The Role of Surface {010} Facets in Improving the NOx Depolluting Activity of TiO2 and Its Application on Building Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Photocatalytic and Depolluting Performance
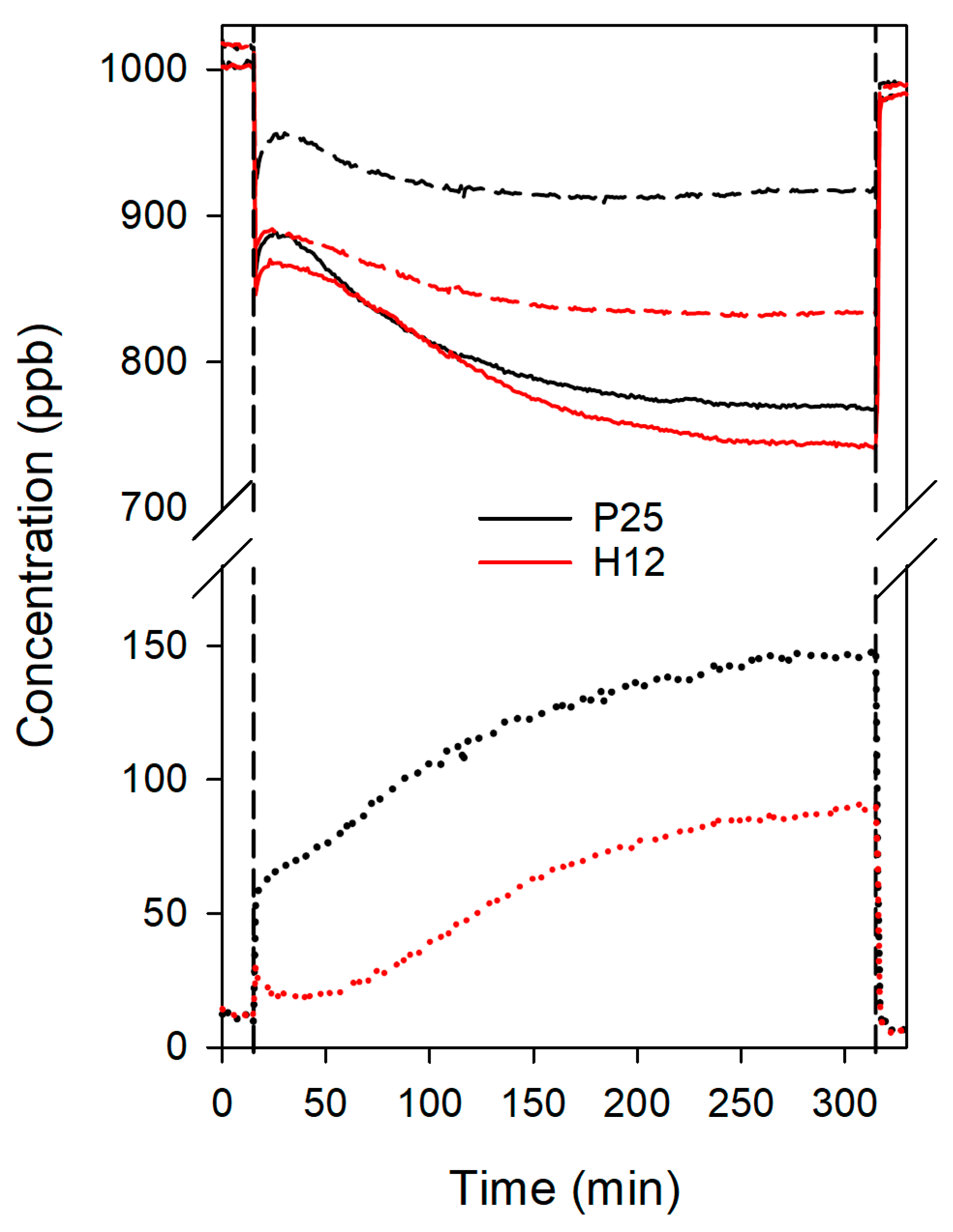
3. Results and Discussion
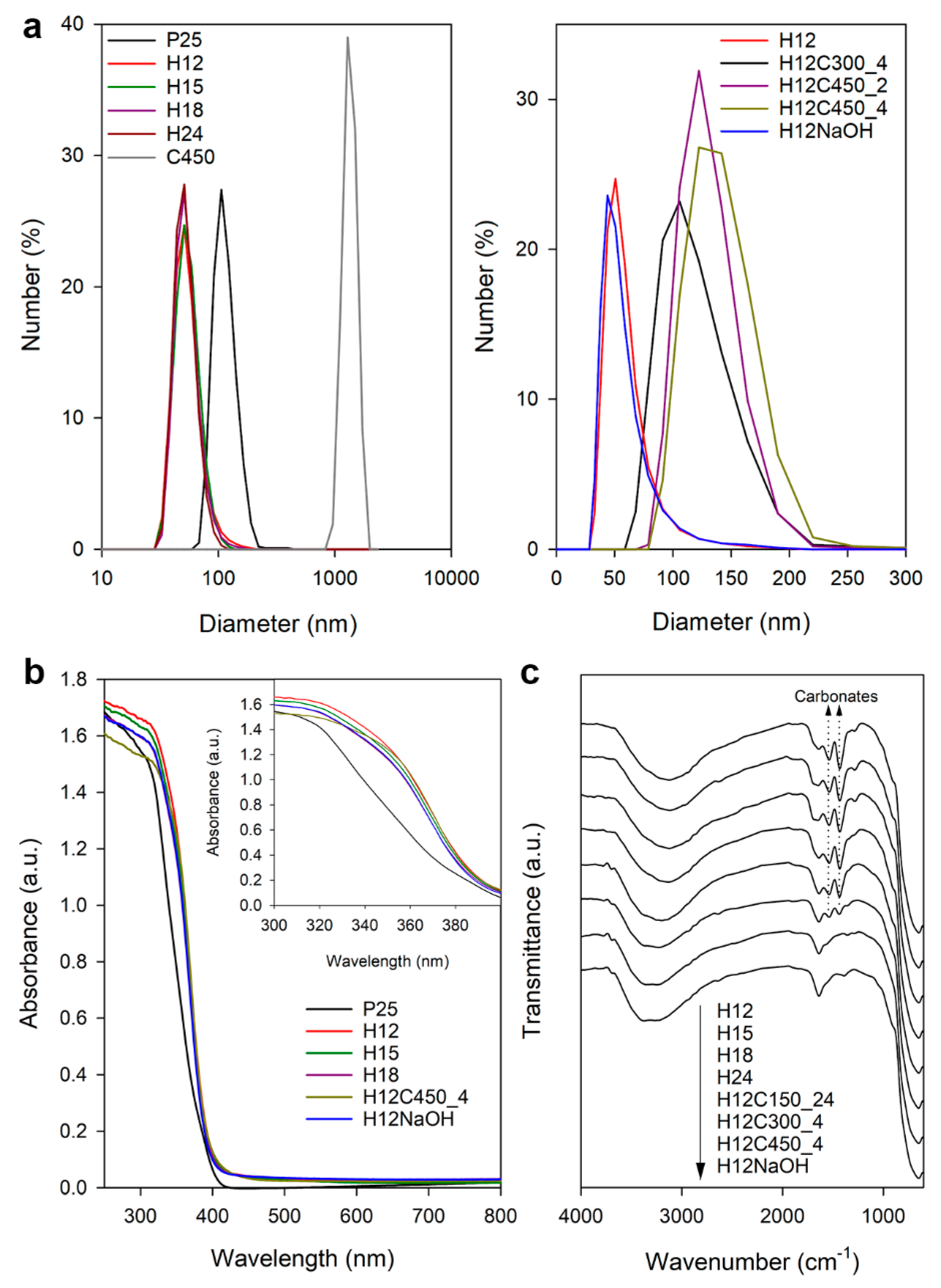
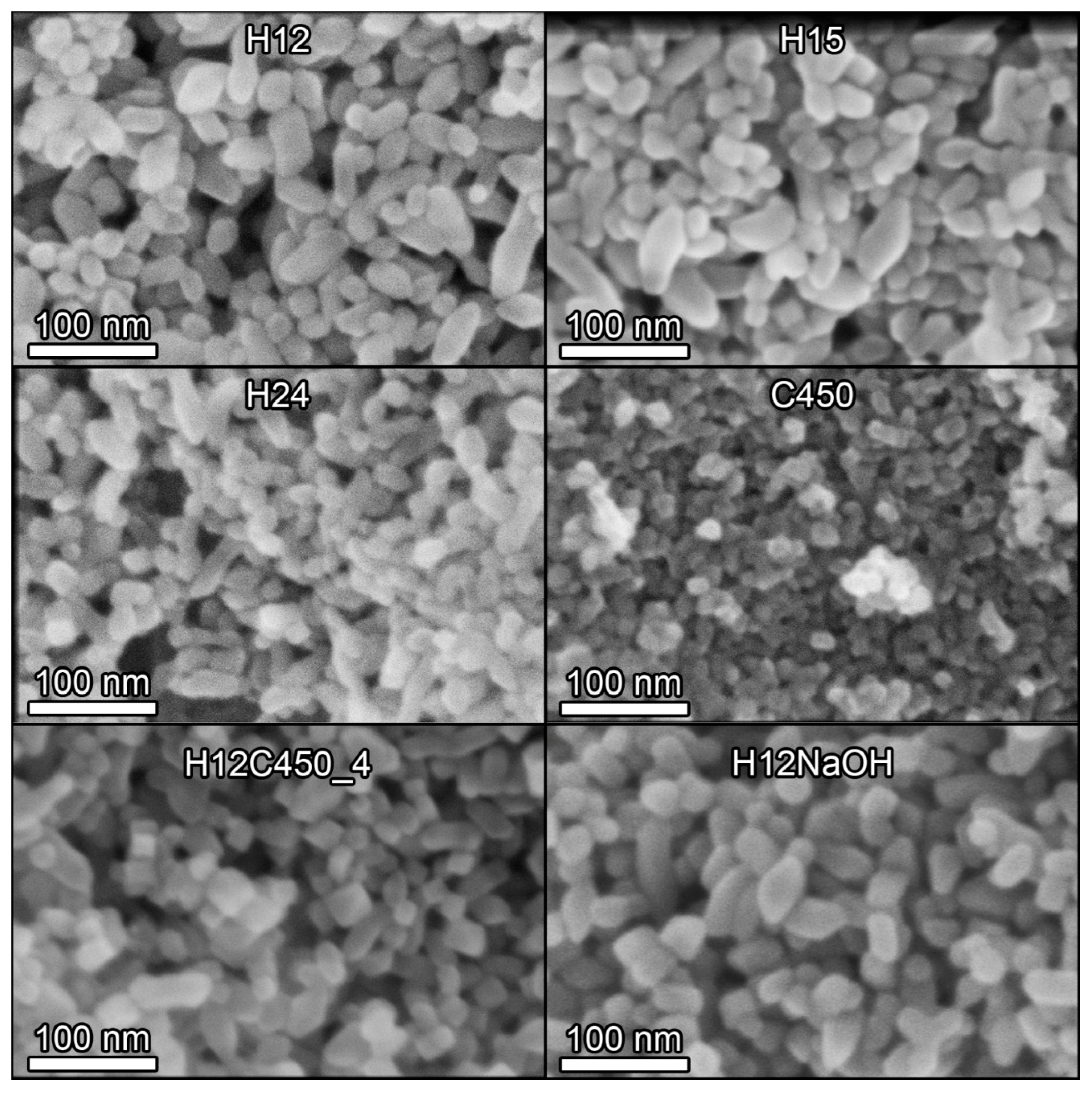
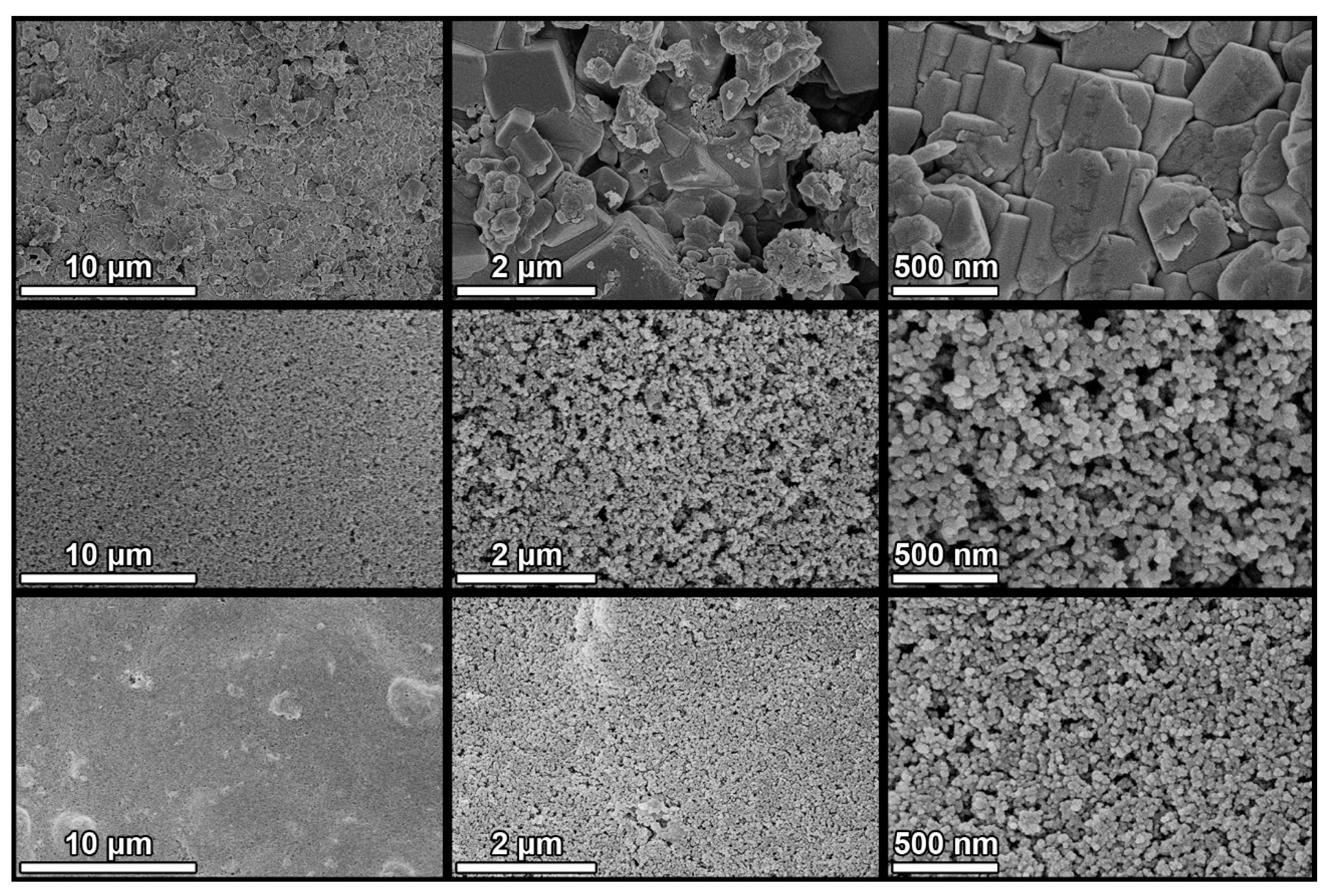
3.1. Particle Aggregation
3.2. UV-Vis and FTIR Spectroscopy
3.3. Textural and Structural Characterization
