] | Questionnaires (ACT, HRQoL SF-12), scale (PSS), hospitalizations and emergency department visits | 19 months | 53 | In both cross-sectional and longitudinal analyses, lower ACT scores correlated with higher indoor NO2 levels, indoor temperature, PM1, PM2.5, and PM10. Emergency department visits were linked to poorer asthma control, physical health, and mental health, as well as higher indoor NO2 ratios and temperatures. | 100% |
| [28] | Clinical data extracted from medical records, exacerbations | 8 months | 79 | Average PM1, PM2.5, and PM10 values were lower in the group of infrequently exacerbating patients than in the group of frequently exacerbating patients. | 100% |
| [22] | Questionnaire (RQ), lung function from medical records | 8 days | 196 | High daily PM2.5 levels, especially in smokers, significantly affected COPD patients’ health. Indoor PM2.5 levels were closely correlated with health conditions, emphasizing the impact of particulate exposure. | 50% |
| [18] | Questionnaire (ATS-DLD-78A), spirometry (FVC, FEV1) | 6 months | 1629 | Formaldehyde and CO levels were associated with cough, phlegm, and wheezing. PM2.5 levels were linked to shortness of breath and overall respiratory symptoms and were associated with a lower risk of cough, phlegm, and bronchitis. | 100% |
| [37] | PEFR | 4 months | 26 | The 10 min RNN model accurately predicted PEFR with an RMSE of 42.5 and MAPE of 14.0, showing how indoor PM2.5 levels impacted PEFR over time. | 75% |
| [32] | Questionnaire, spirometry (FVC, FEV1) | 4 months | 30 | Higher personal NO2 exposure was associated with lower FEV1 (11.3 mL) and lower forced vital capacity (18.0 mL). PM2.5 and NO2 exposure at personal and community levels were also linked to lower FEV1 in participants with higher eosinophil levels. | 100% |
| [11] | Questionnaire (inhaler use), exacerbations | 3 months | 104 | Indoor PM2.5 levels were influenced by lifestyle and economic status in COPD patients and were significantly linked to acute COPD exacerbations. | 75% |
| [21] | Questionnaire (CAT), scale (mMRC), physiological data, exacerbations | 4 months | 67 | The AECOPD predictive model achieved 92% accuracy, 94% sensitivity, and 90% specificity for 7-day predictions, with an AUC above 0.9. Results show lifestyle and environmental data are highly correlated with health conditions. | 100% |
| [17] | PEFR | 6 months | 25 | Transfer machine learning with imbalanced sampling effectively predicted changes in PEFR linked to IAQ, including PM2.5 and carbon dioxide concentrations. | 75% |
| [34] | Questionnaire | 3 months | 80 | The periodic monitoring of IAQ was found to be an effective measure to prevent respiratory allergies among children in an indoor environment. | 75% |
| [42] | Questionnaire, scale | 12 months | 25 adults + 25 children | Indoor PM10, PM2.5, CO2, and VOCs concentrations were lower in EEH. Seasonal adjustments showed consistent indoor temperature and humidity levels in EEH. | 75% |
| [31] | Questionnaire (CAT), scale (mMRC) | 6 months | 63 | PM education led to improved knowledge in the first and third months but not by the sixth month. The experimental group had greater gains initially, with better physical health in the first month, and better psychological health in the sixth month. | 50% |
| [36] | Questionnaire, medication use, unscheduled clinic, emergency department visits, hospitalizations, spirometry (FVC, FEV1) | 2 weeks | 36 | NO2 and PM2.5 elements like Ca, Si, Ni, and Cl were linked to worsened asthma symptoms and increased use of rescue medication in inner-city children with moderate to severe asthma. | 100% |
| [38] | Questionnaires (ACQ, m-AQLQ, EQ-5D-5L), exacerbations, PEFR (FEV1), FeNO, inhaler use and technique score. | Study 1: 3–6 months Study 2: 3 months | Study 1: 30 Study 2: 12 | In study 1, the intervention improved asthma control (ACQ difference 0.70) and reduced exacerbations (HR 0.31). Asthma-related quality of life also improved (mini AQLQ difference 0.53) but forced expiratory volume in 1 s did not change. In study 2, asthma control improved by 0.86 compared to baseline. | 100% |
| [39] | Questionnaire (based on TRACK), PEFR, FeNO | 2 months | 30 | Medication use decreased from 6.9 to 7.12 when using air purifiers, suggesting less medication was needed. Bacterial diversity, measured by the Chao 1 index, was lower with the air purifier on compared to off. | 75% |
| [40] | PEFR, FeNO | 1 month, 3 weeks | 26 | The use of in-home air filtration could be considered as an intervention strategy for IAQ control in asthmatic children’s homes. | 100% |
| [12] | Blood pressure, PEFR, FEV1 | 7 months | 43 | During the heating season, COPD patients had a reduction in forced expiratory volume in the first second (FEV1) associated with PM2.5 | 100% |
| [41] | Questionnaires (ACQ, AQLQ), PEFR, FeNO, spirometry | 3 months | 28 | No significant associations were observed between overnight particulate matter exposure and clinical outcomes measured daily or at study visits. | 100% |
3.2.3. Pollutants
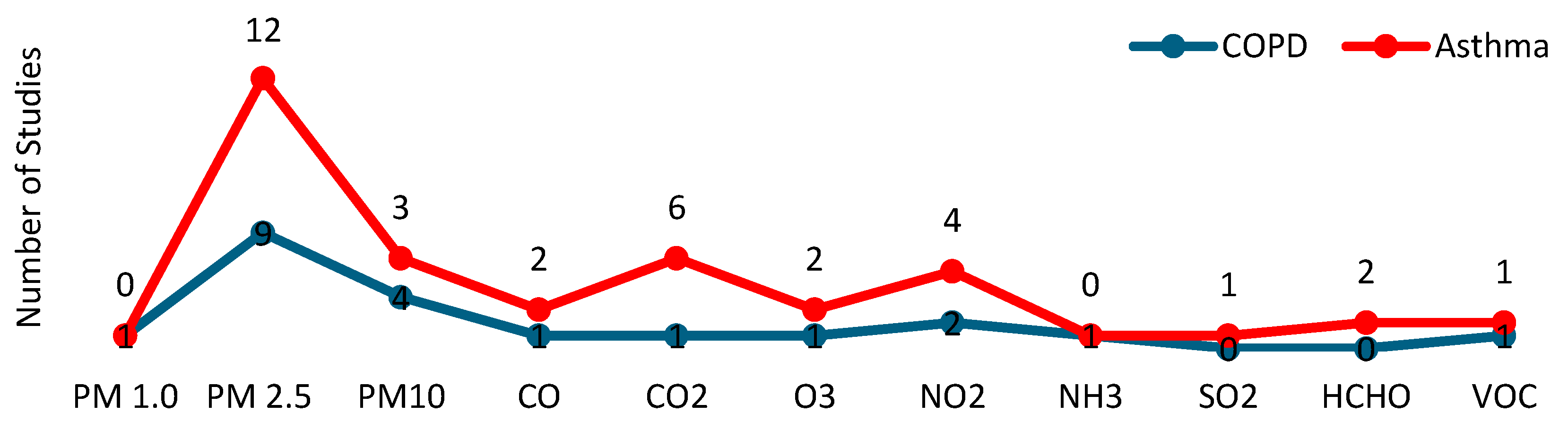
Table 3 presents data on the pollutants researched, sensors technologies and location, calibration, communications technologies, sampling rates, data statistical analyses, and information about whether predictive models and/or machine learning techniques were used. Figure 3 provides a summary of the pollutants and environmental conditions examined across the studies.
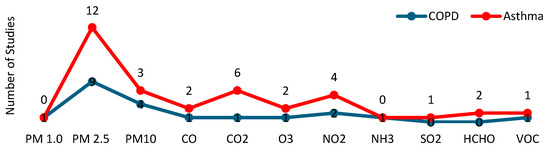
Figure 3.
Pollutants that were studied in the selected articles.
