A New Bundling and Packaging Method Using Nonwoven Polylactide Based on Polymer Shrinkage by Carbon Dioxide
Abstract
1. Introduction
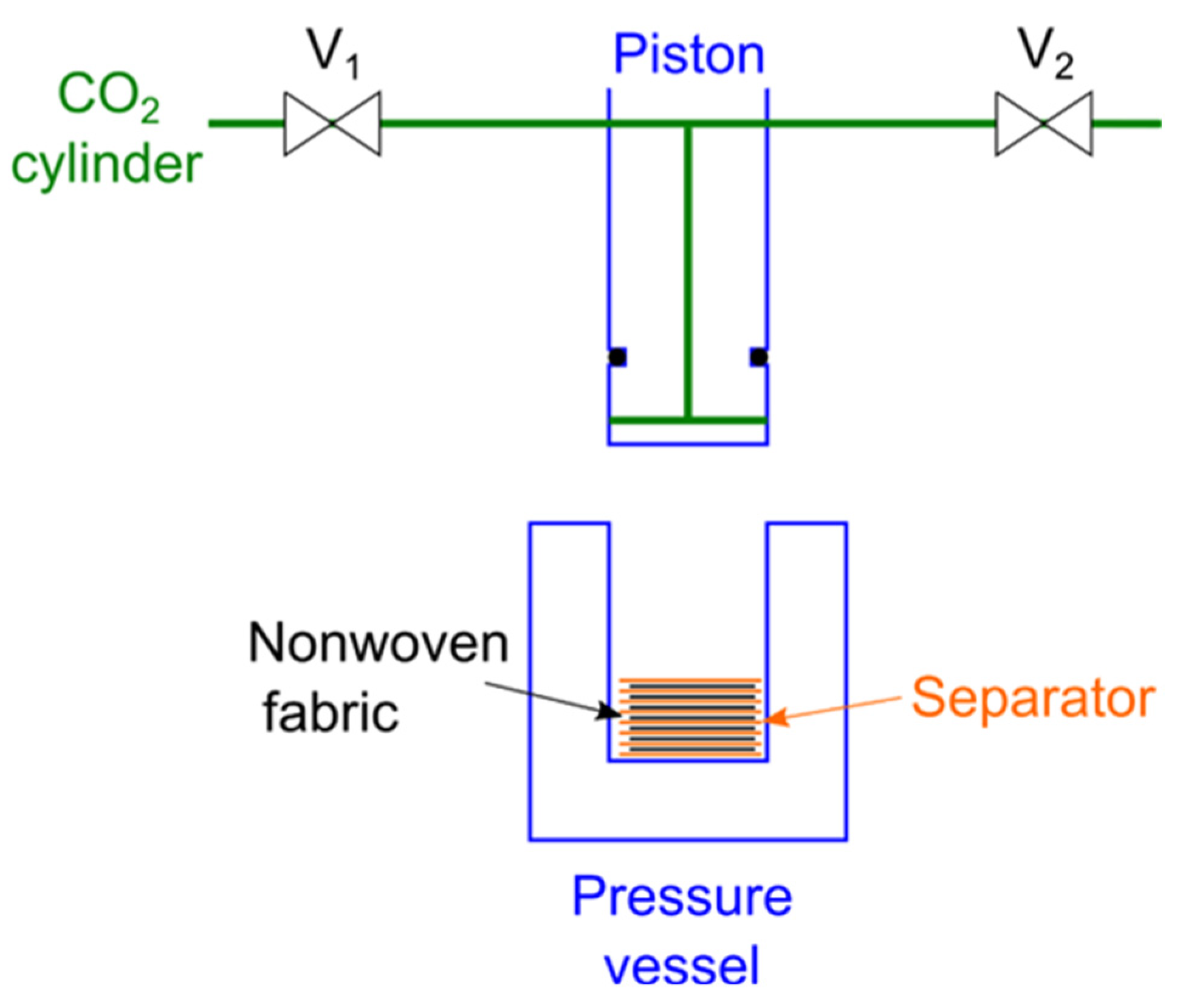
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachs, J.; Schmidt-Traub, G.; Kroll, C.; Lafortune, G.; Fuller, G.; Woelm, F. Sustainable Development Report 2020: The Sustainable Development Goals and COVID-19 Includes the SDG Index and Dashboards; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Review of technological progress in carbon dioxide capture, storage, and utilization. Gas Sci. Eng. 2023, 117, 205070. [Google Scholar] [CrossRef]
- Yusuf, M.; Ibrahim, H. A comprehensive review on recent trends in carbon capture, utilization, and storage techniques. J. Env. Chem. Eng. 2023, 11, 111393. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical carbon dioxide applications in food processing. Food Eng. Rev. 2021, 13, 570–591. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Liu, H.; Liu, B.; Wu, Y.; Wang, L. Solubility prediction of supercritical carbon dioxide in 10 polymers using radial basis function artificial neural network based on chaotic self-adaptive particle swarm optimization and K-harmonic means. RSC Adv. 2015, 5, 45520–45527. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of supercritical carbon dioxide with polymers. I. Crystalline polymers. J. Appl. Polym. Sci. 1996, 59, 695–705. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of supercritical carbon dioxide with polymers. II. Amorphous polymers. J. Appl. Polym. Sci. 1996, 59, 707–717. [Google Scholar] [CrossRef]
- Sarver, J.A.; Kiran, E. Foaming of polymers with carbon dioxide—The year-in-review–2019. J. Supercrit. Fluids 2021, 173, 105166. [Google Scholar] [CrossRef]
- Yu, C.; Wang, Z.; Liu, M.; Li, S. Preparation of drug-loaded composites by supercritical carbon dioxide impregnation. Polym. Sci. Ser. A 2023, 65, 63–71. [Google Scholar] [CrossRef]
