Modification of Al2O3-Based Membranes with Carbon Black for Enhanced Hydrogen Permeation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powder Mixture and Green Compact Preparation
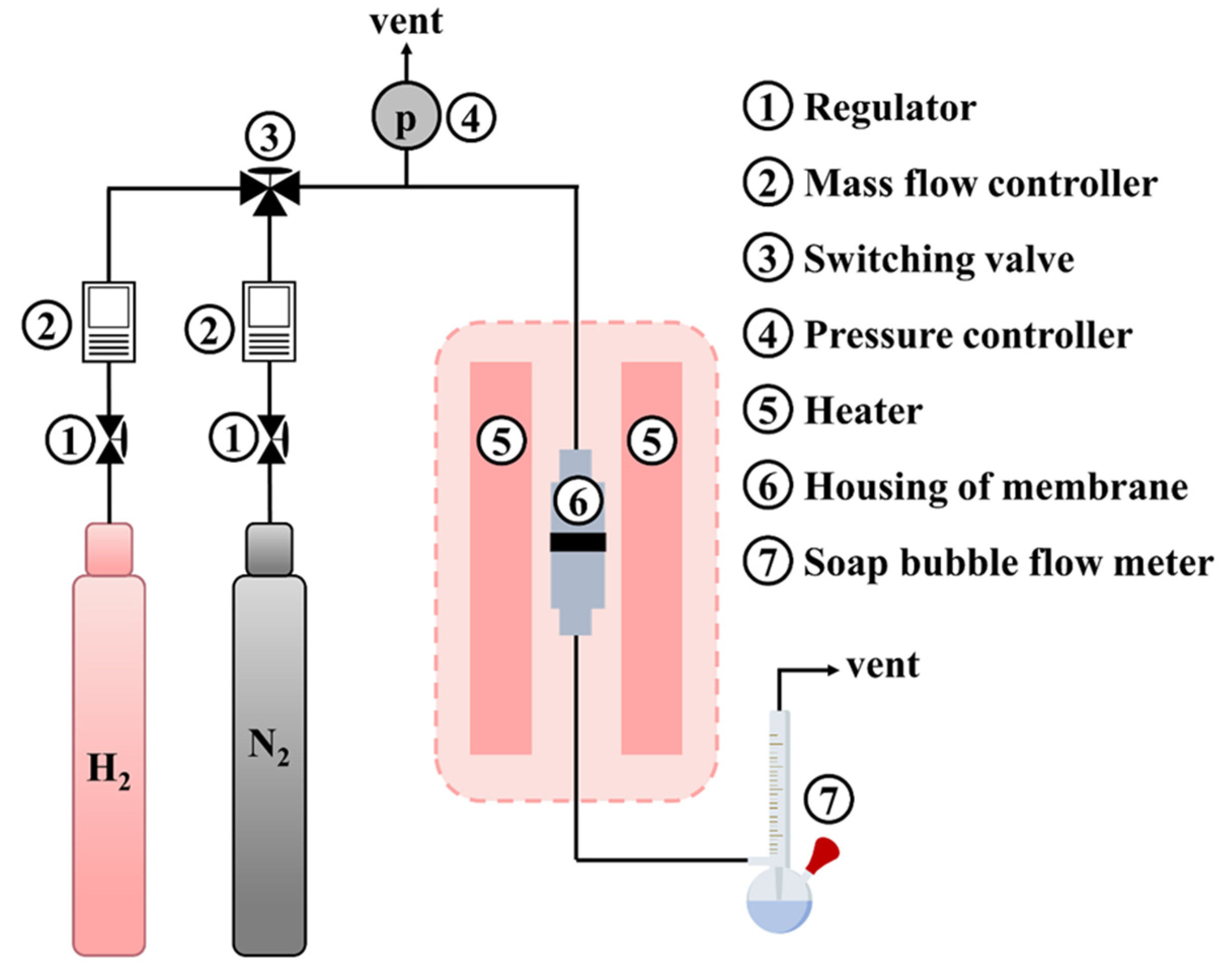
2.3. Characteristic
3. Results
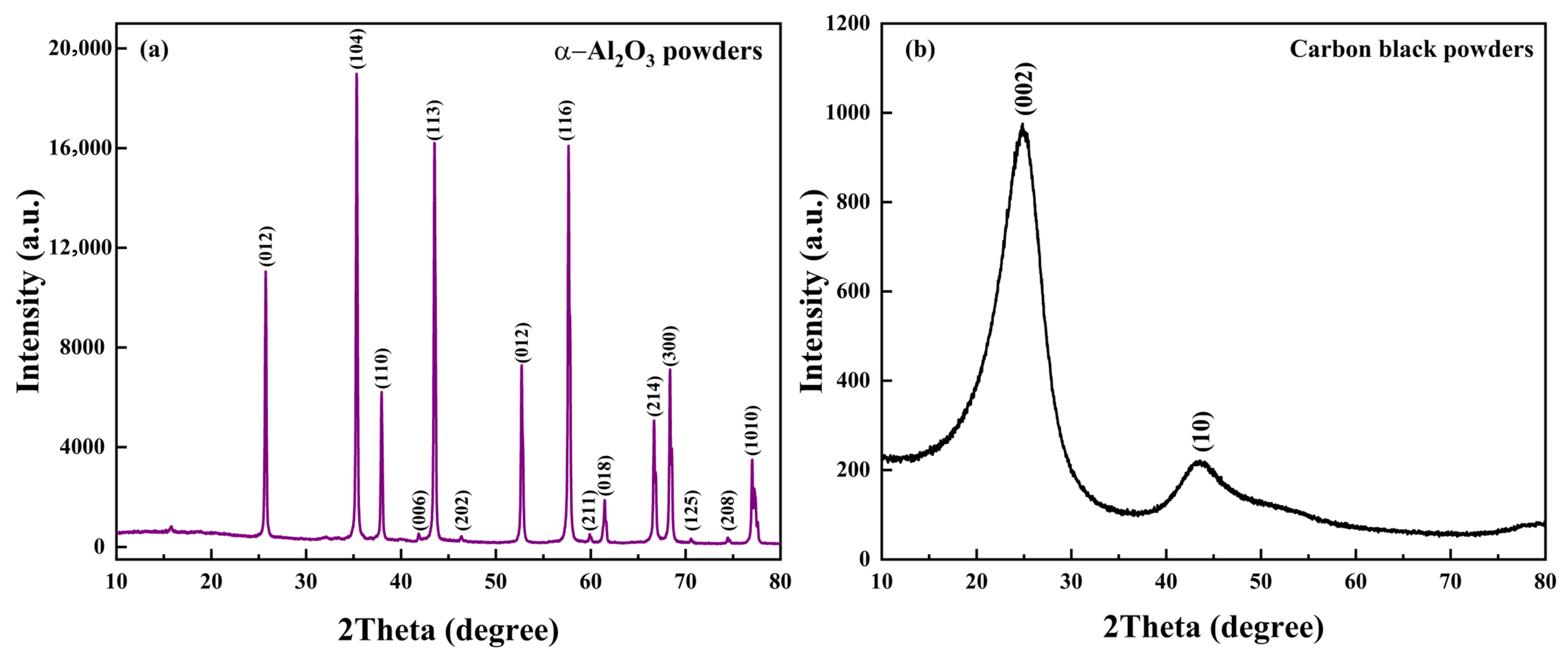
3.1. Phase Composition
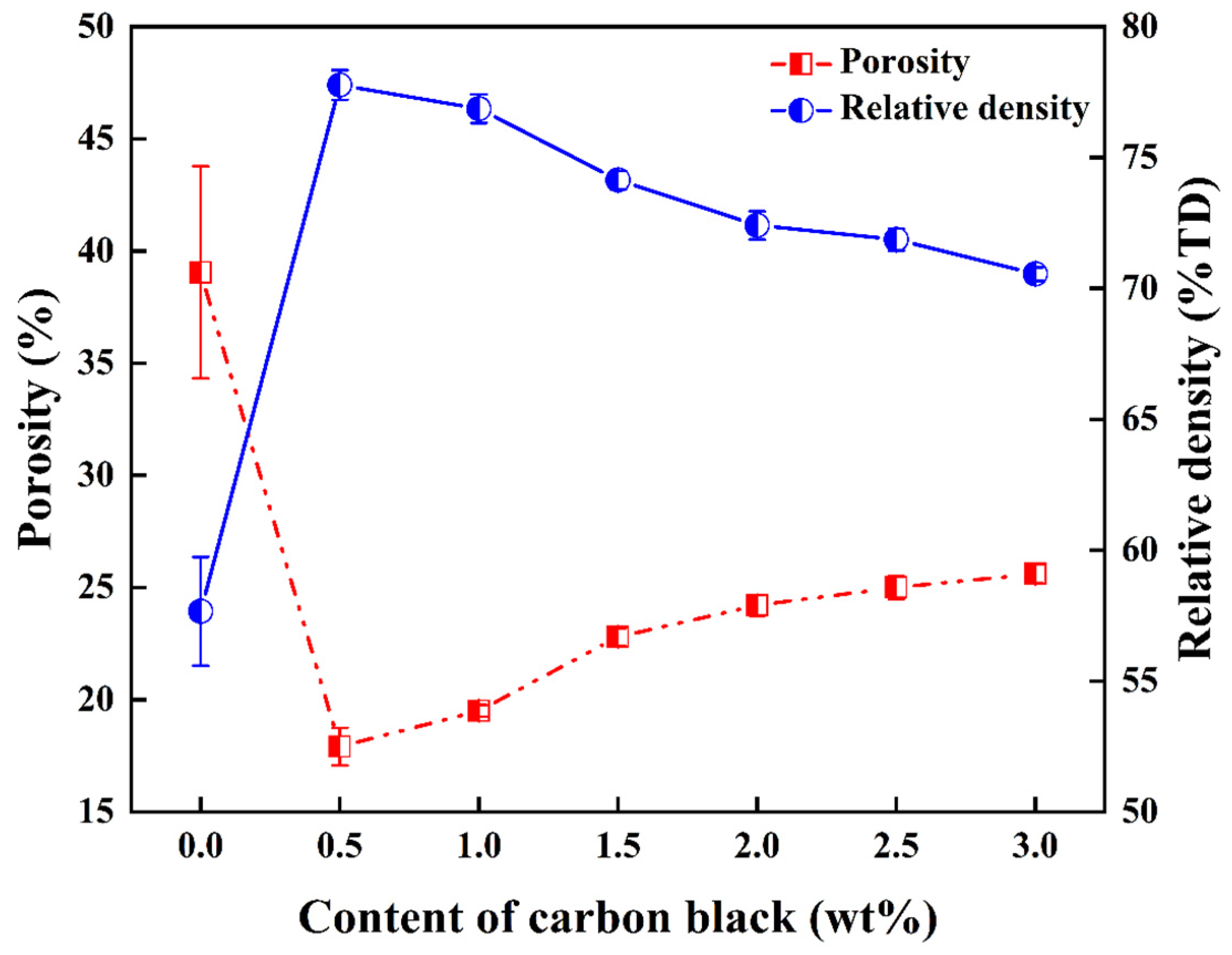
3.2. Densification Properties
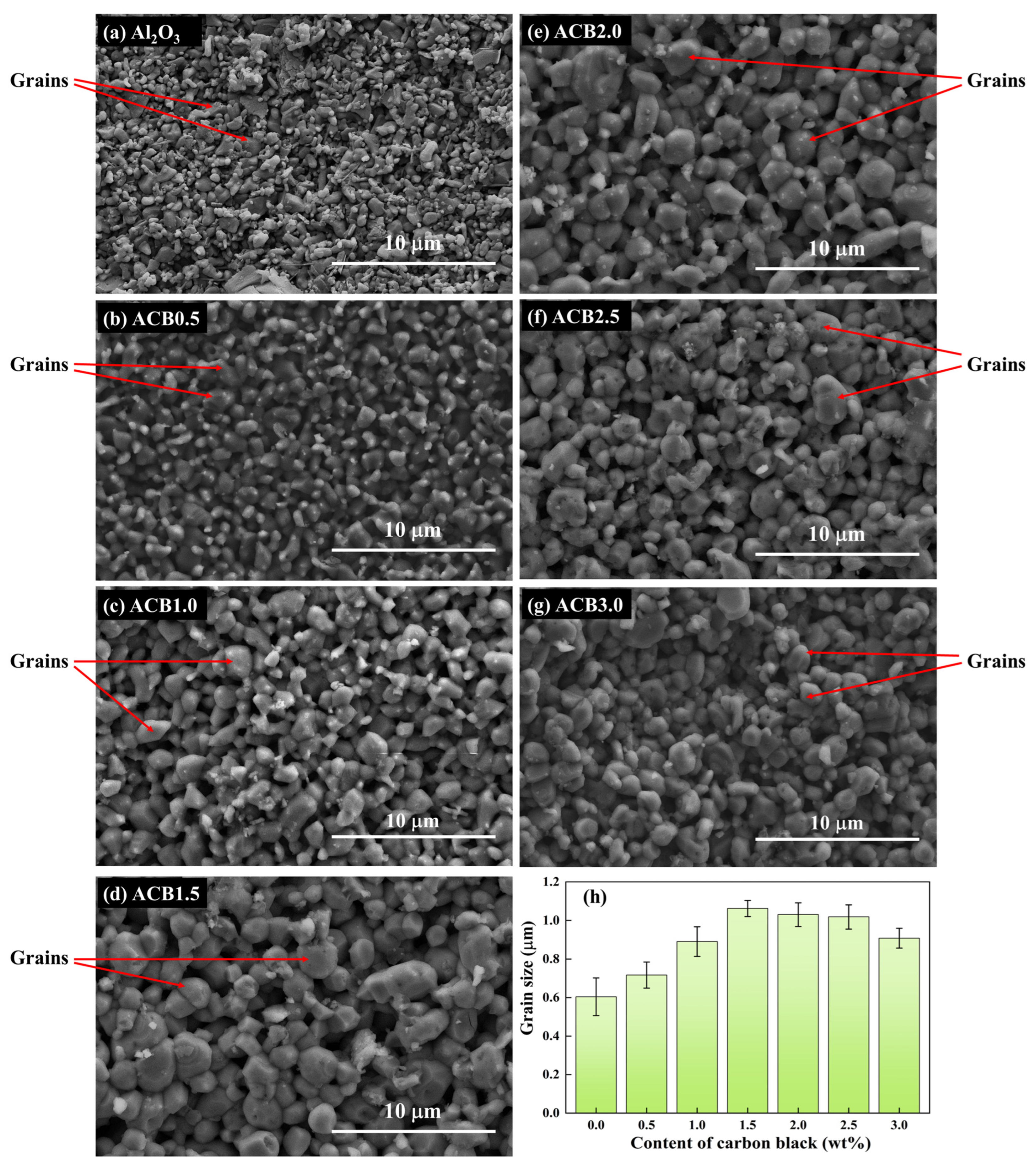
3.3. Surface Morphology
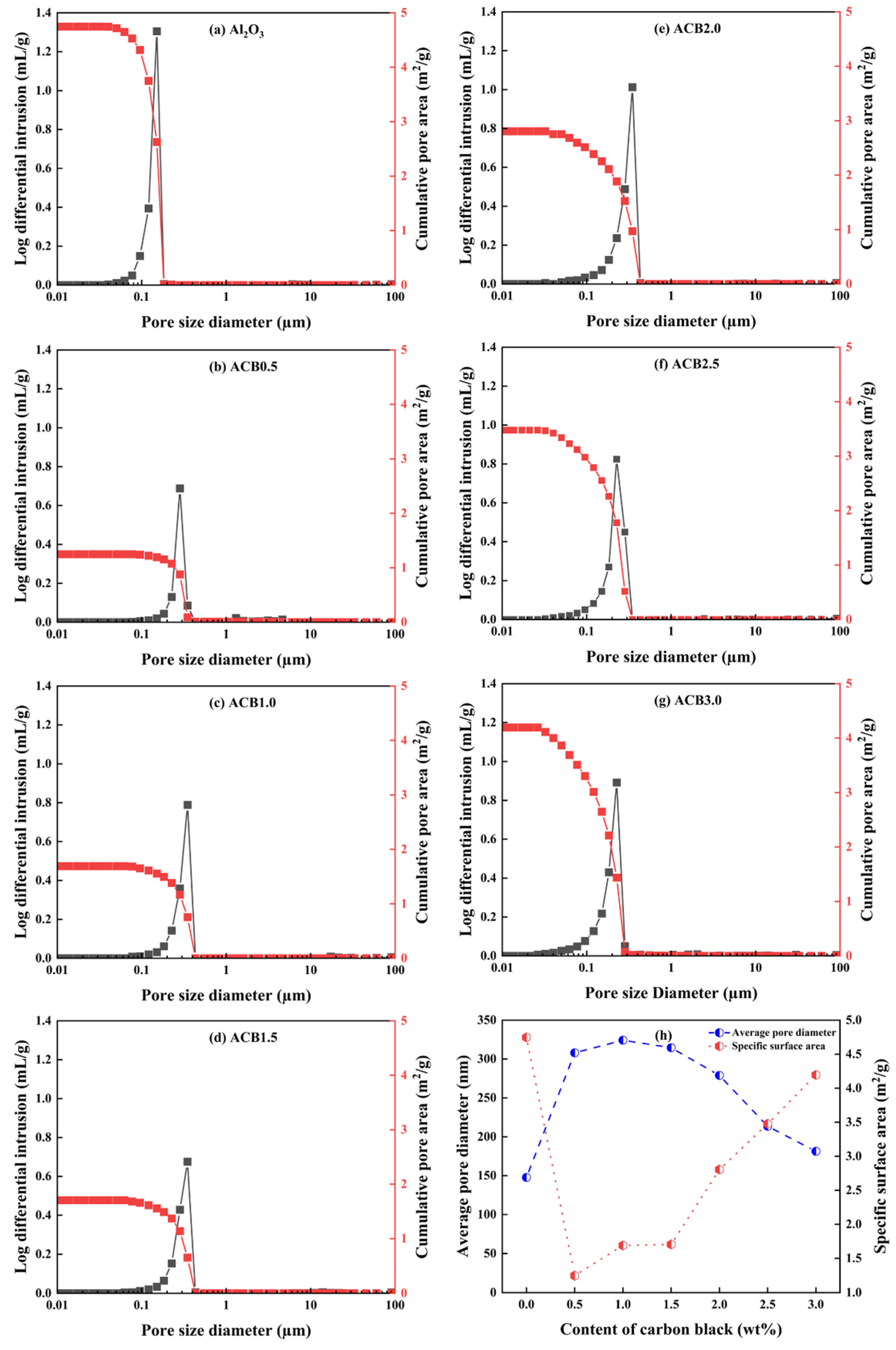
3.4. Pore Distributions and Specific Surface Area
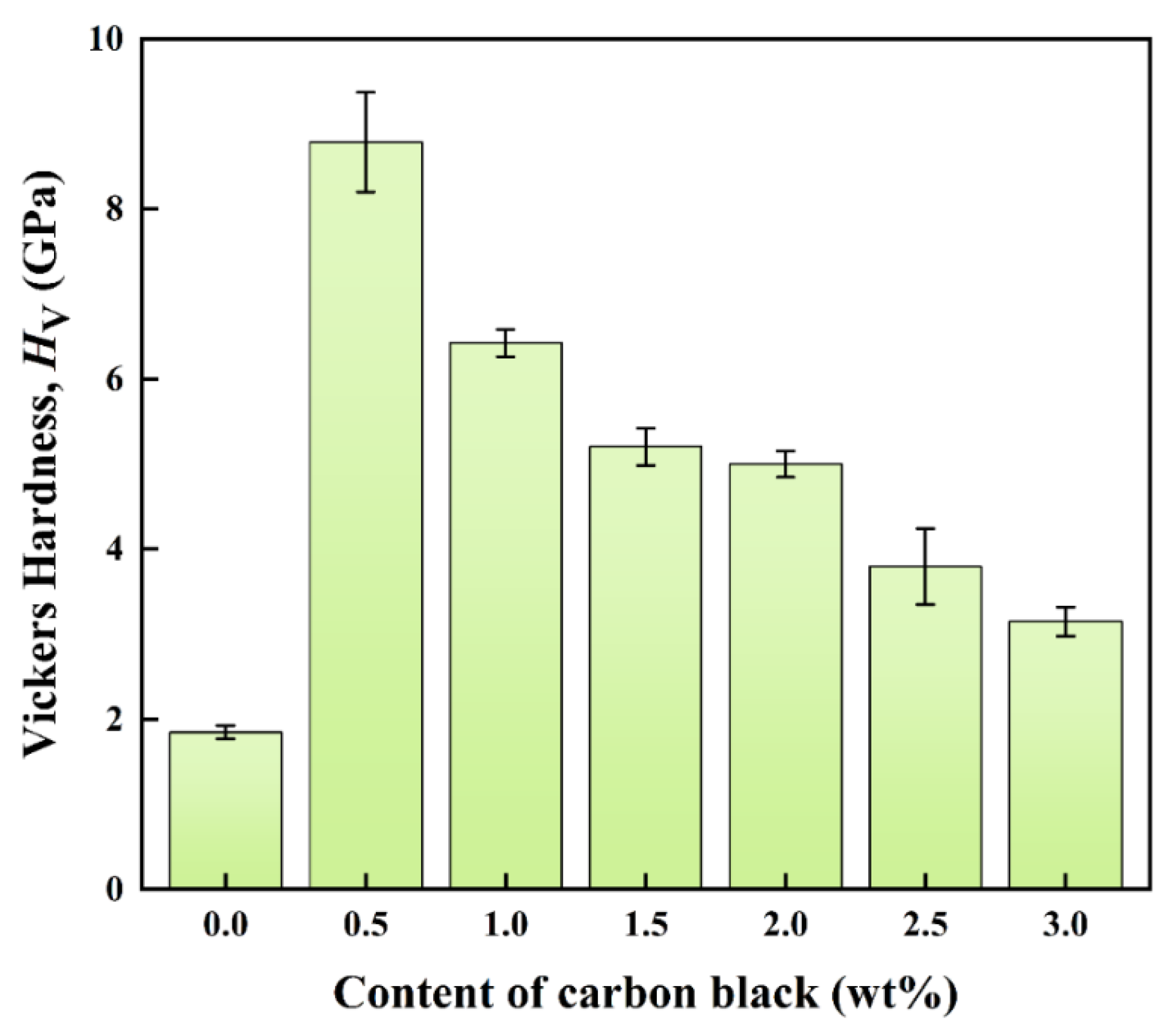
3.5. Mechanical Properties
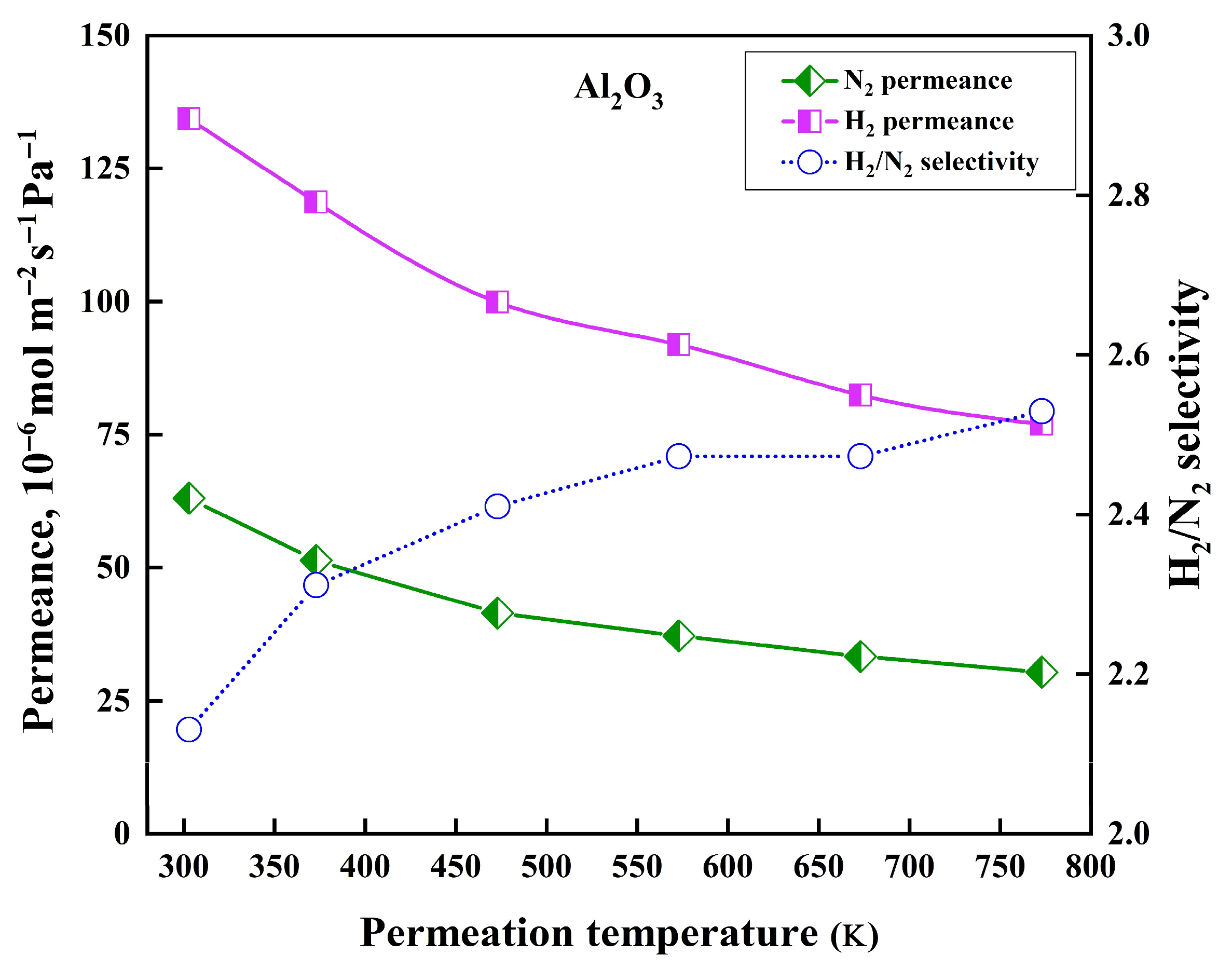
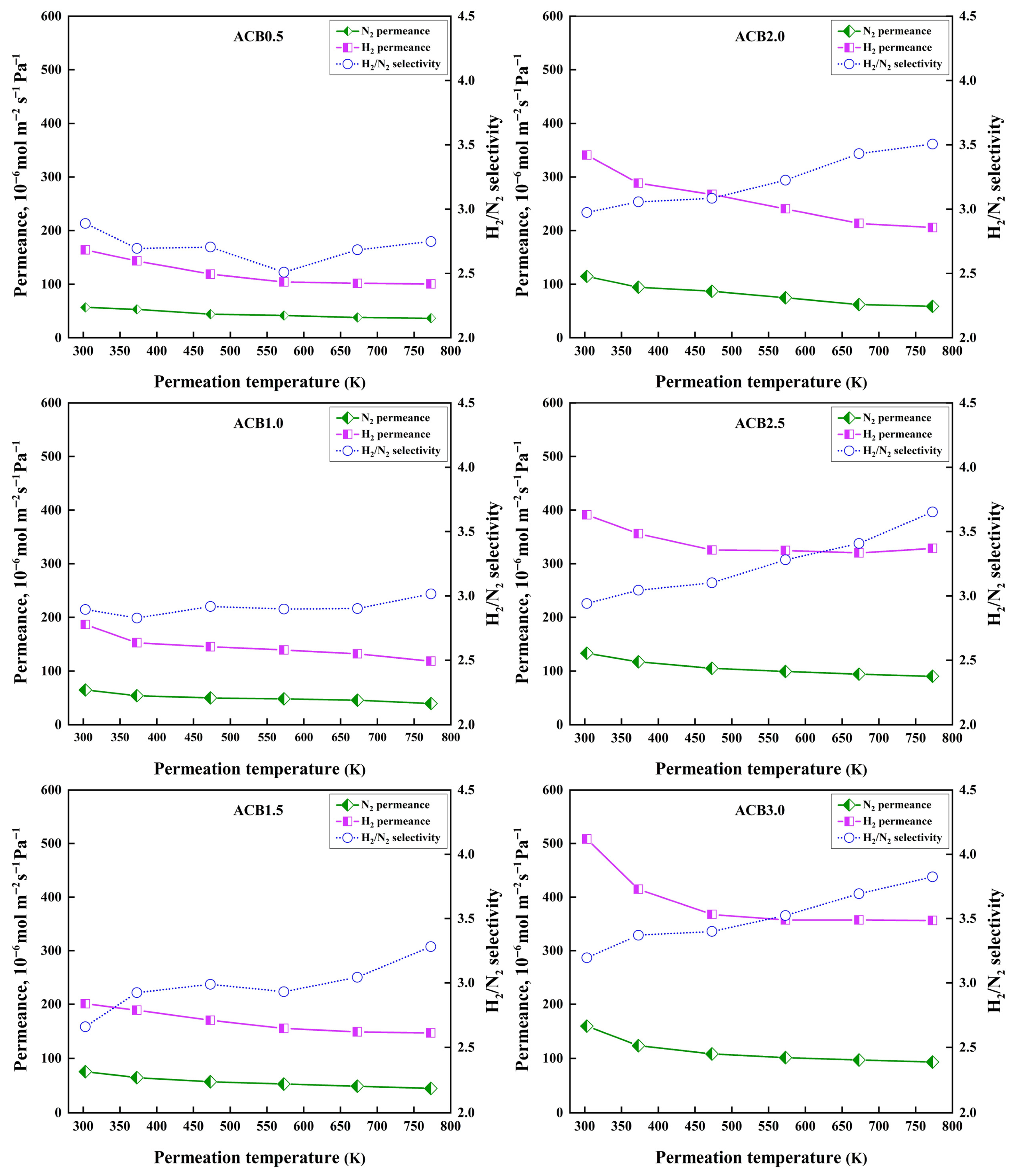
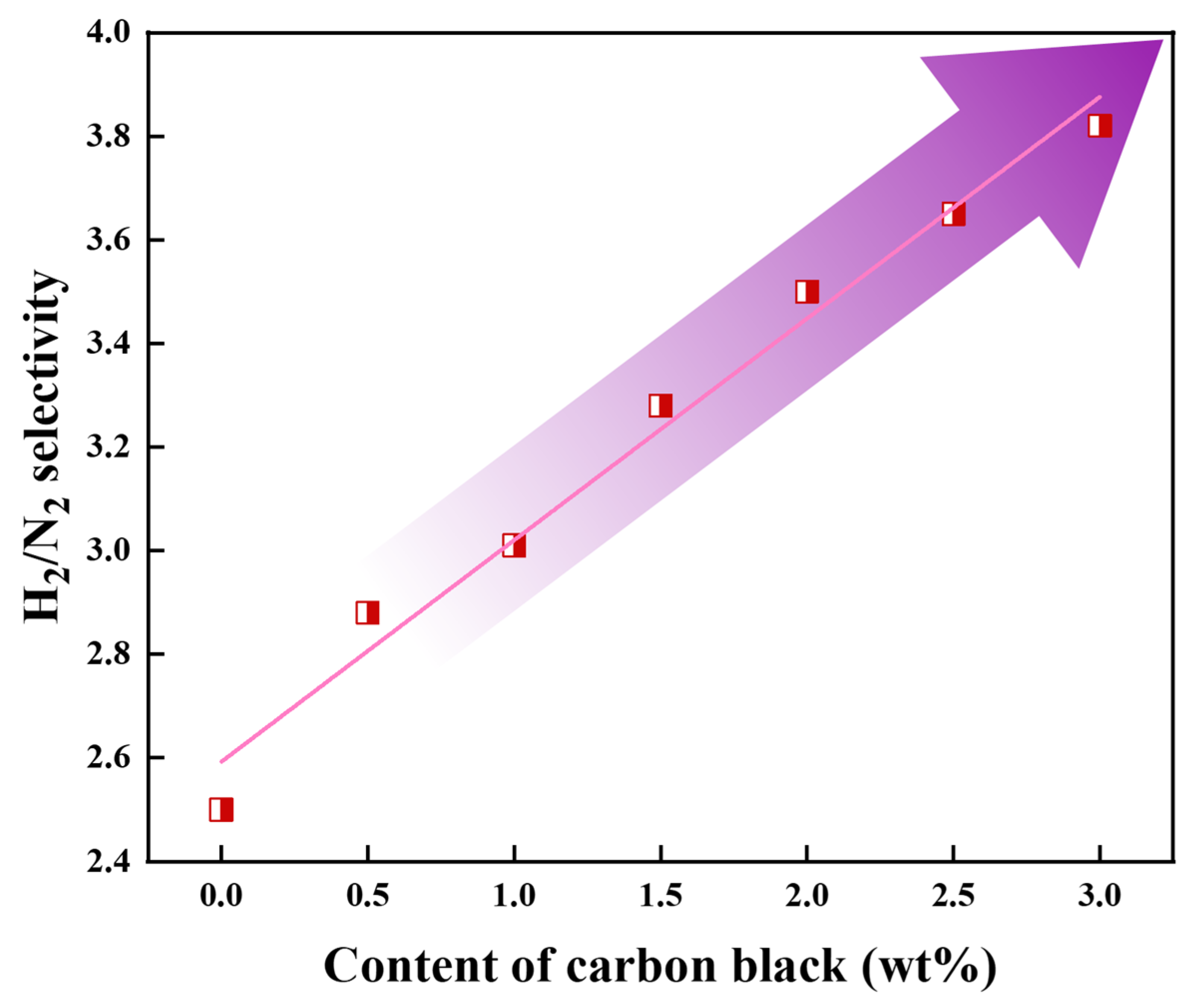
3.6. Gas Permeation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Olguin, G.; Hotza, D.; Seelro, M.A.; Fu, W.; Gao, Y.; Ji, G. Inorganic membranes for in-situ separation of hydrogen and enhancement of hydrogen production from thermochemical reactions. Renew. Sust. Energy Rev. 2022, 160, 112124. [Google Scholar] [CrossRef]
- Moliner, R.; Lázaro, M.J.; Suelves, I. Analysis of the strategies for bridging the gap towards the hydrogen economy. Int. J. Hydrogen Energy 2016, 41, 19500–19508. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The hydrogen economy—Vision or reality? Int. J. Hydrogen Energy 2015, 40, 7903–7919. [Google Scholar] [CrossRef]
- Assareh, E.; Delpisheh, M.; Baldinelli, A.; Cinti, G.; Emami, H.; Lee, M. Integration of geothermal-driven organic rankine cycle with a proton exchange membrane electrolyzer for the production of green hydrogen and electricity. Environ. Sci. Pollut. Res. 2023, 30, 54723–54741. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic membranes for hydrogen separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen production technologies: From fossil fuels toward renewable sources. A mini review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Karousos, D.S.; Qadir, D.; Sapalidis, A.A.; Ahmad, F.; Favvas, E.P. Polymeric, Metallic and carbon membranes for hydrogen separation: A review. Gas Sci. Eng. 2023, 120, 205167. [Google Scholar] [CrossRef]