Among particulate matter, PM2.5 was the most studied pollutant, appearing in all analyzed articles, both in asthma (N = 12) [17,18,33,34,35,36,37,38,39,40,41,42] and COPD (N = 9) [11,12,21,22,28,29,30,31,32].
PM10 was studied in four asthma studies [36,38,41,42] and three COPD studies [28,30,31], while PM1 was only analyzed in one COPD study [28].
Regarding the rest of the pollutants, a total of six studies focused on nitrogen dioxide (NO2), four of which related to asthma [18,35,36,38] and two to COPD [30,32]. Carbon dioxide (CO2) was explored in six asthma studies [17,35,37,39,40,42] and in one COPD study [22]. Carbon monoxide (CO) was studied in three studies, two about asthma [18,35], and one about COPD [22]. Ozone (O3) was analyzed in two asthma studies [35,36] and one COPD study [32]. Formaldehyde (HCHO) was investigated in two asthma studies [18,35]. Finally, volatile organic compounds (VOCs) were studied in one asthma [42] and one COPD study [30]. Sulfur dioxide (SO2) was analyzed in one asthma study [38], and the relation of ammonia (NH3) to COPD was explored in a single study [22].


Table 3.
Pollutants, sensors, calibration, communications, sampling, and algorithms of the included studies.
Table 3.
Pollutants, sensors, calibration, communications, sampling, and algorithms of the included studies.
| Ref. | Pollutants | Sensors | Sensor Location | Calibration | Communications | Sampling | Time Series Processing | Predictive Model | Machine Learning |
|---|---|---|---|---|---|---|---|---|---|
| [29] | PM2.5 | Particle Sensor | Participants’ main area of activity | - | IoT | 1 sample/2 min | Mean ± standard deviation | Yes | No |
| [30] | PM2.5, PM10, NO2, airborne nicotine (VOC) | Particle Sensor, Gas Sensor | Participants’ main area of activity | - | Local Storage | 1 week in three periods: at months 0, 3, and 6. | - | No | No |
| [33] | PM2.5 | Air Quality Monitor | Backpack | Median values for calibration | Local Storage | 1 sample/2 min | Min, max, and mean values | Yes | No |
| [35] | HCHO, CO, CO2, NO2, O3, PM2.5 | Particle Sensor | Participants’ main area of activity | Quarterly colocation calibration | IoT | 1 sample/min | Mean ± standard deviation | No | No |
| [28] | PM1, PM2.5, and PM10 | Air Quality Monitor | Participants’ main area of activity | - | Wi-Fi | 1 sample/min | Min, max, and mean values | Yes | No |
| [22] | PM2.5, CO, CO2, NH3 | Particle Sensor, Gas Sensor, Air Quality Monitor | Participants’ main area of activity | - | Wi-Fi | 1 sample/2 min | Mean values | No | ✘ |
| [18] | HCHO, CO, NO2, PM2.5 | Air Quality Monitor, Particle Sensor | Living room, bedroom, and kitchen | - | Local Storage | 1 sample/5 min | Median values | No | ✘ |
| [37] | PM2.5, CO2 | Particle sensor, Gas Sensor | Bedrooms/living rooms | With a reference device | Local Storage | 1 sample/2 min | Mean values | Yes | Yes |
| [32] | PM2.5, NO2, O3 | Particle Sensor, Gas Sensor | Living room, bedroom, and kitchen | With a reference device | Local Storage | 3 samples/min | Mean ± standard deviation | No | No |
| [11] | PM2.5 | Particle Sensor | Center of the participants’ houses | - | IoT | 1 sample/2 min | Mean ± standard deviation | No | No |
| [21] | PM2.5 | Air Quality Monitor | Participants’ main area of activity | - | IoT | 1 sample/15 min | Mean values | Yes | Yes |
| [17] | PM2.5, CO2 | Particle Sensor, Gas Sensor | Outside, front door, living room, bedroom, and kitchen | - | Local Storage | 1 sample/2 min | Mean values | Yes | Yes |
| [34] | PM2.5 | Dust Sensor | Bedroom | Airflow calibrator | Local Storage | - | Min, max, and mean values | Yes | No |
| [42] | PM10, PM2.5, CO2, VOCs | Air Quality Monitor | Living room | - | IoT | 1 sample/5 min | Mean values | Yes | No |
| [31] | PM10, PM2.5, | Dust Sensor | Outside, front door, living room, bedroom, and kitchen | - | Local Storage | 3 samples/min | Mean ± standard deviation | Yes | No |
| [36] | PM10, PM2.5, NO2, SO2, O3 | Dust Sensor, Gas Sensor | Participants’ main area of activity | - | Local Storage | 3 samples/min | Odds ratios | No | No |
| [38] | NO2, SO2, PM2.5, PM10 | Air Quality Monitor | Bedrooms | - | Bluetooth | 1 sample/2 min | Mean ± standard deviation and median values | No | No |
| [39] | PM2.5, CO2 | Particle Sensor | Living room | - | IoT | 1 sample/2 min | Mean ± standard deviation | No | No |
| [40] | PM2.5, CO2 | Air Quality Monitor, Particle Sensor | Living room | Laboratory test | IoT | 1 sample/2 min | Median values | No | No |
| [12] | PM2.5 | Dust Sensor | Participants’ main area of activity | - | Local Storage | 3 samples/min | Mean ± standard deviation and median values | No | No |
| [41] | PM10, PM2.5 | Air Quality Monitor | Bedroom | - | Wireless | 1 sample/min | Mean values | No | No |
3.3. Quality Assessment
Of all the studies evaluating the impact of IAQ on asthma and COPD, thirteen studies achieved an MMAT score of 100%, seven scored 75%, and two scored 50% (Table 2). Quality limitations in the cohort observational studies included issues such as insufficient details regarding the selection bias of participants [11,18,22], unclear origins of measurement data [17,33,34], unknown validity of measurement tools [22], or the use of non-standardized instruments [31]. Regarding randomized controlled trials, quality variability was noted in cases where there was no clear description of randomization procedures, lack of detailed reporting on blinding methods [39], incomplete outcome data [37], or a high dropout rate.
3.4. Clinical Data
In most of the selected studies, air quality data was supplemented with symptoms or physiological information collected from patients. Symptoms collected through questionnaires were used for asthma [18,33,34,35,36,38,39,41,42] and COPD [11,21,22,29,30,31,32].
In COPD, basic vital signs, such as blood pressure or heart rate [12,21], lung function data [12,22,32], medication adherence [11,12], calories and daily steps [21], and 6 min walk test distance [30] were monitored. In asthma, lung function was measured in [17,18,36,37,38,39,40,41] and medication use was tracked in [33,36,38]. Fractional Exhaled Nitric Oxide (FeNO) was also reported in some asthma studies [38,39,40,41].
Finally, exacerbations were tracked and accounted for both asthma [35,36,38] and COPD [11,21,28,29,30].
3.5. Air Quality Sensors
The sensors used in the various selected studies vary according to the pollutants analyzed, with the following types being the most prominent: IAQ monitors, particle sensors and gas sensors. IAQ monitors collect a range of data from a single point, detecting particulate matter, gases, temperature, and humidity, among other parameters.
A single dust sensor was used in several studies (one study on asthma [34] and four on COPD [11,12,29,31]). Also, a single IAQ monitor was used in some studies (four on asthma [33,38,41,42] and two on COPD [21,28]). One asthma study made use of a particle sensor [39]. The use of multiple sensors was more common, with six studies on asthma [17,18,35,36,37,40] and three on COPD [22,30,32].
The air quality data recorded by the sensors were either stored locally or transmitted from the patient’s home to a central repository. It should be noted that most of the studies did not include details on the protocol for data communication that was used. Data were locally stored in eleven studies (six on asthma [17,18,33,34,36,37] and four on COPD [12,30,31,32]). In general, Wi-Fi and the Internet of Things (IoT) were the most used protocols in the rest of the studies. IoT was used in four asthma [35,39,40,42] and three COPD studies [11,21,29]. Wi-Fi was used in two studies [22,28] and Bluetooth in only one study on asthma [38].