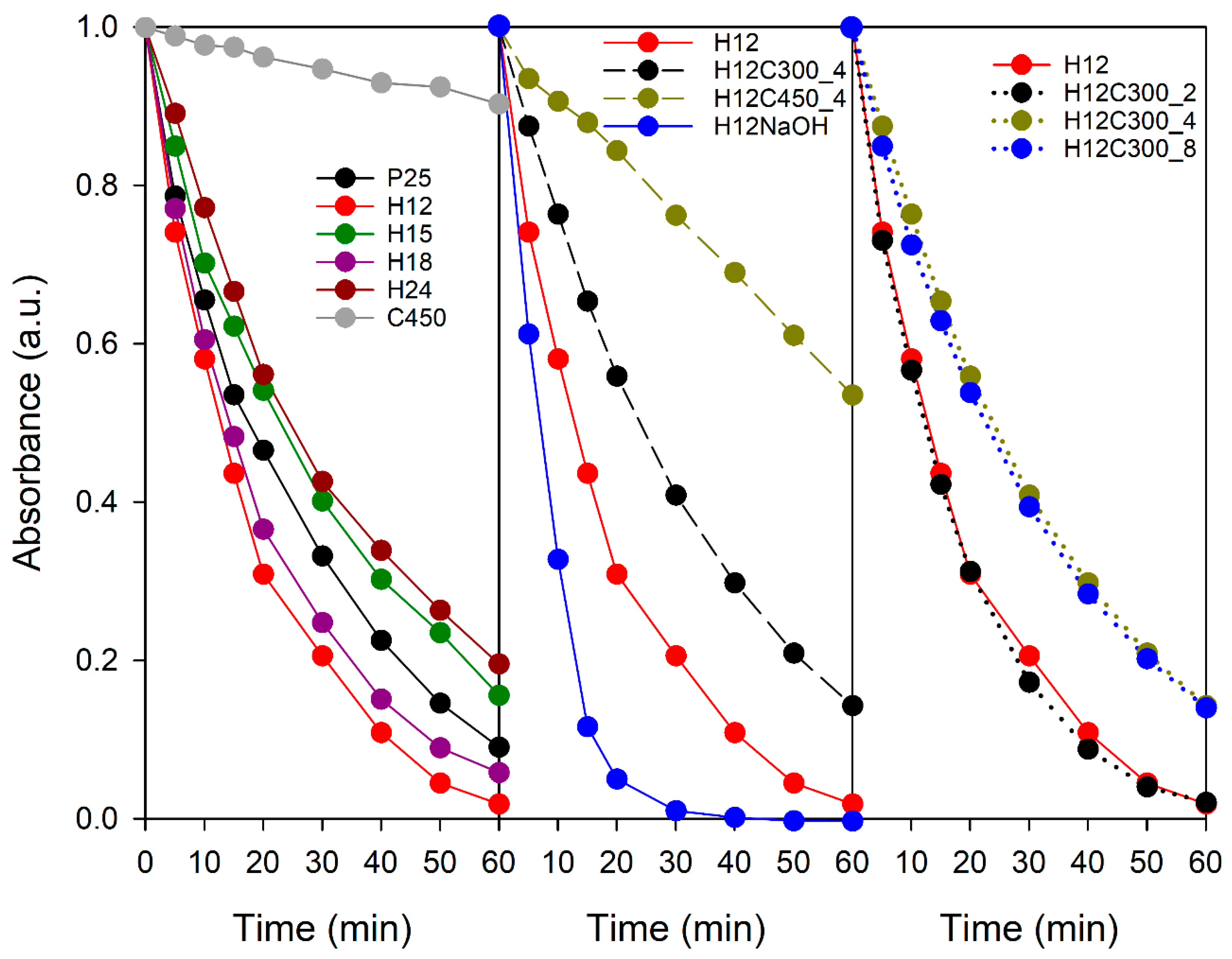
3.4. Photocatalytic and Depolluting Performance
- High specific surface area: A small particle size provides a large surface area, crucial for high photocatalytic activity. Hydrothermal titania’s surface area slightly exceeds that of P25.
- Reduced aggregation: Smaller aggregates favour TiO2-MB contact and light absorption.
- Improved light penetration: Greater transparency of hydrothermal TiO2 dispersions allows deeper light penetration in the volume of the MB solution.
- Phase composition: Hydrothermal titania only presents anatase, a more active phase than rutile, which is also present in P25. However, this combination of phases also produces a positive effect, creating a heterojunction that reduces electron–hole pair recombination. So, it is not clear which situation is more favourable.
- Particle geometry: Elongated particles in hydrothermal titania contain more active facets than those in P25. Large particles formed by oriented attachment can also enhance photocatalytic activity [65].
3.5. Application of Hydrothermal Titania for Producing Functional Building Materials with Depolluting Properties
4. Conclusions
- Increased active sites: The higher density of undercoordinated Ti5c atoms on {010} facets provides more active sites for adsorbing reactant molecules.
- Favourable band alignment: The higher conduction band minimum of {010} facets facilitates the generation of more reducing electrons, which can effectively participate in redox reactions.