- Chehade, G.; Lytle, S.; Ishaq, H.; Dincer, I. hydrogen production by microwave based plasma dissociation of water. Fuel 2020, 264, 11683. [Google Scholar] [CrossRef]
- Antonini, C.; Treyer, K.; Streb, A.; van der Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage—A techno-environmental analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar] [CrossRef]
- Koumi Ngoh, S.; Njomo, D. An overview of hydrogen gas production from solar energy. Renew. Sust. Energy Rev. 2012, 16, 6782–6792. [Google Scholar] [CrossRef]
- Soja, R.J.; Gusau, M.B.; Ismaila, U.; Garba, N.N. Comparative analysis of associated cost of nuclear hydrogen production using iaea hydrogen cost estimation program. Int. J. Hydrogen Energy 2023, 48, 23373–23386. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, X.; Wen, H.; Pei, A. Hydrogen production from offshore wind power in South China. Int. J. Hydrogy Energy 2022, 47, 24558–24568. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Gnanapragasam, N.V.; Reddy, B.V.; Rosen, M.A. Hydrogen production from coal gasification for effective downstream CO2 capture. Int. J. Hydrogen Energy 2010, 35, 4933–4943. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Shahabuddin, M. Production of green hydrogen from desalinated water using membranes: A review. Desalination 2025, 614, 119200. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Basset, J.M.; Zhou, L. Methane decomposition to pure hydrogen and carbon nano materials: State-of-the-art and future perspectives. Int. J. Hydrogen Energy 2020, 45, 15721–15743. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S. Production of High-purity hydrogen by sorption-enhanced steam reforming process of methanol. J. Energy Chem. 2015, 24, 315–321. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, S.; Bai, P.; Hu, H.; Jin, L. Adsorption separation performance of H2/CH4 on ETS-4 by concentration pulse chromatography. J. Energy Chem. 2014, 23, 213–220. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen membrane separation techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Salim, W.; Ho, W.S.W. Hydrogen purification with CO2-selective facilitated transport membranes. Curr. Opin. Chem. Eng. 2018, 21, 96–102. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, F.; Kong, J.; Wang, B.; Li, H.; Yang, Y.; Xing, P. Mechanical properties of geopolymer composite foams reinforced with carbon nanofibers via modified hydrogen peroxide method. Mater. Chem. Phys. 2020, 253, 123258. [Google Scholar] [CrossRef]