3.6. Sensors Calibration
Sensor calibration is often a critical step before device deployment. Despite its relevance, the calibration procedure was not reported in 15 out of the 21 reviewed articles (71%). Eleven percent of COPD studies [32] and seventeen percent of asthma-related articles [34,37] utilized a reference device as a calibration standard. Other calibration approaches were only described in asthma studies, with 8% of articles [33] using mean values, 8% quarterly collocation [35], and 8% conducting a laboratory test for calibration purposes [40].
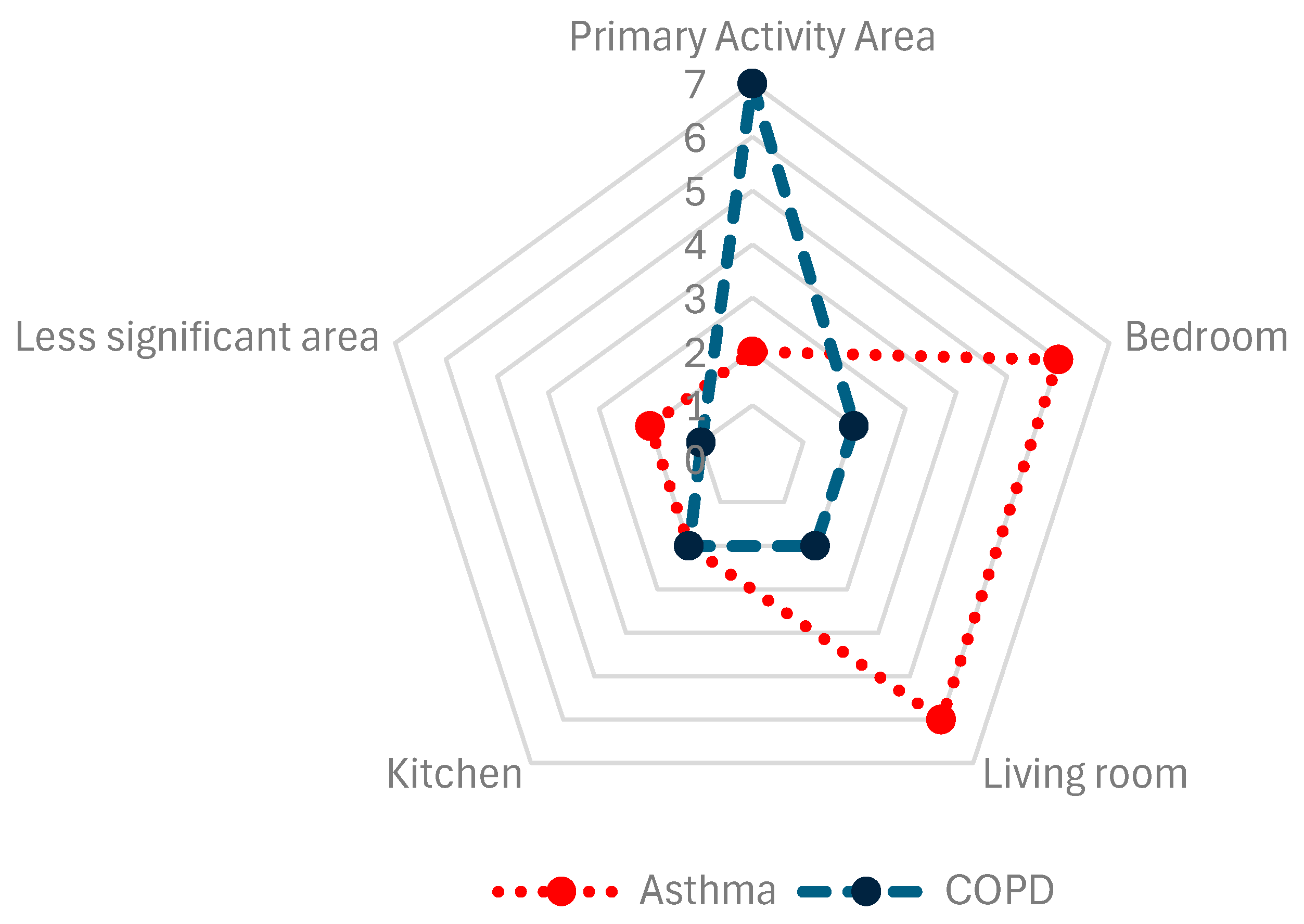
A relevant aspect to consider is the placement of sensors in the different areas of the patient’s home (Figure 4). In 17% of asthma articles [35,36] and 78% of COPD articles [11,12,21,22,28,29,30], sensors were placed in the patient’s center or primary activity area, although the criteria for determining this area were not detailed. Sensors were placed in the bedrooms in 50% of asthma articles [17,18,34,37,38,41] and 22% of COPD articles [31,32]. Living rooms were selected in 50% of asthma articles [17,18,37,39,40,42] and 22% of COPD articles [31,32]. To a lesser extent, sensors were placed in kitchens in 17% of asthma articles [17,18] and 22% of COPD articles [31,32]. Other locations for sensors accounted for 17% of asthma [17,33] and 11% of COPD articles [31].
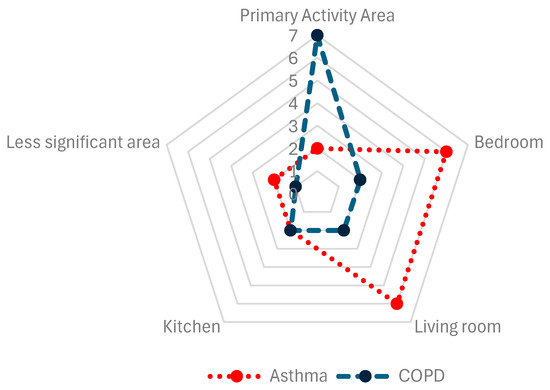
Figure 4.
Sensor placement, compiled from the selected studies.
3.7. Data Processing
The data processing and analysis of the information collected through sensors was conducted heterogeneously. When reported, sampling was performed at intervals equal to or greater than one minute in 83% of asthma articles [17,18,33,35,37,38,39,40,41,42] and in 44% of COPD articles [11,21,22,28]. In asthma studies, the sampling rate was greater than one sample per minute in one (8%) study [36]. In COPD, this happened in three studies (33%) [12,31,32]. The sampling rate was unreported in one asthma [34] and one COPD study [30].
Data samples were processed differently. Mainly, the mean and/or standard deviation were calculated in 75% of asthma articles [17,33,34,35,37,38,39,41,42] and in 89% of COPD studies [11,12,21,22,28,29,31,32]. Following this, median values were used in 25% of asthma studies [18,38,40] and in 11% of COPD articles [12]. Odds ratios were reported to be used in 8% of asthma articles [36], while minimum and maximum values were estimated in 16% of asthma articles [33,34] and in 11% of COPD articles [28].
3.8. Predictive Models and Machine Learning
In the scoped studies, 16% of asthma articles [17,37] and 10% of COPD articles [21] developed predictive models using machine learning. Over 25% of asthma articles [33,34,42] and 33% of COPD articles [28,29,31] implemented predictive models using traditional statistics. Seven (58%) of asthma articles [18,35,36,38,39,40,41] and five (55%) of COPD articles [11,12,22,30,32] did not include a predictive model.
3.9. Disease Progression and Pollutants
3.9.1. Particulate Matter
In the context of air pollutants, particulate matter is present in various forms depending on its size. These contaminants can significantly affect patients with respiratory diseases, potentially exacerbating their conditions if air quality is poor.
Of the nine studies aimed at exploring indoor particulate matter and its relation to COPD, only one (10%) explored PM1, one (10%) explored PM10, and nine (100%) analyzed PM2.5. In the case of asthma, one article (10%) addressed the influence of PM1, three (30%) evaluated PM10, and twelve (92%) analyzed PM2.5.
3.9.2. Gaseous Pollutants
CO2 and NO2 were the most studied gases. The effects of CO2 were evaluated in 50% of asthma studies [17,35,37,39,40,42] and 11% of COPD studies [22]. Concerning NO2, it was explored in 33% of the studies on asthma [18,35,36,38] and 22% of studies on COPD [30,32]. The impact of CO on asthma was assessed in 17% of articles [18,35] while its impact on COPD was evaluated in 11% of the reviewed studies [22].