- Enhanced charge separation: Differences in work function between different crystal facets can promote the formation of intra- and interparticle heterojunctions, reducing charge carrier recombination and improving photocatalytic efficiency.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Environment Agency (EEA). European Union Emission Inventory Report 1990–2022—Under the UNECE Convention on Long-Range Transboundary Air Pollution (Air Convention); European Environment Agency (EEA): Copenhagen, Denmark, 2024. [Google Scholar]
- European Environment Agency (EEA). Harm to Human Health from Air Pollution in Europe: Burden of Disease 2023; European Environment Agency (EEA): Copenhagen, Denmark, 2023. [Google Scholar]
- Russell, H.S.; Frederickson, L.B.; Hertel, O.; Ellermann, T.; Jensen, S.S. A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts 2021, 11, 675. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; de la Cruz, A.M. Enhancement of Photocatalytic Properties of TiO2 for NO Photo-Oxidation by Optimized Sol–Gel Synthesis. Res. Chem. Intermed. 2016, 42, 7065–7084. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Nguyen, V.H.; Lasek, J.; Wu, J.C.S. Titania Nanosheet Photocatalysts with Dominantly Exposed (001) Reactive Facets for Photocatalytic NOx Abatement. Appl. Catal. B Environ. 2017, 219, 391–400. [Google Scholar] [CrossRef]
- Luna, M.; Cruceira, Á.; Díaz, A.; Gatica, J.M.; Mosquera, M.J. Influence of Gold Nanoparticles Size for Photocatalytic NO Oxidation in Low Loading Au/TiO2 Catalysts. Environ. Technol. Innov. 2023, 30, 103070. [Google Scholar] [CrossRef]
- Kuppusamy, M.; Kim, S.W.; Lee, K.P.; Jo, Y.J.; Kim, W.J. Development of TiO2–CaCO3 Based Composites as an Affordable Building Material for the Photocatalytic Abatement of Hazardous NOx from the Environment. Nanomaterials 2024, 14, 136. [Google Scholar] [CrossRef]
- Hu, Y.; Song, X.; Jiang, S.; Wei, C. Enhanced Photocatalytic Activity of Pt-Doped TiO2 for NOx Oxidation Both under UV and Visible Light Irradiation: A Synergistic Effect of Lattice Pt4+ and Surface PtO. Chem. Eng. J. 2015, 274, 102–112. [Google Scholar] [CrossRef]
- Kuppusamy, M.; Passi, M.; Sundaram, S.K.; Vadivel, G.; Rathinasamy, M.; Lee, K.P.; Kim, W.J. Synergistic Effect of Li, La Co-Doping on Photocatalytic Activity of BaTiO3 Ferroelectric Material for Effective Degradation of Toxic NOx for Environmental Remediation. J. Environ. Chem. Eng. 2024, 12, 112801. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs Thermal Exfoliation of G-C3N4 for NOx Removal under Visible Light Irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Singh, L.P.; Dhaka, R.K.; Ali, D.; Tyagi, I.; Sharma, U.; Banavath, S.N. Remediation of Noxious Pollutants Using Nano-Titania-Based Photocatalytic Construction Materials: A Review. Environ. Sci. Pollut. Res. 2021, 28, 34087–34107. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.; Deng, X. NOx Removal Ability of Photocatalytic Cement-Based Materials with Porous Structure. J. Clean. Prod. 2022, 377, 134396. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Photocatalytic Activity of TiO2-SiO2 Nanocomposites Applied to Buildings: Influence of Particle Size and Loading. Appl. Catal. B Environ. 2013, 134–135, 205–221. [Google Scholar] [CrossRef]
- Khannyra, S.; Luna, M.; Gil, M.L.A.; Addou, M.; Mosquera, M.J. Self-Cleaning Durability Assessment of TiO2/SiO2 Photocatalysts Coated Concrete: Effect of Indoor and Outdoor Conditions on the Photocatalytic Activity. Build. Environ. 2022, 211, 108743. [Google Scholar] [CrossRef]
- Pinho, L.; Rojas, M.; Mosquera, M.J. Ag–SiO2–TiO2 Nanocomposite Coatings with Enhanced Photoactivity for Self-Cleaning Application on Building Materials. Appl. Catal. B Environ. 2015, 178, 144–154. [Google Scholar] [CrossRef]
- Luna, M.; Mosquera, M.J.; Vidal, H.; Gatica, J.M. Au-TiO2/SiO2 Photocatalysts for Building Materials: Self-Cleaning and de-Polluting Performance. Build. Environ. 2019, 164, 106347. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. Use of Au/N-TiO2/SiO2 Photocatalysts in Building Materials with NO Depolluting Activity. J. Clean. Prod. 2020, 243, 118633. [Google Scholar] [CrossRef]
- Khannyra, S.; Gil, M.L.A.; Addou, M.; Mosquera, M.J. Dye Decomposition and Air De-Pollution Performance of TiO2/SiO2 and N-TiO2/SiO2 Photocatalysts Coated on Portland Cement Mortar Substates. Environ. Sci. Pollut. Res. 2022, 29, 63112–63125. [Google Scholar] [CrossRef] [PubMed]
- Khannyra, S.; Mosquera, M.J.; Addou, M.; Gil, M.L.A. Cu-TiO2/SiO2 Photocatalysts for Concrete-Based Building Materials: Self-Cleaning and Air de-Pollution Performance. Constr. Build. Mater. 2021, 313, 125419. [Google Scholar] [CrossRef]
- Luna, M.; Delgado, J.J.; Romero, I.; Montini, T.; Almoraima Gil, M.L.; Martínez-López, J.; Fornasiero, P.; Mosquera, M.J. Photocatalytic TiO2 Nanosheets-SiO2 Coatings on Concrete and Limestone: An Enhancement of de-Polluting and Self-Cleaning Properties by Nanoparticle Design. Constr. Build. Mater. 2022, 338, 127349. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.M.; Cheng, H.-M. Titanium Dioxide Crystals with Tailored Facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mao, J.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Synthesis of Anatase TiO2 Nanocrystals with {101}, {001} or {010} Single Facets of 90% Level Exposure and Liquid-Phase Photocatalytic Reduction and Oxidation Activity Orders. J. Mater. Chem. A 2013, 1, 10532–10537. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.M.; Cheng, H.-M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chemie Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Xiang, Q.; Yu, J. Effect of Calcination Temperature on Morphology and Photocatalytic Activity of Anatase TiO2 Nanosheets with Exposed {001} Facets. Appl. Catal. B Environ. 2011, 104, 275–281. [Google Scholar] [CrossRef]
- Tandon, S.P.; Gupta, J.P. Measurement of Forbidden Energy Gap of Semiconductors by Diffuse Reflectance Technique. Phys. Status Solidi 1970, 38, 363–367. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Jensen, H.; Joensen, K.D.; Jørgensen, J.-E.; Pedersen, J.S.; Søgaard, E.G. Characterization of Nanosized Partly Crystalline Photocatalysts. J. Nanoparticle Res. 2004, 6, 519–526. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- EN 15886:2010; Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces. Ente Nazionale Italiano di Unificazione: Milan, Italy, 2010.