- De Araújo, M.J.G.; Villarroel-Rocha, J.; De Souza, V.C.; Sapag, K.; Pergher, S.B.C. Carbon foams from sucrose employing different metallic nitrates as blowing agents: Application in CO2 capture. J. Anal. Appl. Pyrolysis 2019, 141, 104627. [Google Scholar] [CrossRef]
- Kusdhany, M.I.M.; Ma, Z.; Mufundirwa, A.; Li, H.W.; Sasaki, K.; Hayashi, A.; Lyth, S.M. Hydrogen and carbon dioxide uptake on scalable and inexpensive microporous carbon foams. Microporous Mesoporous Mater. 2022, 343, 112141. [Google Scholar] [CrossRef]
- Shim, D.H.; Jung, S.S.; Kim, H.S.; Cho, H.; Kim, J.K.; Kim, T.G.; Yoon, S.J. Effect of carbon nanotubes on the properties of spark plasma sintered ZrO2/CNT composites. Arch. Metall. Mater. 2015, 60, 1315–1318. [Google Scholar] [CrossRef]
- He, J.; Wu, H.; Zhong, L.; Zhong, Q.; Yang, Q.; Ye, X.; Liu, Z.; Kang, Y. Mechanical properties and thermal conductivity of lightweight and high-strength carbon-graphite thermal insulation materials. J. Mater. Sci. 2022, 57, 4166–4179. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 27062–27087. [Google Scholar] [CrossRef]
- Hasan, I.U.; Othmen, A.B.; Onaizi, S.A. CO2 separation from gas mixtures using metal organic frameworks-based mixed matrix membranes: A comprehensive review. Gas. Sci. Eng. 2025, 138, 205604. [Google Scholar] [CrossRef]
- Silva, A.M.A.; Britto de Faria, A.C.; de Lima Ribeiro, C.; Athayde, D.D.; Paulo da Silva, E.; Magalhães dos Santos, G.; Fernando de Sousa Lima, L.; Nunes de Souza, R.; Pereira da Silva, S.L.; Mohallem, N.D.S. Review of zeolite membranes for natural gas processing and treatment: State of the art and future perspectives. Gas. Sci. Eng. 2023, 117, 205056. [Google Scholar] [CrossRef]
- Karim, S.S.; Ahsan, M.; Farrukh, S.; Fan, X.; Yu, Z. Multi-layer composite (MLC) membranes gas transport models and separation mechanisms. In Multi-Layer Composite (MLC) Membranes for Gas Separation; Lecture Notes in Energy; Springer: Cham, Switzerland, 2025; Volume 102, pp. 159–185. [Google Scholar] [CrossRef]