HCHO was only considered in two studies, both on asthma [18,35]. NH3 was evaluated in the context of COPD in only one article [22]. Finally, the association of SO2 with respiratory pathologies was explored only on asthma, in two studies [36,38]. The effects of O3 on asthma progression were evaluated in 16% of the studies [35,36], while its relationship to COPD was studied in 11% of the selected articles [32]. Finally, the link between VOCs and disease progression was studied in two studies, one on asthma [42] and one on COPD [30].
4. Discussion
Worldwide, indoor air pollution contributes to over four million annual deaths [43]. This preventable exposure has significant respiratory health effects. To the best of our knowledge no prior research has explored the application of indoor monitoring technologies, sensors, predictive models, and AI in analyzing COPD and asthma progression.
We conducted a systematic review to describe the evolution of and current research on the potential effects of IAQ on COPD and asthma progression. Specifically, we examined studies that collected and analyzed air quality data, biomedical parameters, and clinical information, to identify links between certain pollutants and the triggering of acute respiratory exacerbations.
To this purpose, we conducted a systematic search and article collection using the PRISMA methodology. This search resulted in 21 studies that met our inclusion criteria, providing a solid evidence base for this analysis.
4.1. IAQ, Asthma, and COPD
The relationship between IAQ and respiratory diseases such as asthma and COPD has been the subject of increasing scientific interest in recent decades. While earlier studies focused primarily on outdoor air pollution, it is now recognized that IAQ significantly impacts respiratory health. Factors such as tobacco smoke, cleaning products, VOCs, dust mites, and fine particulate matter have been identified as key elements that can exacerbate asthma and COPD in vulnerable individuals.
Advances in indoor pollutant measurement and a deeper understanding of the biological mechanisms behind these diseases have driven this field’s evolution. Notably, studies on the association between IAQ and asthma and COPD progression have increased since 2019, with a particularly pronounced rise in asthma research.
In asthma, researchers have shown that exposure to certain allergens and pollutants in indoor air can trigger attacks [43,44]. Recent studies have examined how humidity, mold, and chemicals in the home affect the severity of asthma symptoms. It has been observed that children and adults living in environments with poor IAQ are more likely to develop asthma or suffer exacerbations of the disease [18,33,34,35,36,38,39,40,42]. The development of predictive systems that consider IAQ as an input for the early detection of acute episodes has been explored recently [17,33,34,37,42]. This strategy has achieved promising results in the early detection of increased asthma symptoms [33], and in predicting changes in peak expiratory flow rate using artificial intelligence techniques [37].
Also, COPD is affected by IAQ, with a high correlation found between IAQ and COPD acute exacerbations [21,28]. Traditionally, tobacco smoke has been recognized as the main risk factor, but recent research has highlighted the importance of other indoor pollutants, such as the biofuels used for cooking in rural areas or the dust generated in industrial settings [45,46]. Household ultrafine particles have been associated with a greater risk to cardiovascular health [47] and with a decline in cardiopulmonary function in COPD [12]. As with asthma, research efforts in recent years have focused on conducting behavioral interventions to improve COPD clinical outcomes [11,29,31], including the development of predictive models [11,21,29].
4.2. Sensors for IAQ Monitoring
Asthma studies measured significantly fewer indoor air quality (IAQ) parameters, and consequently used fewer sensors, compared to COPD studies. This difference may stem from the distinct disease trajectories. COPD patients often experience gradual and subtle clinical changes, necessitating a wider range of sensors to detect subtle deteriorations. In contrast, asthma exacerbations are typically more abrupt, and patients are often more aware of their triggers, enabling quicker and more direct responses, thus reducing the need for extensive sensor arrays.
Overall, there is a trend towards simplifying device configurations, favoring integrated multi-sensor units with data transmission capabilities [18,28,37,40,41,42]. The combination of air quality monitors, which integrated multiparameter measurements, and dust and gas sensors were the most frequent approach both in asthma and COPD.
Calibration is a prevalent technique employed to minimize measurement errors. Prior to deployment, sensor performance is evaluated against a reference standard, and a calibration model is developed by identifying and addressing potential error sources. While sensor calibration is fundamental to the accurate assessment of IAQ, a substantial proportion of the reviewed studies (over 70%) failed to provide details regarding calibration procedures. This lack of transparency undermines the reliability of reported pollutant concentrations and hinders the ability to conduct meaningful cross-study comparisons. Consequently, future research efforts must prioritize the implementation and transparent reporting of comprehensive calibration protocols, which should include rigorous pre-deployment validation and a schedule for periodic recalibration to ensure sustained data accuracy.
Furthermore, post-deployment calibration, where the calibration model is periodically evaluated to ensure accuracy, was not detailed in any study. The selection of low-cost air pollutants sensors and the lack of detailed calibration processes were identified as significant gaps, posing a challenging task in this context [48,49,50].
A limited adoption of the IoT in IAQ monitoring was detected, despite its numerous advantages. While only 33% of reviewed studies utilize IoT for real-time IAQ monitoring, technology offers significant benefits, including continuous data collection, low power consumption, scalability, remote accessibility, cloud integration, and cost-effectiveness. IoT is crucial in building next-generation systems [51] and IoT platforms have the potential to leverage artificial intelligence and machine learning for predictive modeling, early warning systems, and optimize air quality management [52], key for managing CRD. With 50% of studies relying on local data storage, remote and centralized monitoring strategies are also compromised. Therefore, promoting the integration of IoT protocols in future IAQ monitoring studies is vital to overcome these limitations and harness advanced technologies for improved CRD care, aligning with the need for personalized early warning systems and recommendations.
4.3. Disease Progression and Pollutants
4.3.1. Particulate Matter
Airborne particles are a form of air pollution that can have a significant impact on human health, particularly due to particulate matter (PM) [53]. PM was a key in IAQ studies due to its various household sources, including heaters, cooking, cleaning products, personal hygiene products, and tobacco.
PM1, ultrafine particles smaller than 1 micron, are generated through combustion, cooking, infiltration of outdoor air pollution, household products, HVAC systems, electronic devices, building materials, mold, and resuspension of settled dust caused by movement or cleaning activities. Only one reviewed study [28] has explored the correlation between PM1 and COPD exacerbations, reporting higher PM1 levels in homes with frequent exacerbations.
Particulate matter with a diameter of 2.5 microns or smaller (PM2.5) shares similar sources with PM1. This pollutant was explored in all reviewed studies, both in asthma and COPD, and was linked to respiratory symptoms, including shortness of breath and decreased Forced Expiratory Volume in one second (FEV1) [12,18,32] as well as to higher eosinophil levels [32]. COPD, a lifestyle that involves living in an environment with varying levels of PM2.5, has been associated with a significant impact on patient health [11,12,30]. Exposure to this particulate matter was found to be lower in patients who experience infrequent COPD exacerbations [2]. Furthermore, a decrease in PM2.5 exposure improved patient health, whether through medical intervention or the use of filters, among other methods [29,30]. Similarly, studies on asthma found that increased PM2.5 concentrations were associated with airway inflammation, exacerbated symptoms, including cough, phlegm, and bronchitis [17,18,33,37], and impacting PEFR [37], as well as deteriorating quality of life [41]. The ability of PM2.5 to penetrate deeply into the lungs and affect the respiratory system has been pointed out as a critical factor in asthma exacerbation [17]. Reducing PM2.5 levels, both during the day and at night, has proven potential to prevent emergency visits due to uncontrolled symptoms [35,36] and to reduce medication use [39]. Daily monitoring of pollutants, coupled with medical interventions, is considered an effective strategy for asthma management. Daily monitoring of pollutants and patient conditions was revealed as an effective preventive measure for better controlling the patient’s asthmatic state [38]. However, research on the association between PM2.5 exposure variability and asthma clinical outcomes has yielded inconsistent results, with some studies finding no significant associations. This disparity may be attributed to factors such as natural PM2.5 variability, study duration, and specific environmental conditions [35].