- ISO 22197-1:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide 2016. International Organization for Standardization: Geneva, Switzerland, 2016.
- Kwon, D.; Lee, S.H.; Kim, J.; Yoon, T.H. Dispersion, Fractionation and Characterization of Sub-100nm P25 TiO2 Nanoparticles in Aqueous Media. Toxicol. Environ. Health Sci. 2010, 2, 78–85. [Google Scholar] [CrossRef]
- Liao, D.L.; Wu, G.S.; Liao, B.Q. Zeta Potential of Shape-Controlled TiO2 Nanoparticles with Surfactants. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Kosmulski, M. Zeta Potentials in Nonaqueous Media: How to Measure and Control Them. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 159, 277–281. [Google Scholar] [CrossRef]
- Miyazaki, M.; Sugawara, Y.; Li, Y.J. Charge Behavior of Terminal Hydroxyl on Rutile TiO2(110). Langmuir 2021, 37, 10588–10593. [Google Scholar] [CrossRef]
- Yurdakal, S.; Çetinkaya, S.; Augugliaro, V.; Palmisano, G.; Soria, J.; Sanz, J.; Torralvo, M.J.; Livraghi, S.; Giamello, E.; Garlisi, C. Alkaline Treatment as a Means to Boost the Activity of TiO2 in Selective Photocatalytic Processes. Catal. Sci. Technol. 2020, 10, 5000–5012. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, Optical and Morphological Analyses of Pristine Titanium Di-Oxide Nanoparticles—Synthesized via Sol-Gel Route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Mino, L.; Cesano, F.; Scarano, D.; Spoto, G.; Martra, G. Molecules and Heterostructures at TiO2 Surface: The Cases of H2O, CO2, and Organic and Inorganic Sensitizers. Res. Chem. Intermed. 2019, 45, 5801–5829. [Google Scholar] [CrossRef]
- Ayers, M.R.; Hunt, A.J. Titanium Oxide Aerogels Prepared from Titanium Metal and Hydrogen Peroxide. Mater. Lett. 1998, 34, 290–293. [Google Scholar] [CrossRef]
- Pellegrino, F.; Morra, E.; Mino, L.; Martra, G.; Chiesa, M.; Maurino, V. Surface and Bulk Distribution of Fluorides and Ti3+ Species in TiO2 Nanosheets: Implications on Charge Carrier Dynamics and Photocatalysis. J. Phys. Chem. C 2020, 124, 3141–3149. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent Advances in the Textural Characterization of Hierarchically Structured Nanoporous Materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Fan, Z.; Meng, F.; Gong, J.; Li, H.; Ding, Z.; Ding, B. One-Step Hydrothermal Synthesis of Mesoporous Ce-Doped Anatase TiO2 Nanoparticles with Enhanced Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2016, 27, 11866–11872. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, G.; Fang, J.; Chen, J. Synthesis, Characterization, and Photocatalysis of Well-Dispersible Phase-Pure Anatase TiO2 Nanoparticles. Int. J. Photoenergy 2013, 2013, 726872. [Google Scholar] [CrossRef]
- Abdel-Monem, Y.K. Efficient Nanophotocatalyt of Hydrothermally Synthesized Anatase TiO2 Nanoparticles from Its Analogue Metal Coordinated Precursor. J. Mater. Sci. Mater. Electron. 2016, 27, 5723–5728. [Google Scholar] [CrossRef]
- Lee, D.-H.; Cho, G.; Lim, H.M.; Kim, D.S.; Kim, C.; Lee, S.-H. Comparisons of Particle Size Measurement Method for Colloidal Silica. J. Ceram. Process. Res. 2013, 14, 274–278. [Google Scholar]
- Balázs, N.; Srankó, D.F.; Dombi, A.; Sipos, P.; Mogyorósi, K. The Effect of Particle Shape on the Activity of Nanocrystalline TiO2 Photocatalysts in Phenol Decomposition. Part 2: The Key Synthesis Parameters Influencing the Particle Shape and Activity. Appl. Catal. B Environ. 2010, 96, 569–576. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Šubrt, J.; Štengl, V.; Dianez, M.J.; Sayagues, M.J. Photoactivity of Anatase-Rutile TiO2 Nanocrystalline Mixtures Obtained by Heat Treatment of Homogeneously Precipitated Anatase. Appl. Catal. B Environ. 2005, 58, 193–202. [Google Scholar] [CrossRef]