- Lee, N.R.; Lee, S.S.; Kim, K.I.; Kim, W.G.; Ju, H.; Kim, D.M.; Hong, T.W. Fabrications and evaluations of hydrogen permeation on Al2O3/CeO2/Graphene (ACG) composites membrane by Hot Press Sintering (HPS). Int. J. Hydrogen Energy 2013, 38, 7654–7658. [Google Scholar] [CrossRef]
- Terra, N.M.; Bessa, L.P.; Cardoso, V.L.; Reis, M.H.M. Graphite coating on alumina substrate for the fabrication of hydrogen selective membranes. Int. J. Hydrogen Energy 2018, 43, 1534–1544. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Y.; Wu, Y.; Wang, T.; Qiu, J. Preparation and Characterization of Supported Ordered Nanoporous Carbon Membranes for Gas Separation. J. Appl. Polym. Sci. 2014, 131, 39925. [Google Scholar] [CrossRef]
- Nie, J.; Okada, F.; Kita, H.; Tanaka, K.; Mihara, T.; Kondo, D.; Yamashita, Y.; Yahagi, N. Fabrication of carbon molecular sieve membranes supported on a novel porous carbon fiber. Energy Fuels 2022, 36, 7147–7157. [Google Scholar] [CrossRef]
- Yousef, S.; Tuckute, S.; Tonkonogovas, A.; Stankevičius, A.; Mohamed, A. Ultra-permeable cnts/pes membranes with a very low cnts content and high H2/N2 and CH4/N2 selectivity for clean energy extraction applications. J. Mater. Res. Technol 2021, 15, 5114–5127. [Google Scholar] [CrossRef]
- Molaei, M.J. Synthesis and application of carbon quantum dots derived from carbon black in bioimaging. J. Fluoresc. 2023, 34, 213–226. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Wen, J.; Mo, S.; Wang, J.; Zhang, Z.; Wang, G.; Liu, M.; Liu, H. Single-atom doping in carbon black nanomaterials for photothermal antibacterial applications. Cell. Rep. Phys. Sci. 2021, 2, 100535. [Google Scholar] [CrossRef]
- Kosinska, A.; Balakin, B.V.; Kosinski, P. Use of biodegradable colloids and carbon black nanofluids for solar energy applications. AIP Adv. 2021, 11, 055214. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Shirai, Y.; Okazaki, M.; Maruyama, K. A novel gas sensor from crystalline polymer-grafted carbon black: Responsibility of electric resistance of composite from crystalline polymer-grafted carbon black against solvent vapo. Polym. Bull. 1999, 42, 425–431. [Google Scholar] [CrossRef]