PM10, particulate matter with a diameter of 10 microns or smaller, is found indoors due to renovation, construction or demolition activities, cleaning activities, dust, and resuspension of airborne particles. In COPD, lower levels of PM10 were associated with fewer exacerbations [28], and better physical and psychological health [31]. In asthma, the effects of PM10 on children with moderate to severe asthma were explored, but asthma severity was not associated with this specific indoor pollutant [36].
4.3.2. Gaseous Pollutants
Several selected articles explored the effects of CO2, NO2, CO, HCHO, NH3, SO2, and O3 on asthma and COPD progression. Indoor CO, often present in homes using fuels such as natural gas for cooking or heating, have been associated with cough, phlegm, and wheezing in asthma patients [18]. However, no direct evidence was found linking CO to COPD in the reviewed studies.
Most of the selected studies focused on analyzing CO2 and its effects on asthma. The reviewed studies showed that no significant effects were found on respiratory symptoms or lung function.
NO2, mainly from gas stoves and heating systems, showed significant associations with asthma symptoms in children. In the reviewed studies, NO2 was linked to worsened asthma symptoms and increased use of rescue medication in inner-city children with moderate to severe asthma [36], and to increased emergency department visits [35]. In COPD, some studies suggest a possible relationship between NO2 exposure and symptoms, lung function, and the risk of exacerbation [30,32].
HCHO can be released indoors by pressed wood products, adhesives, paints, varnishes, and lacquers, new carpets or synthetic textiles, emissions from cleaning products and disinfectants, and degradation of building materials. The impact of HCHO on respiratory health is well documented, and its presence indoors has been associated with asthma and respiratory symptoms [35], phlegm, wheezing, and coughing [18].
The main sources of indoor NH3 are cleaning products, fertilizers, decomposition of organic matter, emissions from pets and humans, and cooking and processed foods. NH3 was monitored in one study on COPD, but it was not linked to any health outcome [22].
Indoor O3 originates from electronic devices, photochemical reactions between indoor pollutants and sunlight, ozone-generating air purifiers, and outdoor air infiltration. Irritating effects of O3 on the respiratory tract can contribute to the exacerbation of asthma conditions [20]. Although ozone forms mainly outdoors, it can penetrate buildings and affect IAQ [54]. In COPD, the studies reviewed did not provide much specific information on the relationship between O3 exposure and disease progression [32]. In asthma, significant associations between poorly controlled asthma and measures of residential O3 were reported in [35].
VOCs are very prevalent inside households and are commonly caused by cleaning products, paints, solvents, air fresheners and aerosols, new furniture, carpets, wood products, adhesives, and glues, and use of pesticides and repellents. VOCs have been linked to increased risk and severity of asthma, rhinitis, eczema, and airway inflammation. Sensitivity to VOCs can vary widely among people, and some individuals may experience symptoms at lower exposure levels than others. However, the reviewed sources offered limited specific information on VOCs and disease progression. Only in asthma, a higher risk of allergic rhinitis in adults was associated with VOCs [42].
4.4. Predictive Models and AI
COPD and asthma pose a large burden on healthcare [3,55]. While these CRD cannot be cured, various treatments and approaches can help manage symptoms, improve patient’s quality of life, and reduce the risk of adverse outcomes, such as exacerbation. Many severe exacerbations, which contribute to significant morbidity, increased healthcare utilization, disability, and mortality are deemed preventable with appropriate outpatient care [56]. A challenging area of research nowadays is based on the development of AI-based models for predicting severe exacerbations of asthma and COPD. Current models conventionally use physiological information gathered from questionnaires and sensors, but they lack the accuracy needed to reduce severe exacerbations and improve outcomes [23,56]. The identification of novel predictors that can enable the early detection of deterioration has been marked as a goal for home telemonitoring to improve clinical accuracy and prevent hospital admissions, improve the quality of life of patients, and reduce health resource utilization [23].
Machine learning has gained ground in the field of air pollution epidemiology [57]. Over the past decade, research has focused on the impact of outdoor air pollution on respiratory diseases, demonstrating the significant role of outdoor environmental factors in exacerbation prediction [58,59]. Nevertheless, patients may spend much time indoors and consequently can be affected by indoor air pollution levels [59,60,61].
In this context, machine learning models could use IAQ data to better predict asthma and COPD exacerbations, enabling early interventions and personalized management strategies. However, despite its potential to find interactions between pollutant exposition and disease progression and its potential clinical utility in COPD and asthma [59,62], the application of AI techniques together with IAQ remains underexplored, as evidenced by the low number of studies that addressed this research area. Such developments require the use of interdisciplinary and rigorous methodological approaches, including connecting air quality measurements with health markers during the study period (e.g., through validated health questionnaires, measures of lung function, physiological information gathered using sensors, traceability of exacerbations, medication use and consumption of healthcare resources). Specifically, disease exacerbation episodes were only recorded in 25% of the asthma studies and 50% of the COPD studies. These episodes mark a decline in patient health and account for much of the economic burden on health services [63,64], and their follow-up enables the development of supervised machine learning algorithms [56,65].
4.5. Methodological Considerations
Randomized Controlled Trials (RCTs) are the gold standard in clinical research due to their ability to minimize bias, ensure comparability between groups, and establish causal relationships. However, only 29% conducted randomized controlled studies.
Collecting longitudinal data on IAQ, environmental factors, and health outcomes can be costly and time-consuming, especially for large-scale studies. However, longitudinal studies covering longer periods are needed to assess long-term exposure to different indoor air pollutants and their impact on specific populations, such as children, the elderly, and people with pre-existing respiratory conditions. However, 71% of the studies had a duration of less than 6 months. Most studies were based on point data from surveys or monthly/quarterly measurements of indoor air pollutants, rather than continuous monitoring. A six-month air quality study may miss seasonal variations, limiting its ability to capture long-term trends [66]. Studies of short duration can be influenced by temporary events potentially skewing results. Additionally, geographic and temporal representativeness may be restricted, affecting the study’s overall reliability. As a consequence, longitudinal studies of more than one year are needed to better understand the risks of chronic exposure and disease progression.
The distribution of sample sizes within the reviewed literature revealed that 38% of studies (8 out of 21) included 30 or fewer participants. Such limited sample sizes inherently restrict statistical power, potentially compromising the robustness of observed effects and limiting the generalizability of findings. To address these limitations, future research should prioritize larger, multi-center studies to improve the representativeness of study populations and reduce inter-study variability. Regarding the inclusion of spatial variability in the analyses, significant spatial differences in reported health symptoms have been identified in different geographic areas [67]. Geographic variability needs to be considered in future research to understand how regional characteristics, ventilation patterns, and pollution sources influence IAQ and health outcomes [66]. Differences in lifestyle habits, building materials, and sources of pollution can affect the external validity of the results. In addition, the heterogeneity in demographic and health characteristics of participants can limit the generalizability of the findings. Variations in disease severity, age, gender, and other individual factors can influence the response to indoor air pollution. Additionally, the lack of representation of certain socioeconomic or ethnic groups can increase the bias of the results, preventing a comprehensive assessment of the impact of indoor air on the health of all patients.
Finally, from a lower-level technical perspective, the frequency with which air quality data are collected can be a significant limitation. In studies with sporadic measurements, the data may not capture important variations in air quality over time. Pollution peaks and daily or seasonal fluctuations can be overlooked if data are collected only at wide intervals, affecting the accuracy of the assessment of respiratory health impacts.
As a result, there is a clear and compelling need to address the lack of standardization in IAQ monitoring in studies involving patients with CRD. Heterogeneity in measurement protocols, sensor calibration, data communication, sampling rates, and analysis methods makes comparison between studies difficult and limits the ability to draw robust conclusions. To overcome these limitations, a more uniform and rigorous framework for future research is needed, ranging from the definition of unified measurement guidelines (e.g., reaching a consensus on which IAQ parameters should be considered) to the adoption of standardized calibration protocols and the promotion of data interoperability through common communication protocols. In addition, the geographic and socioeconomic variability of the populations under study must be considered.
