Abstract
This study investigates the use of multi-field electrostimulation with the Fesia Grasp device for hand rehabilitation in patients with Multiple Sclerosis (MS). This research aims to evaluate the effectiveness of this novel approach in improving hand function and dexterity in MS patients. A cohort of MS patients with varying degrees of hand impairment underwent a structured rehabilitation program using the Fesia Grasp device, which delivers targeted electrical stimulation to specific muscle groups. Outcome measures assessed multiple aspects of hand function, including gross and fine motor skills, strength, and functional independence, at baseline, post-intervention, and 1-month follow-up. The main finding was a sustained between-group improvement in gross manual dexterity, measured by the Box and Block Test, at 1-month follow-up (p = 0.008, η2ₚ = 0.429). Secondary analyses showed task-specific gains in the experimental group, with significant intragroup improvements in Jebsen–Taylor Hand Function Test items related to simulated feeding (p = 0.012) and lifting light objects (p = 0.036), and a trend toward better performance in stacking checkers (p = 0.069) and faster page-turning (p = 0.046) after the intervention. Other outcomes showed non-significant changes favoring the experimental group. This research contributes to the growing body of evidence supporting the use of advanced electrostimulation techniques in neurological rehabilitation and offers promising implications for enhancing the quality of life for individuals with MS-related hand dysfunction.
1. Introduction
Multiple sclerosis (MS) is the most prevalent demyelinating disease of the central nervous system (CNS) and currently stands as the leading cause of non-traumatic neurological disability among young adults in developed countries [1]. It is an autoimmune, inflammatory, and neurodegenerative disorder in which the immune system attacks the myelin—the lipid-rich sheath that insulates axons—impairing efficient neural signal conduction. Demyelination and axonal degeneration lead to progressive and often irreversible neurological impairment [2].
Globally, MS affects approximately 2.5 million individuals, with an estimated 68,577 cases in Spain. The disease shows a marked female predominance, with over twice as many women affected compared to men [3]. Clinical manifestations are highly variable depending on the location and extent of CNS lesions. MS is classified into four main clinical courses: clinically isolated syndrome, relapsing–remitting MS (RRMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS), with RRMS being the most frequent initial presentation [4].
Common symptoms include sensory disturbances, motor deficits, cerebellar signs, cognitive dysfunction, bladder or sexual dysfunction, and pain. Notably, upper limb impairment—particularly affecting hand function—is frequent, with patients experiencing paresthesia, spasticity, muscle weakness, tremor, and coordination issues [5]. These symptoms have a significant impact on hand function, daily activities, and overall quality of life [5].
Despite its relevance, rehabilitation of the upper limb, especially the hand, has traditionally received less attention compared to other motor functions. Therapeutic approaches aim to preserve and enhance hand function by addressing strength, fine and gross motor skills, coordination, and motor imagery [6].
Neurorehabilitation has evolved into a multidisciplinary field, integrating emerging technologies to complement conventional therapies such as mirror therapy, cognitive stimulation, neurodynamic, and task-specific training [7]. Among these technologies, functional electrical stimulation (FES) has gained prominence since the 1980s as a therapeutic modality for neurological conditions [8,9]. FES involves the application of electrical impulses to specific muscle groups to induce functional contractions and promote movement.
Initially used for correcting foot drops in post-stroke patients by stimulating the ankle dorsiflexors, FES has significantly advanced with the introduction of multi-field electrodes that allow more selective and precise stimulation. Within this context, the Fesia Grasp device represents a significant technological advancement. It consists of a stimulator with a multi-field electrode capable of generating up to ten isolated flexion and extension movements in the wrist, thumb, and fingers, thus enhancing targeted muscle activation and improving motor control [10,11].
Several studies support the effectiveness of the Fesia Grasp in stroke patients, demonstrating improvements in muscle strength, coordination, and upper limb function [12,13]. However, there is a notable lack of scientific evidence regarding its use in people with MS. This research gap underlines the importance of evaluating its efficacy in this population. Therefore, the main objective of this study is to evaluate the impact of the Fesia Grasp device on hand functionality in people with multiple sclerosis, focusing on variables such as manual dexterity, grip strength, motor imagery, and functional task performance.
2. Materials and Methods
2.1. Study Design
This is a randomized controlled trial (RCT) with parallel groups and a longitudinal design, conducted at the Faculty of Health Sciences of the University of Burgos. The study was a single center, with blind assessments performed by evaluators who were not involved in recruitment, randomization, or intervention. Participants were randomly assigned into two parallel groups (experimental and control), using stratified randomization by sex with the RAND function in Microsoft Excel.
2.2. Participants
Participants were recruited via collaboration with the Burgos Multiple Sclerosis Association (AFAEM), university networks, and social media. Inclusion criteria: (1) clinical diagnosis of MS with upper limb involvement (hand/wrist) and a Quick DASH score < 100; (2) able to complete the Nine-Hole Peg Test (NHPT); (3) intact skin on the arm used for stimulation; (4) EDSS score between 0–8; (5) age ≥ 18 years; (6) signed informed consent. Exclusion criteria: relapse phase; other neurological comorbidities (e.g., stroke, cognitive impairment, neglect); recent botulinum toxin injection; presence of pacemakers or metallic implants; epilepsy; active tumors or topical lesions; pregnancy; or participation in other studies. Participants could withdraw voluntarily, due to adverse events, non-compliance, or failure to complete the planned sessions. Reasons for withdrawal were documented.
2.3. Intervention
The experimental group underwent 12 sessions (3/week for 4 weeks, 30 min/session) with the Fesia Grasp device on the affected arm (or the less impaired arm when function was severely limited). Sessions followed two protocols:
- Habituation protocol (first 10 min of session 1): Gradual adaptation to electrical stimulation.