- Xie, H.; Yang, Q.; Sun, X.; Yang, J.; Huang, Y. Gas sensor arrays based on polymer-carbon black to detect organic vapors at low concentration. Sens. Actuators B Chem. 2006, 113, 887–891. [Google Scholar] [CrossRef]
- Grießl, D.; Huber, K.; Scherbauer, R.; Kwade, A. Dispersion kinetics of carbon black for the application in lithium-ion batteries. Adv. Powder Technol. 2021, 32, 2280–2288. [Google Scholar] [CrossRef]
- Gauthier, N.; Agrawal, A.; Dubrunfaut, O.; Franger, S.; Lestriez, B.; Badot, J.C.; Assaud, L. Carbon black/electrolyte interface study by electromagnetic and electrochemical techniques in γAl2O3/poly(vinylidene fluoride)/carbon black composite materials: Application for lithium-ion batteries. Electrochim. Acta 2023, 437, 141495. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of carbon black reinforcement of rubber: Perspective on the original polymer nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.W.; Zheng, Q.R. Methane adsorption on the graphene sheets, activated carbon and carbon black. Appl. Therm. Eng. 2016, 108, 605–613. [Google Scholar] [CrossRef]
- Youssry, M.; Madec, L.; Soudan, P.; Cerbelaud, M.; Guyomard, D.; Lestriez, B. Non-Aqueous carbon black suspensions for lithium-based redox flow batteries: Rheology and simultaneous rheo-electrical behavior. Phys. Chem. Chem. Phys. 2013, 15, 14476–14486. [Google Scholar] [CrossRef]
- Çelik, A.; Çağlar, G.; Çelik, Y. Fabrication of porous Al2O3 ceramics using carbon black as a pore forming agent by spark plasma sintering. Ceram. Int. 2022, 48, 28181–28190. [Google Scholar] [CrossRef]
- Manisa, V.K.; Porter, M.; Whiting, M.J.; Dorey, R.A. Effect of processing on the stability and electrical properties of pressureless sintered graphene oxide–alumina composites. Ceram. Int. 2022, 48, 15839–15847. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Xu, Q.; Yang, M.; Zhang, S.; Goto, T.; Tu, R. Effect of TZP nanoparticles synthesized by RCVD on mechanical properties of ZTA composites sintered by SPS. J. Eur. Ceram. Soc. 2022, 42, 3550–3558. [Google Scholar] [CrossRef]