4.6. Opportunities and Challenges
The applications of air quality sensor-based monitoring technologies to the management of respiratory diseases such as COPD and asthma bring research gaps that must be considered for the further development of this field. The reviewed studies revealed several issues, challenges, opportunities, and limitations that will be discussed in this section.
Several key insights emerged from this review that should be considered in future studies, including the following:
- (a)
- the lack of methodological standardization in studies.
- (b)
- the need for long-term longitudinal trials that consider the spatial variability, individualized measures of exposure to pollutants, and the heterogeneity in demographic and health characteristics of patients.
- (c)
- the key to connecting monitored air quality measurements with respiratory health markers.
- (d)
- the lack of personalized early warning and recommendations systems that combine environmental monitoring with artificial intelligence techniques.
In general, more studies are needed to understand the interaction between exposure to indoor air pollutants and the occurrence of specific respiratory symptoms. It is crucial to identify which pollutants are most associated with specific symptoms so that targeted intervention strategies can be developed. In addition, while the relationship between exposure to pollutants and exacerbation of symptoms has been demonstrated, the exact threshold at which certain pollutants become critical to respiratory health is still unknown. In addition, the study of combinations of pollutants, which are often present in indoor spaces, and their possible synergistic effects, is a challenge yet to be addressed.
The placement of air quality sensors within the home is crucial for obtaining representative data. Sensors placed in specific locations may not accurately reflect conditions in other areas of the home where patients spend time. The lack of standardization in sensor placement can lead to underestimation or overestimation of the levels of pollutants to which patients are exposed. In this regard, monitoring personal exposure, which varies according to microenvironments and individual activities, could have significant implications for improving health outcomes [33]. Portable sensors could provide individualized measures of exposure to pollutants, not only indoors but also outdoors. Currently, advances in sensors and smart devices allow for accurate measurements, but mass deployment of these technologies in homes and work environments remains limited. Developing more accessible and low-cost tools that can be used by the public appears to be a crucial need.
Integrating indoor air pollutant measurements, physiological monitoring devices, patient diaries, and medical records offers a unique opportunity to study exposure-health relationships more accurately. However, this integrated approach was considered only in 33% of the studies.
Finally, a paramount challenge lies in translating scientific knowledge into effective global public policies. Although some countries have adopted regulations to improve IAQ, many regions worldwide, particularly in low- and middle-income countries, face significant limitations in access to healthy housing and IAQ-enhancing technologies.
5. Conclusions
Consistent exposure to certain indoor pollutants has been repeatedly linked to increased symptom severity and exacerbation frequency in vulnerable asthma and COPD patients. This review has examined the impact of air quality on disease management, emphasizing the importance of monitoring and controlling indoor environments to protect respiratory health.
Research on IAQ and its impact on asthma and COPD remains an evolving field with significant open questions and challenges. This systematic review underscores the critical need for larger, multi-center studies and standardized IAQ monitoring protocols in studies involving patients with CRD. The identified research gaps, including the lack of unified measurement guidelines, calibration standards, and data communication protocols, significantly compromise data accuracy and comparability and impede the ability to draw robust conclusions and translate research findings into clinical practice.
Additionally, the relatively short duration of most studies hinders the ability to assess long-term health effects and seasonal pollution variations, while the limited use of technologies such as IoT restricts the potential for real-time monitoring and predictive modeling. Expanding AI applications in IAQ research could improve early detection of health risks and enhance precision medicine. Furthermore, a more comprehensive evaluation of specific sources of indoor pollution (such as emissions from new furniture, home renovations, and allergen exposure) may provide deeper insights into their role in the development of respiratory diseases from early life.
Addressing these limitations and leveraging emerging technological advancements could lead to a more profound understanding of the complex relationship between IAQ and respiratory health. Recent innovations, novel methodologies, and increasing public awareness present promising opportunities to develop effective prevention and management strategies to improve respiratory health. These advances may pave the way for personalized approaches to disease management and support policy and social initiatives aimed at improving the health and well-being of individuals affected by CRD.
Author Contributions
The authors confirm that all listed authors meet the requirements for authorship. P.C.-M.: investigation; methodology; writing—original draft preparation; formal analysis; writing—review and editing; visualization. D.S.-L.: investigation; methodology; formal analysis; writing—review and editing. A.L.-J.: review and editing. D.S.-M.: investigation; methodology; conceptualization; writing—original draft preparation; formal analysis; writing—review and editing; visualization; supervision; project administration; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This contribution has been supported by grant PID2021-126810OB-I00 funded by MICIU/AEI/10.13039/501100011033 and by ERDF/EU.
Institutional Review Board Statement
This manuscript is a review article and does not involve a protocol requiring approval by the relevant institutional review board or ethics committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ACQ | Asthma Control Questionnaire |
| ACT | Asthma Control Test |
| AECOPD | Acute Exacerbation of Chronic Obstructive Pulmonary Disease |
| AQ | Air Quality |
| AQI | Air Quality Index |
| AQLQ | Asthma Quality of Life Questionnaire |
| ATS-DLD-78A | American Thoracic Society Division of Lung Diseases Questionnaire for Detecting Respiratory Symptoms in Welders |
| AUC | Area Under the Receive Operating Characteristic Curve |
| BCSS | Breathlessness, Cough and Sputum Scale |
| CAT | COPD Assessment Test |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRD | Chronic Respiratory Diseases |
| DNN | Deep Neural Networks |
| EEH | Energy-efficient homes |
| FeNO | Fractional Exhaled Nitric Oxide |
| FEV1 | Forced Expiratory Volume during the first second |
| FVC | Forced Vital Capacity |
| HCHO | Formaldehyde |
| HEPA | High-Efficiency Particulate Air |
| HRQoL | Health-Related Quality of Life |
| IAQ | Indoor Air Quality |
| IoT | Internet of Things |
| MAPE | Mean Absolute Percentage Error |
| mMRC | Modified Medical Research Council Dyspnea Scale |
| NH3 | Ammonia |
| NO2 | Nitrogen Dioxide |
| NRCT | Non-randomized Control Trial |
| O3 | Ozone |
| OCS | Observational Cohort Study |
| PEFR | Peak Expiratory Flow Rate |
| PM | Particulate Matter |
| RCT | Randomized Control Trial |
| RH | Relative Humidity |
| RMSE | Root Mean Square Error |
| RNN | Recurrent Neural Networks |
| RQ | Respiratory Questionnaire |
| SGRQ | St. George’s Respiratory Questionnaire |
| SO2 | Sulfur Dioxide |
| T | Temperature |
| VOCs | Volatile Organic Compounds |
References
- World Health Organization. Chronic Respiratory Diseases. Available online: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1 (accessed on 16 July 2024).