- Kiran, E. Supercritical fluids and polymers—The year in review—2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Bahrami, M.; Ranjbarian, S. Production of micro- and nano-composite particles by supercritical carbon dioxide. J. Supercrit. Fluids 2007, 40, 263–283. [Google Scholar] [CrossRef]
- Gu, Z.; Zhao, B.; Zhang, L.; Bao, J. Supercritical carbon dioxide foamed thermoplastic polyester elastomer with poly(lactic acid) blending: Shrinkage reduction and expansion ratio improvement. Colloid Polym. Sci. 2025, 303, 67–80. [Google Scholar] [CrossRef]
- Aizawa, T. A new method for producing porous polymer materials using carbon dioxide and a piston. J. Supercrit. Fluids 2018, 133, 38–41. [Google Scholar] [CrossRef]
- Aizawa, T. Fabrication of porosity-controlled polyethylene terephthalate porous materials using a CO2-assisted polymer compression method. RSC Adv. 2018, 8, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T.; Matsuura, S.-I. Fabrication of enzyme-loaded cartridges using CO2-assisted polymer compression. Technologies 2021, 9, 85. [Google Scholar] [CrossRef]
- Russell, S.J. Handbook of Nonwovens, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2022. [Google Scholar]
- Aizawa, T. Application of CO2-assisted polymer compression to polylactic acid and the relationship between crystallinity and plasticization. Compounds 2021, 1, 75–82. [Google Scholar] [CrossRef]
- Kalkan-Sevinc, Z.; Strobel, C.T. Material characterization of heat shrinkable film. J. Test Eval. 2015, 43, 1531–1539. [Google Scholar] [CrossRef]
- ASTM International Official Website. Available online: https://astm.org/ (accessed on 20 January 2025).
- International Organization for Standardization Official Webcite. Available online: https://iso.org/ (accessed on 20 January 2025).