- Kageyama, N.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Synthesis and Characterization of a silica-alumina composite membrane and its application in a membrane reactor. Sep. Purif. Technol. 2018, 195, 437–445. [Google Scholar] [CrossRef]
- Singh, M.; Vander Wal, R. Nanostructure quantification of carbon blacks. C-J. Carbon Res. 2018, 5, 2. [Google Scholar] [CrossRef]
- Ostyn, N.R.; Steele, J.A.; De Prins, M.; Sree, S.P.; Chandran, C.V.; Wangermez, W.; Vanbutsele, G.; Seo, J.W.; Roeffaers, M.B.J.; Breynaert, E.; et al. Low-temperature activation of carbon black by selective photocatalytic oxidation. Nanoscale Adv. 2019, 1, 2873–2880. [Google Scholar] [CrossRef]
- Pawlyta, M.; Rouzaud, J.N.; Duber, S. Raman microspectroscopy characterization of carbon blacks: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Roh, J.S. Analysis of activation process of carbon black based on structural parameters obtained by XRD analysis. Crystals 2021, 11, 153. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Alahmari, T.S.; Laoui, T.; Hakeem, A.S.; Patel, F. Mechanical and thermal evaluation of aluminum hybrid nanocomposite reinforced with alumina and graphene oxide. Nanomaterials 2021, 11, 1225. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, R.; Bao, C.; Mu, M.; Wang, K. Fabrication of alumina ceramics with high flexural strength using stereolithography. Int. J. Adv. Manuf. Technol. 2023, 128, 2983–2994. [Google Scholar] [CrossRef]
- Rivero-Antúnez, P.; Zamora-Ledezma, C.; Sánchez-Bajo, F.; Moreno-López, J.C.; Anglaret, E.; Morales-Flórez, V. Sol–gel method and reactive SPS for novel alumina–graphene ceramic composites. J. Eur. Ceram. Soc. 2023, 43, 1064–1077. [Google Scholar] [CrossRef]
- Lei, L.; Pan, F.; Lindbråthen, A.; Zhang, X.; Hillestad, M.; Nie, Y.; Bai, L.; He, X.; Guiver, M.D. Carbon hollow fiber membranes for a molecular sieve with precise-cutoff ultramicropores for superior hydrogen separation. Nat. Commun. 2021, 12, 268. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.H.; Macquarrie, D.J. Tuneable Porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef]