- Labaki, W.W.; Han, M.K. Chronic respiratory diseases: A global view. Lancet Respir. Med. 2020, 8, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef] [PubMed]
- Mattila, T.; Vasankari, T.; Herse, F.; Leskelä, R.-L.; Erhola, M.; Avellan-Hietanen, H.; Toppila-Salmi, S.; Haahtela, T. Contrasting healthcare costs of COPD and asthma in elderly. Respir. Med. 2023, 220, 107477. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Khanam, R.; Kabir, E.; Jürges, H. The Healthcare Cost Burden of Asthma in Children: A Longitudinal Population-Based Study. Value Health 2023, 26, 1201–1209. [Google Scholar] [CrossRef]
- Wedzicha, J.A. Impact of Chronic Obstructive Pulmonary Disease Exacerbations on Patients and Payers. Proc. Am. Thorac. Soc. 2006, 3, 218–221. [Google Scholar] [CrossRef]
- Halpin, D.M.; Miravitlles, M.; Metzdorf, N.; Celli, B. Impact and prevention of severe exacerbations of COPD: A review of the evidence. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2891–2908. [Google Scholar] [CrossRef]
- Graham, L.M.; Eid, N. The impact of asthma exacerbations and preventive strategies. Curr. Med. Res. Opin. 2015, 31, 825–835. [Google Scholar] [CrossRef]
- Loftus, P.A.; Wise, S.K. Epidemiology and economic burden of asthma. Int. Forum Allergy Rhinol. 2015, 5, S7–S10. [Google Scholar] [CrossRef]
- Kim, H.; Na, G.; Park, S.; Ra, S.W.; Kang, S.-Y.; Kim, H.-C.; Lee, S.W. The impact of life behavior and environment on particulate matter in chronic obstructive pulmonary disease. Environ. Res. 2021, 198, 111265. [Google Scholar] [CrossRef]
- Chi, R.; Chen, C.; Li, H.; Pan, L.; Zhao, B.; Deng, F.; Guo, X. Different health effects of indoor- and outdoor-originated PM 2.5 on cardiopulmonary function in COPD patients and healthy elderly adults. Indoor Air 2019, 29, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Lu, W. Exposure to nitrogen dioxide and chronic obstructive pulmonary disease (COPD) in adults: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2018, 25, 15133–15145. [Google Scholar] [CrossRef] [PubMed]
- Ghozikali, M.G.; Heibati, B.; Naddafi, K.; Kloog, I.; Conti, G.O.; Polosa, R.; Ferrante, M. Evaluation of Chronic Obstructive Pulmonary Disease (COPD) attributed to atmospheric O3, NO2, and SO2 using Air Q Model (2011–2012 year). Environ. Res. 2016, 144, 99–105. [Google Scholar] [CrossRef]
- Huang, W.; Wu, J.; Lin, X. Ozone Exposure and Asthma Attack in Children. Front. Pediatr. 2022, 10, 830897. [Google Scholar] [CrossRef]
- Agache, I.; Canelo-Aybar, C.; Annesi-Maesano, I.; Cecchi, L.; Rigau, D.; Rodríguez-Tanta, L.Y.; Nieto-Gutierrez, W.; Song, Y.; Cantero-Fortiz, Y.; Roqué, M.; et al. The impact of outdoor pollution and extreme temperatures on asthma-related outcomes: A systematic review for the EAACI guidelines on environmental science for allergic diseases and asthma. Allergy 2024, 79, 1725–1760. [Google Scholar] [CrossRef]
- Bae, W.D.; Kim, S.; Park, C.-S.; Alkobaisi, S.; Lee, J.; Seo, W.; Park, J.S.; Park, S.; Lee, S.; Lee, J.W. Performance improvement of machine learning techniques predicting the association of exacerbation of peak expiratory flow ratio with short term exposure level to indoor air quality using adult asthmatics clustered data. PLoS ONE 2021, 16, e0244233. [Google Scholar] [CrossRef]
- Rabbani, U.F.; Razzaq, S.F.; Irfan, M.F.; Semple, S.; Nafees, A.A.F. Indoor Air Pollution and Respiratory Health in a Metropolitan City of Pakistan. J. Occup. Environ. Med. 2022, 64, 761–765. [Google Scholar] [CrossRef]
- Bhat, G.S.; Shankar, N.; Kim, D.; Song, D.J.; Seo, S.; Panahi, I.M.S.; Tamil, L. Machine Learning-Based Asthma Risk Prediction Using IoT and Smartphone Applications. IEEE Access 2021, 9, 118708–118715. [Google Scholar] [CrossRef]
- Dong, Q.; Li, B.; Downen, R.S.; Tran, N.; Chorvinsky, E.; Pillai, D.K.; Zaghloul, M.E.; Li, Z. A Cloud-Connected NO2 and Ozone Sensor System for Personalized Pediatric Asthma Research and Management. IEEE Sens. J. 2020, 20, 15143–15153. [Google Scholar] [CrossRef]
- Wu, C.-T.; Li, G.-H.; Huang, C.-T.; Cheng, Y.-C.; Chen, C.-H.; Chien, J.-Y.; Kuo, P.-H.; Kuo, L.-C.; Lai, F. Acute Exacerbation of a Chronic Obstructive Pulmonary Disease Prediction System Using Wearable Device Data, Machine Learning, and Deep Learning: Development and Cohort Study. JMIR Mhealth Uhealth 2021, 9, e22591. [Google Scholar] [CrossRef]
- John, B.J.; Harish, C.; Lawrence, C.C.; Krishnakumar, S.; Divakaran, S.; Premkumar, J.; Kanmani, P.G.; Sabarivani, A.; Jagadeesan, A.K. Monitoring indoor air quality using smart integrated gas sensor module (IGSM) for improving health in COPD patients. Environ. Sci. Pollut. Res. Int. 2023, 30, 28889–28902. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Morillo, D.; A Fernandez-Granero, M.; Leon-Jimenez, A. Use of predictive algorithms in-home monitoring of chronic obstructive pulmonary disease and asthma: A systematic review. Chronic Respir. Dis. 2016, 13, 264–283. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme; Peninsula Medical School: Lancaster, UK, 2006. [Google Scholar]
- Egger, M.; Zellweger-Zähner, T.; Schneider, M.; Junker, C.; Lengeler, C.; Antes, G. Language bias in randomised controlled trials published in English and German. Lancet 1997, 350, 326–329. [Google Scholar] [CrossRef]
- Bălă, G.-P.; Timar, B.; Gorun, F.; Motisan, R.; Pescaru, C.; Tudorache, E.; Marc, M.; Manolescu, D.; Citu, C.; Oancea, C. The Impact of Air Pollution on Frequent Exacerbations among COPD Patients: An Observational Study on the Population of Western Romania. J. Clin. Med. 2022, 11, 4352. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.-C.; Jang, Y.; Lee, J.B.; Lee, J.S.; Oh, Y.-M.; Ji, H.W.; Jung, J.Y.; Lee, S.W. Randomised controlled trial of a behavioural intervention to reduce exposure to PM2.5 in patients with COPD. Environ. Int. 2023, 181, 108286. [Google Scholar] [CrossRef]
- Hansel, N.N.; Putcha, N.; Woo, H.; Peng, R.; Diette, G.B.; Fawzy, A.; Wise, R.A.; Romero, K.; Davis, M.F.; Rule, A.M.; et al. Randomized Clinical Trial of Air Cleaners to Improve Indoor Air Quality and Chronic Obstructive Pulmonary Disease Health: Results of the CLEAN AIR Study. Am. J. Respir. Crit. Care Med. 2022, 205, 421–430. [Google Scholar] [CrossRef]
- Guo, S.-E.; Chi, M.-C.; Hwang, S.-L.; Lin, C.-M.; Lin, Y.-C. Effects of Particulate Matter Education on Self-Care Knowledge Regarding Air Pollution, Symptom Changes, and Indoor Air Quality among Patients with Chronic Obstructive Pulmonary Disease. Int. J. Environ. Res. Public Health 2020, 17, 4103. [Google Scholar] [CrossRef]
- Nurhussien, L.; Kang, C.-M.; Koutrakis, P.; Coull, B.A.; Rice, M.B. Air Pollution Exposure and Daily Lung Function in Chronic Obstructive Pulmonary Disease: Effect Modification by Eosinophil Level. Ann. Am. Thorac. Soc. 2022, 19, 728–736. [Google Scholar] [CrossRef]
- McCarron, A.; Semple, S.; Braban, C.F.; Gillespie, C.; Swanson, V.; Price, H.D. Personal exposure to fine particulate matter (PM2.5) and self-reported asthma-related health. Soc. Sci. Med. 2023, 337, 116293. [Google Scholar] [CrossRef] [PubMed]
- Prasasti, C.I.; Haryanto, B.; Latif, M.T. Association of VOCs, PM2.5 and household environmental exposure with children’s respiratory allergies. Air Qual. Atmos. Health 2021, 14, 1279–1287. [Google Scholar] [CrossRef]