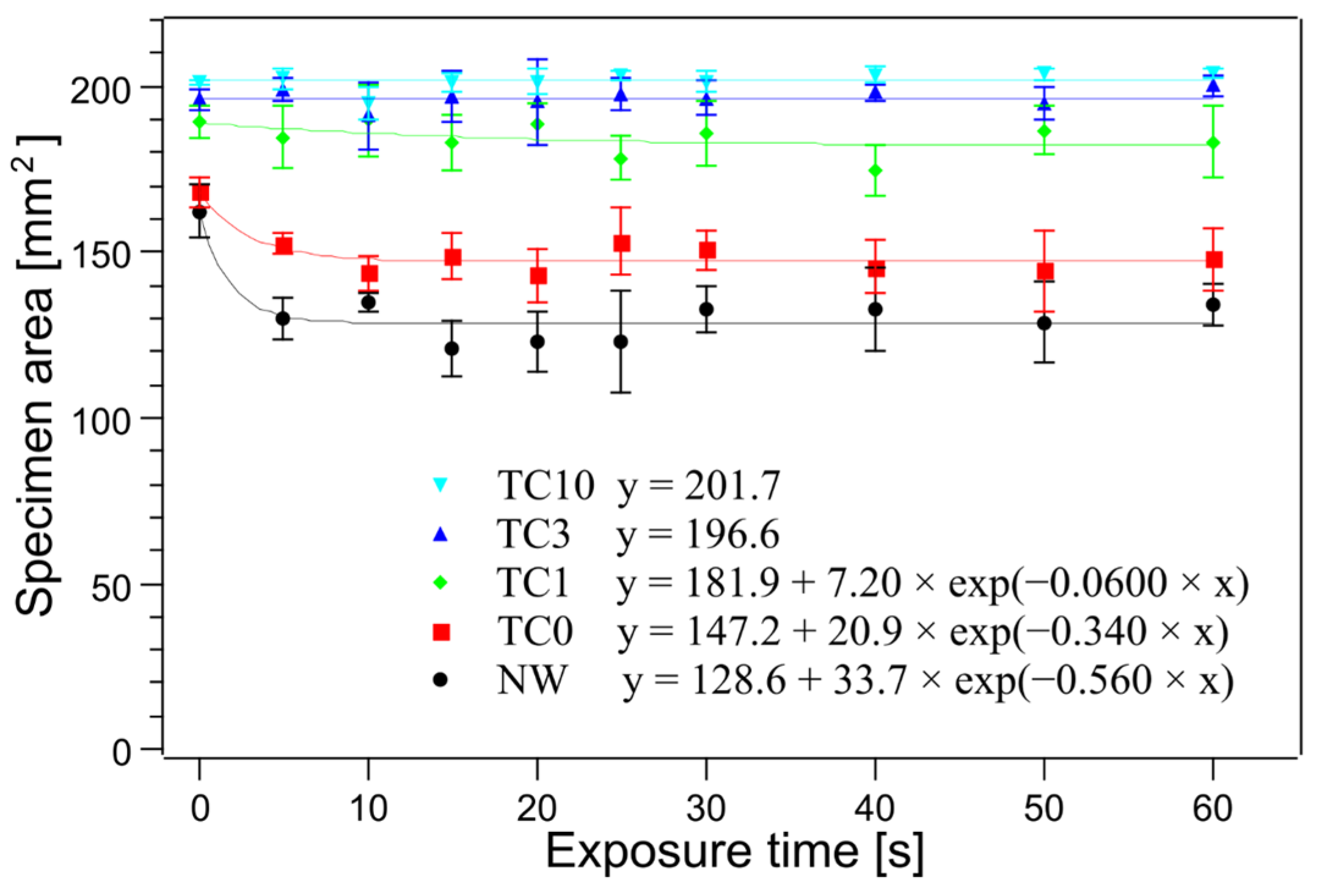

| Time [s] | Area of NW [mm2] | Area of TC0 [mm2] | Area of TC1 [mm2] | Area of TC3 [mm2] | Area of TC10 [mm2] |
|---|---|---|---|---|---|
| 0 | 162.3 ± 8.1 | 168.0 ± 4.6 | 189.2 ± 5.1 | 195.9 ± 2.9 | 201.1 ± 0.7 |
| 5 | 130.0 ± 6.1 | 152.5 ± 3.2 | 184.6 ± 9.3 | 199.1 ± 3.8 | 202.4 ± 3.2 |
| 10 | 134.8 ± 3.1 | 143.8 ± 5.2 | 189.7 ± 10.7 | 191.0 ± 10.3 | 194.9 ± 5.1 |
| 15 | 120.8 ± 8.6 | 148.8 ± 7.0 | 183.2 ± 8.5 | 197.0 ± 7.9 | 201.2 ± 2.9 |
| 20 | 123.0 ± 9.4 | 143.2 ± 8.0 | 188.8 ± 6.4 | 195.4 ± 12.9 | 201.4 ± 3.8 |
| 25 | 123.0 ± 15.4 | 153.1 ± 10.1 | 178.3 ± 6.8 | 197.8 ± 5.1 | 203.3 ± 1.4 |
| 30 | 132.8 ± 6.9 | 150.8 ± 6.0 | 185.7 ± 9.6 | 196.4 ± 5.3 | 201.5 ± 2.9 |
| 40 | 132.7 ± 12.4 | 145.5 ± 8.2 | 174.5 ± 7.7 | 198.1 ± 2.6 | 203.5 ± 2.4 |
| 50 | 128.8 ± 12.0 | 144.3 ± 12.1 | 186.8 ± 7.6 | 195.0 ± 5.0 | 203.6 ± 1.5 |
| 60 | 133.9 ± 6.2 | 148.0 ± 9.3 | 183.3 ± 11.0 | 200.1 ± 3.1 | 204.1 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aizawa, T. A New Bundling and Packaging Method Using Nonwoven Polylactide Based on Polymer Shrinkage by Carbon Dioxide. Technologies 2025, 13, 49. https://doi.org/10.3390/technologies13020049
Aizawa T. A New Bundling and Packaging Method Using Nonwoven Polylactide Based on Polymer Shrinkage by Carbon Dioxide. Technologies. 2025; 13(2):49. https://doi.org/10.3390/technologies13020049
Chicago/Turabian StyleAizawa, Takafumi. 2025. "A New Bundling and Packaging Method Using Nonwoven Polylactide Based on Polymer Shrinkage by Carbon Dioxide" Technologies 13, no. 2: 49. https://doi.org/10.3390/technologies13020049
APA StyleAizawa, T. (2025). A New Bundling and Packaging Method Using Nonwoven Polylactide Based on Polymer Shrinkage by Carbon Dioxide. Technologies, 13(2), 49. https://doi.org/10.3390/technologies13020049