- Wu, C.C.; Rice, R.W. Porosity dependence of wear and other mechanical properties on fine-grain Al2O3 and B4C. Ceram. Eng. Sci. Proc. 1985, 6, 977. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, L.; Liu, L.; Ding, Z. Zeolitic imidazolate framework membranes with a high h2 permeance fabricated on a macroporous support with novel spherical porous hybrid materials. Ind. Eng. Chem. Res. 2021, 60, 1387–1395. [Google Scholar] [CrossRef]
- Nian, P.; Cao, Y.; Li, Y.; Zhang, X.; Wang, Y.; Liu, H.; Zhang, X. Preparation of a pure zif-67 membrane by self-conversion of cobalt carbonate hydroxide nanowires for H2 separation. CrystEngComm 2018, 20, 2440–2448. [Google Scholar] [CrossRef]
- Yu, C.Y.; Sea, B.K.; Lee, D.W.; Park, S.J.; Lee, K.Y.; Lee, K.H. effect of nickel deposition on hydrogen permeation behavior of mesoporous γ-alumina composite membranes. J. Colloid Interface Sci. 2008, 319, 470–476. [Google Scholar] [CrossRef]
- Lee, H.K.; Suda, H.; Haraya, K. Gas permeation properties in a composite mesoporous alumina ceramic membrane. Kor. J. Chem. Eng. 2005, 22, 721–728. [Google Scholar] [CrossRef]
- Briceño, K.; Iulianelli, A.; Montané, D.; Garcia-Valls, R.; Basile, A. Carbon molecular sieve membranes supported on non-modified ceramic tubes for hydrogen separation in membrane reactors. Int. J. Hydrogen Energy 2012, 37, 13536–13544. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/matrimid® mixed-matrix membranes. J. Memb. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Hynek, S.; Fuller, W.; Bentley, J. Hydrogen storage by carbon sorption. Int. J. Hydrogen Energy 1997, 22, 601–610. [Google Scholar] [CrossRef]
- Kaneko, T.; Toyama, T.; Kojima, Y.; Nishimiya, N. temperature dependence of hydrogen adsorption on Pd-modified carbon blacks and their enthalpy-entropy changes. C-J. Carbon Res. 2022, 8, 16. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Roth, S. Hydrogen adsorption in different carbon nanostructures. Carbon 2005, 43, 2209–2214. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Liu, Z.; Wan, H.L. Microwave-heating synthesis and gas separation performance of NaA zeolite membrane. Chin. J. Chem. 2005, 23, 28–31. [Google Scholar] [CrossRef]