- Kang, I.; McCreery, A.; Azimi, P.; Gramigna, A.; Baca, G.; Hayes, W.; Crowder, T.; Scheu, R.; Evens, A.; Stephens, B. Impacts of residential indoor air quality and environmental risk factors on adult asthma-related health outcomes in Chicago, IL. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Schachter, E.N.; Rohr, A.; Habre, R.; Koutrakis, P.; Moshier, E.; Nath, A.; Coull, B.; Grunin, A.; Kattan, M. Indoor air pollution and respiratory health effects in inner city children with moderate to severe asthma. Air Qual. Atmos. Health 2020, 13, 247–257. [Google Scholar] [CrossRef]
- Woo, J.; Lee, J.-H.; Kim, Y.; Rudasingwa, G.; Lim, D.H.; Kim, S. Forecasting the Effects of Real-Time Indoor PM2.5 on Peak Expiratory Flow Rates (PEFR) of Asthmatic Children in Korea: A Deep Learning Approach. IEEE Access 2022, 10, 19391–19400. [Google Scholar] [CrossRef]
- Khusial, R.J.; Honkoop, P.J.; Usmani, O.; Soares, M.; Simpson, A.; Biddiscombe, M.; Meah, S.; Bonini, M.; Lalas, A.; Polychronidou, E.; et al. Effectiveness of myAirCoach: A mHealth Self-Management System in Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 1972–1979.e8. [Google Scholar] [CrossRef]
- Lee, G.H.; Kim, J.H.; Kim, S.; Lee, S.; Lim, D.H. Effects of Indoor Air Purifiers on Children with Asthma. Yonsei Med. J. 2020, 61, 310–316. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Park, S.; Rudasingwa, G.; Lee, S.; Yu, S.; Lim, D.H. Association between Peak Expiratory Flow Rate and Exposure Level to Indoor PM2.5 in Asthmatic Children, Using Data from the Escort Intervention Study. Int. J. Environ. Res. Public Health 2020, 17, 7667. [Google Scholar] [CrossRef]
- Hussain, S.; Parker, S.; Edwards, K.; Finch, J.; Jeanjean, A.; Leigh, R.; Gonem, S. Effects of indoor particulate matter exposure on daily asthma control. Ann. Allergy Asthma Immunol. 2019, 123, 375–380.e3. [Google Scholar] [CrossRef]
- Lim, A.-Y.; Yoon, M.; Kim, E.-H.; Kim, H.-A.; Lee, M.J.; Cheong, H.-K. Effects of mechanical ventilation on indoor air quality and occupant health status in energy-efficient homes: A longitudinal field study. Sci. Total Environ. 2021, 785, 147324. [Google Scholar] [CrossRef]
- Raju, S.; Siddharthan, T.; McCormack, M.C. Indoor Air Pollution and Respiratory Health. Clin. Chest Med. 2020, 41, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Bruce, N.; Perez-Padilla, R.; Albalak, R. Indoor air pollution in developing countries: A major environmental and public health challenge. Bull. World Health Organ. 2000, 78, 1078–1092. [Google Scholar] [PubMed]
- Kurmi, O.P.; Semple, S.; Simkhada, P.; Smith, W.C.S.; Ayres, J.G. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: A systematic review and meta-analysis. Thorax 2010, 65, 221–228. [Google Scholar] [CrossRef]
- Raju, S.; Woo, H.; Koehler, K.; Fawzy, A.; Liu, C.; Putcha, N.; Balasubramanian, A.; Peng, R.D.; Lin, C.T.; Lemoine, C.; et al. Indoor Air Pollution and Impaired Cardiac Autonomic Function in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2023, 207, 721–730. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Villani, M.G.; Aleixandre, M.; Bonavitacola, F. Field calibration of a cluster of low-cost commercially available sensors for air quality monitoring. Part B: NO, CO and CO2. Sens. Actuators B Chem. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Karagulian, F.; Barbiere, M.; Kotsev, A.; Spinelle, L.; Gerboles, M.; Lagler, F.; Redon, N.; Crunaire, S.; Borowiak, A. Review of the Performance of Low-Cost Sensors for Air Quality Monitoring. Atmosphere 2019, 10, 506. [Google Scholar] [CrossRef]
- De Vito, S.; Esposito, E.; Salvato, M.; Popoola, O.; Formisano, F.; Jones, R.; Di Francia, G. Calibrating chemical multisensory devices for real world applications: An in-depth comparison of quantitative machine learning approaches. Sens. Actuators B Chem. 2017, 255, 1191–1210. [Google Scholar] [CrossRef]
- Barot, V.; Kapadia, V. Air Quality Monitoring Systems using IoT: A Review. In Proceedings of the 2020 International Conference on Computational Performance Evaluation (ComPE), Shillong, India, 2–4 July 2020; pp. 226–231. [Google Scholar] [CrossRef]
- Ullo, S.L.; Sinha, G.R. Advances in Smart Environment Monitoring Systems Using IoT and Sensors. Sensors 2020, 20, 3113. [Google Scholar] [CrossRef]
- Kumar, A.; Dhawan, S.; Bhatt, S.; Saxena, A.; Khare, M.; Dubey, S.K.; Mehta, D.S. Ambient particulate matter monitoring using bright field imaging-based sensor. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos. Environ. 2011, 45, 275–288. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.-H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abdelmasseh, M.; et al. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef]
- Zeng, S.; Arjomandi, M.; Tong, Y.; Liao, Z.C.; Luo, G. Developing a Machine Learning Model to Predict Severe Chronic Obstructive Pulmonary Disease Exacerbations: Retrospective Cohort Study. J. Med. Internet Res. 2022, 24, e28953. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, C.; Jabbar, M.S.M.; Zaïane, O.; Osornio-Vargas, A. A systematic review of data mining and machine learning for air pollution epidemiology. BMC Public Health 2017, 17, 907. [Google Scholar] [CrossRef] [PubMed]
- YoussefAgha, A.H.; Jayawardene, W.P.; Lohrmann, D.K.; El Afandi, G.S. Air pollution indicators predict outbreaks of asthma exacerbations among elementary school children: Integration of daily environmental and school health surveillance systems in Pennsylvania. J. Environ. Monit. 2012, 14, 3202–3210. [Google Scholar] [CrossRef]
- Ratcliff, G.E.; Matheny, M.E.; Brown, J.R.; Sullivan, I.; Richmond, B.W.; Paulin, L.M.; Conger, A.K.; Davis, S.E. Integrating Clinical and Air Quality Data to Improve Prediction of COPD Exacerbations. AMIA Annu. Symp. Proc. 2024, 2023, 1209–1217. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10785856 (accessed on 14 February 2025).
- Hansel, N.N.; McCormack, M.C.; Belli, A.J.; Matsui, E.C.; Peng, R.D.; Aloe, C.; Paulin, L.; Williams, D.L.; Diette, G.B.; Breysse, P.N. In-Home Air Pollution Is Linked to Respiratory Morbidity in Former Smokers with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 1085–1090. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Winquist, A.; Kirrane, E.; Klein, M.; Strickland, M.; Darrow, L.A.; Sarnat, S.E.; Gass, K.; Mulholland, J.; Russell, A.; Tolbert, P. Joint Effects of Ambient Air Pollutants on Pediatric Asthma Emergency Department Visits in Atlanta, 1998–2004. Epidemiology 2014, 25, 666–673. [Google Scholar] [CrossRef]
- Perera, P.N.; Armstrong, E.P.; Sherrill, D.L.; Skrepnek, G.H. Acute Exacerbations of COPD in the United States: Inpatient Burden and Predictors of Costs and Mortality. COPD J. Chronic Obstr. Pulm. Dis. 2012, 9, 131–141. [Google Scholar] [CrossRef]
- Rennard, S.I.; Farmer, S.G. Exacerbations and Progression of Disease in Asthma and Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2004, 1, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chen, W.; Jia, X.; Jia, Y.; Liu, C. Machine learning for prediction of asthma exacerbations among asthmatic patients: A systematic review and meta-analysis. BMC Pulm. Med. 2023, 23, 278. [Google Scholar] [CrossRef]
- Xydi, I.; Saharidis, G.; Kalantzis, G.; Pantazopoulos, I.; Gourgoulianis, K.I.; Kotsiou, O.S. Assessing the Impact of Spatial and Temporal Variability in Fine Particulate Matter Pollution on Respiratory Health Outcomes in Asthma and COPD Patients. J. Pers. Med. 2024, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Managi, S. Spatial Variability of the Relationship between Air Pollution and Well-being. Sustain. Cities Soc. 2022, 76, 103447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).