- Collazzo, G.C.; Jahn, S.L.; Carreño, N.L.V.; Foletto, E.L. Temperature and Reaction Time Effects on the Structural Properties of Titanium Dioxide Nanopowders Obtained via the Hydrothermal Method. Braz. J. Chem. Eng. 2011, 28, 265–272. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A. Fully Quantitative X-Ray Characterisation of Evonik Aeroxide TiO2 P25®. Mater. Lett. 2014, 122, 345–347. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Bhattacharya, S.S. Size Effect on the Lattice Parameters of Nanocrystalline Anatase. Appl. Phys. Lett. 2009, 95, 191906. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What Is Degussa (Evonic) P25? Crystalline Composition Analysis, Reconstruction from Isolated Pure Particles and Photocatalytic Activity Test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and Energetics of Stoichiometric TiO2 Anatase Surfaces. Phys. Rev. B—Condens. Matter Mater. Phys. 2001, 63, 1554091–1554099. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Ren, Z.; Qian, G.; Wang, Z. One-Dimension TiO2 Nanostructures: Oriented Attachment and Application in Dye-Sensitized Solar Cell. CrystEngComm 2014, 16, 1681–1686. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, A.; Pecharromán, C.; Faraldos, M.; Bahamonde, A. Strong Effect of Light Scattering by Distribution of TiO2 Particle Aggregates on Photocatalytic Efficiency in Aqueous Suspensions. Chem. Eng. J. 2021, 403, 126186. [Google Scholar] [CrossRef]
- Chen, M.; Ma, J.; Zhang, B.; Wang, F.; Li, Y.; Zhang, C.; He, H. Facet-Dependent Performance of Anatase TiO2 for Photocatalytic Oxidation of Gaseous Ammonia. Appl. Catal. B Environ. 2018, 223, 209–215. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Xi, J.; Ji, Z. {001} Facets of Anatase TiO2 Show High Photocatalytic Selectivity. Mater. Lett. 2012, 79, 259–262. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, X.X.; Su, J.; Wang, P.P.; Zhou, L.J.; Li, G.D. Synthesis and Photocatalytic Activity of Porous Anatase TiO2 Microspheres Composed of {010}-Faceted Nanobelts. Dalton Trans. 2013, 42, 4365–4368. [Google Scholar] [CrossRef]
- Pan, J.; Wu, X.; Wang, L.; Liu, G.; Lu, G.Q.; Cheng, H.M. Synthesis of Anatase TiO2 Rods with Dominant Reactive {010} Facets for the Photoreduction of CO2 to CH4 and Use in Dye-Sensitized Solar Cells. Chem. Commun. 2011, 47, 8361–8363. [Google Scholar] [CrossRef]
- Xu, H.; Reunchan, P.; Ouyang, S.; Tong, H.; Umezawa, N.; Kako, T.; Ye, J. Anatase TiO2 Single Crystals Exposed with High-Reactive {111} Facets toward Efficient H2 Evolution. Chem. Mater. 2013, 25, 405–411. [Google Scholar] [CrossRef]
- Meng, F.; Lai, Y.; Cheng, Z.; Ding, Y.; Sun, M.; Zhang, S.; Zhong, Q. Distinguishing the Roles of Anatase TiO2 Nanocrystals with {101}, {010} or {001} Facets Catalyzed O3/H2O2 for Low-Temperature NO Oxidation. Mol. Catal. 2023, 549, 113513. [Google Scholar] [CrossRef]
- Mishra, S.B.; Nanda, B.R.K. Facet Dependent Catalytic Activities of Anatase TiO2 for CO2 Adsorption and Conversion. Appl. Surf. Sci. 2020, 531, 147330. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Y.; Gao, T.; Zhang, J.; Sun, X.; Zhou, G. Fabrication of Anatase TiO2 Tapered Tetragonal Nanorods with Designed {100}, {001} and {101} Facets for Enhanced Photocatalytic H2 Evolution. Int. J. Hydrogen Energy 2017, 42, 21775–21785. [Google Scholar] [CrossRef]
- Du, Y.E.; Du, Y.E.; Du, Y.E.; Niu, X.; He, J.; Liu, L.; Liu, Y.; Chen, C.; Yang, X.; Feng, Q. Hollow Square RodLike Microtubes Composed of Anatase Nanocuboids with Coexposed {100}, {010}, and {001} Facets for Improved Photocatalytic Performance. ACS Omega 2020, 5, 14147–14156. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.Y. Synthesis and Crystal Growth Mechanism of Titanium Dioxide Nanorods. Wuji Cailiao Xuebao/J. Inorg. Mater. 2012, 27, 45–48. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. One-Pot Synthesis of Au/N-TiO2 Photocatalysts for Environmental Applications: Enhancement of Dyes and NOx Photodegradation. Powder Technol. 2019, 355, 793–807. [Google Scholar] [CrossRef]
- Ohko, Y.; Nakamura, Y.; Negishi, N.; Matsuzawa, S.; Takeuchi, K. Photocatalytic Oxidation of Nitrogen Monoxide Using TiO2 Thin Films under Continuous UV Light Illumination. J. Photochem. Photobiol. A Chem. 2009, 205, 28–33. [Google Scholar] [CrossRef]