- Zeynali, R.; Ghasemzadeh, K.; Iulianelli, A.; Basile, A. Experimental evaluation of graphene oxide/TiO2-alumina nanocomposite membranes performance for hydrogen separation. Int. J. Hydrogen Energy 2020, 45, 7479–7487. [Google Scholar] [CrossRef]
- Kumar, R.; Kamakshi; Kumar, M.; Awasthi, K. Functionalized Pd-decorated and aligned MWCNTs in polycarbonate as a selective membrane for hydrogen separation. Int. J. Hydrogen Energy 2016, 41, 23057–23066. [Google Scholar] [CrossRef]
- Ribeiro, S.R.F.L.; Bessa, L.P.; Cardoso, V.L.; Reis, M.H.M. Enhanced hydrogen permeance through graphene oxide membrane deposited on asymmetric spinel hollow fiber substrate. Int. J. Hydrogen Energy 2022, 47, 9616–9626. [Google Scholar] [CrossRef]

| Membranes | H2 Permeance (× 10−6 mol m−2 s−1 Pa−1) | H2/N2 Selectivity | References |
|---|---|---|---|
| TiO2-ZrO2/carbon molecular sieve-tubular | 6.7–8.2 | 3–3.5 | [65] |
| TiO2-Al2O3/graphene oxide-tubular | 0.30 | 9.00 | [71] |
| Polycarbonate/Pd-carbon nanotubes-flat | 0.49 | 4.19 | [72] |
| MgAl2O4/graphene oxide-hollow fiber | 0.82 | 3.30 | [73] |
| Al2O3 carbon black membrane (ACB3.0) | 356 | 3.82 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hankoy, M.; Rodchom, M.; Vichaphund, S.; Atong, D.; Zhang, J.; Kitiwan, M.; Tunthawiroon, P. Modification of Al2O3-Based Membranes with Carbon Black for Enhanced Hydrogen Permeation. Technologies 2025, 13, 491. https://doi.org/10.3390/technologies13110491
Hankoy M, Rodchom M, Vichaphund S, Atong D, Zhang J, Kitiwan M, Tunthawiroon P. Modification of Al2O3-Based Membranes with Carbon Black for Enhanced Hydrogen Permeation. Technologies. 2025; 13(11):491. https://doi.org/10.3390/technologies13110491
Chicago/Turabian StyleHankoy, Montree, Mana Rodchom, Supawan Vichaphund, Duangduen Atong, Jianfeng Zhang, Mettaya Kitiwan, and Phacharaphon Tunthawiroon. 2025. "Modification of Al2O3-Based Membranes with Carbon Black for Enhanced Hydrogen Permeation" Technologies 13, no. 11: 491. https://doi.org/10.3390/technologies13110491
APA StyleHankoy, M., Rodchom, M., Vichaphund, S., Atong, D., Zhang, J., Kitiwan, M., & Tunthawiroon, P. (2025). Modification of Al2O3-Based Membranes with Carbon Black for Enhanced Hydrogen Permeation. Technologies, 13(11), 491. https://doi.org/10.3390/technologies13110491