- Bloh, J.Z.; Folli, A.; Macphee, D.E. Photocatalytic NOx Abatement: Why the Selectivity Matters. RSC Adv. 2014, 4, 45726–45734. [Google Scholar] [CrossRef]
- Luna, M.; Gonzalez-Hidalgo, A.; Diaz, A.; Goma, D.; Gatica, J.M.; Mosquera, M.J. Strong Metal-Support Interaction (SMSI) in Au/TiO2 Photocatalysts for Environmental Remediation Applications: Effectiveness Enhancement and Side Effects. J. Environ. Chem. Eng. 2023, 11, 109947. [Google Scholar] [CrossRef]
- Bertolotti, F.; Vivani, A.; Moscheni, D.; Ferri, F.; Cervellino, A.; Masciocchi, N.; Guagliardi, A. Structure, Morphology, and Faceting of TiO2 Photocatalysts by the Debye Scattering Equation Method. The P25 and P90 Cases of Study. Nanomaterials 2020, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering Coexposed {001} and {101} Facets in Oxygen-Deficient TiO2 Nanocrystals for Enhanced CO2 Photoreduction under Visible Light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J.; Grossi, A. Indicators and Ratings for the Compatibility Assessment of Conservation Actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]


| Sample | Final Step | Post-Treatment |
|---|---|---|
| H12 | Hydrothermal 200 °C 12 h | No |
| H15 | Hydrothermal 200 °C 15 h | No |
| H18 | Hydrothermal 200 °C 18 h | No |
| H24 | Hydrothermal 200 °C 24 h | No |
| C450 | Drying + calcination at 450 °C 3 h | No |
| H12C150_24 | Hydrothermal 200 °C 12 h | 150 °C 24 h |
| H12C300_2 | Hydrothermal 200 °C 12 h | 300 °C 2 h |
| H12C300_4 | Hydrothermal 200 °C 12 h | 300 °C 4 h |
| H12C300_8 | Hydrothermal 200 °C 12 h | 300 °C 8 h |
| H12C450_2 | Hydrothermal 200 °C 12 h | 450 °C 2 h |
| H12C450_4 | Hydrothermal 200 °C 12 h | 450 °C 4 h |
| H18C450_2 | Hydrothermal 200 °C 18 h | 450 °C 2 h |
| H18C450_4 | Hydrothermal 200 °C 18 h | 450 °C 4 h |
| H12NaOH | Hydrothermal 200 °C 12 h | NaOH 0.1 M |
| Sample | DLS | N2 Physisorption | XRD Size (nm) | SEM Size | ||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Z (mV) | SBET (m2/g) | Vp (cm3/g) | (101) | (004) | Short (nm) | Long (nm) | |
| P25 | 115 ± 25 | 27.8 ± 5.8 | 57 | 0.21 | 25.0 ± 0.4 | 19.1 ± 0.8 | - | - |
| H12 | 55 ± 13 | 39.7 ± 7.6 | 61 | 0.22 | 23.1 ± 0.2 | 38.7 ± 1.1 | 22 ± 5 | 61 ± 22 |
| H15 | 55 ± 14 | 39.3 ± 7.4 | - | - | 21.9 ± 0.2 | 40.3 ± 1.2 | 20 ± 4 | 55 ± 22 |
| H18 | 55 ± 12 | 40.0 ± 7.9 | 68 | 0.26 | 24.1 ± 0.3 | 39.2 ± 1.1 | - | - |
| H24 | 53 ± 11 | 39.5 ± 7.6 | - | - | 21.6 ± 0.4 | 25.4 ± 1.5 | 17 ± 4 | 56 ± 22 |
| C450 | 1347 ± 200 | 16.6 ± 4.8 | - | - | - | - | 16 ± 4 | - |
| H12C300_4 | 114 ± 30 | - | - | - | 21.1 ± 0.2 | 30.4 ± 0.8 | - | - |
| H12C450_2 | 127 ± 24 | - | - | - | - | - | - | - |
| H12C450_4 | 137 ± 30 | 17.9 ± 4.9 | 64 | 0.25 | 22.1 ± 0.2 | 31.4 ± 0.7 | 23.0 ± 4 | 54 ± 16 |
| H12NaOH | 53 ± 13 | −38.6 ± 7.3 | 62 | 0.30 | 23.4 ± 0.3 | 38.2 ± 1.0 | 22 ± 5 | 62 ± 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, M.; Cruces, J.L.; Gatica, J.M.; Cruceira, A.; Cifredo, G.A.; Vidal, H.; Mosquera, M.J. The Role of Surface {010} Facets in Improving the NOx Depolluting Activity of TiO2 and Its Application on Building Materials. Technologies 2025, 13, 52. https://doi.org/10.3390/technologies13020052
Luna M, Cruces JL, Gatica JM, Cruceira A, Cifredo GA, Vidal H, Mosquera MJ. The Role of Surface {010} Facets in Improving the NOx Depolluting Activity of TiO2 and Its Application on Building Materials. Technologies. 2025; 13(2):52. https://doi.org/10.3390/technologies13020052
Chicago/Turabian StyleLuna, Manuel, Jose L. Cruces, José M. Gatica, Alvaro Cruceira, Gustavo A. Cifredo, Hilario Vidal, and María J. Mosquera. 2025. "The Role of Surface {010} Facets in Improving the NOx Depolluting Activity of TiO2 and Its Application on Building Materials" Technologies 13, no. 2: 52. https://doi.org/10.3390/technologies13020052
APA StyleLuna, M., Cruces, J. L., Gatica, J. M., Cruceira, A., Cifredo, G. A., Vidal, H., & Mosquera, M. J. (2025). The Role of Surface {010} Facets in Improving the NOx Depolluting Activity of TiO2 and Its Application on Building Materials. Technologies, 13(2), 52. https://doi.org/10.3390/technologies13020052