- Task-oriented training (sessions 1–12): After configuring the electrode fields for wrist, thumb, and finger movements, participants performed repetitive tasks (e.g., open/close hand, wrist extension, index flexion) followed by functional tasks (e.g., grasping objects, squeezing a ball, pinch precision). Training was adapted to individual needs and progressively intensified.
The control group maintained their usual daily routines without Fesia Grasp intervention, which included continuing their standard care such as occupational therapy, physical therapy, or both, according to individual needs.
2.4. Procedure
The study included three phases: (1) preparation (ethical approval, participant recruitment); (2) data collection (pre-test, intervention, post-test, 1-month follow-up) with blinded outcome assessments conducted by evaluators not involved in recruitment, randomisation, or intervention; and (3) data analysis and reporting. The procedure followed the previously published protocol [14], ensuring consistency and methodological rigor throughout all phases of the study (Figure 1).
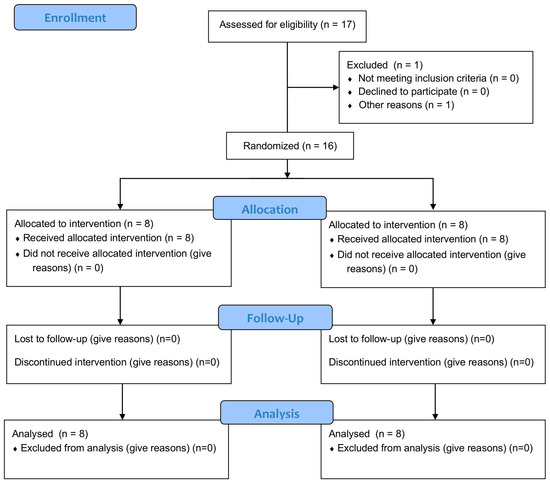
Figure 1.
Flow diagram.
2.5. Outcome Measures
Validated tools were used to assess hand function comprehensively:
- Jebsen–Taylor Hand Function Test (JTHFT) [15]: Evaluates hand function through seven standardized, timed subtests simulating daily activities, including writing a sentence (item 1), turning over cards (item 2), picking up small common objects (item 3), stacking checkers (item 4), simulated feeding (item 5), and moving light (item 6) and heavy objects (item 7). Lower total times indicate better hand function. It demonstrates high internal consistency (Cronbach’s alpha = 0.96 for the dominant hand, 0.92 for the non-dominant hand).
- Nine-Hole Peg Test (NHPT) [16]: Assesses fine motor dexterity by measuring the time required to place and remove nine pegs from corresponding holes. Shorter completion times indicate better manual dexterity. The test has demonstrated high inter-rater and test–retest reliability (r = 0.86–0.98) [17].
- Box and Block Test (BBT) [18]: Measures gross manual dexterity by counting the number of wooden blocks (2.5 cm) transferred from one compartment of a divided box to another within one minute. Higher scores reflect better gross motor skills. Test–retest reliability is high (intraclass correlation coefficients ranging from 0.89 to 0.97) [19].
- Baseline Pinch Gauge: Assesses grip and pinch strength by converting the force applied into a numeric reading in kilograms. It offers excellent test–retest reliability, with intraclass correlation coefficients above 0.90 [15].
- Functional Independence Measure (FIM) [20]: Evaluates the level of functional independence in individuals across 18 items, rated on a 7-point scale (1 = total assistance, 7 = complete independence), with total scores ranging from 18 to 126. The scale includes two subdomains: motor (13 items) and cognitive (5 items). Higher scores indicate greater independence. It has strong internal consistency (Cronbach’s alpha = 0.93) [21].
All evaluations were conducted at three time points: baseline, post-intervention, and 1-month follow-up. Data was collected using a standardized sheet that included demographic, clinical, and observational data.
2.6. Statistical Analysis
Analyses were performed using IBM SPSS Statistics v28 [22]. Descriptive statistics were used to summarize sociodemographic and clinical variables. To assess distributional assumptions, normality was evaluated with the Shapiro–Wilk test, and homogeneity of variances with Levene’s test. For variables that met the assumption of normality, parametric tests were applied; otherwise, non-parametric tests were used.
Between-group comparisons at baseline were conducted with independent samples Student’s t-tests (for normally distributed variables) or Mann–Whitney U tests (for non-normal distributions). For repeated measures, within-group changes were analyzed using paired samples t-tests (parametric) or Wilcoxon signed-rank tests (non-parametric). Between-group differences in progression (difference scores post–pre and follow-up–post) were evaluated using one-way ANOVAs for parametric variables and Kruskal–Wallis tests for non-parametric variables. When baseline differences were detected, ANCOVA was performed with the pretest score as a covariate.
The analysis followed a per-protocol approach. All participants completed the intervention and assessments; therefore, no withdrawals or missing data required imputation methods. Effect sizes were reported as partial eta squared (η2ₚ) for ANOVA/ANCOVA. Given the exploratory nature of the study and the small sample size, no corrections for multiple comparisons were applied. The significance threshold was set at p < 0.05.
As a sensitivity analysis, results were cross-checked with non-parametric tests when distributional assumptions were not met, and the overall interpretation of findings remained consistent.
2.7. Ethical Considerations
The study was approved by the Ethics Committee of the University of Burgos (IO 6/2024). Informed consent was obtained from all participants prior to. The trial was registered at ClinicalTrials.gov (ID: NCT06638775).
3. Results
3.1. Descriptive Analysis
The sample consisted of 16 individuals diagnosed with MS, evenly distributed between a Control Group (CG) and an Experimental Group (EG), with 8 participants in each. The mean age was 51.62 years (SD = 11.36). Of the participants, 56.3% were women. Regarding the type of MS, the primary progressive form was predominant in the CG (50%), while the secondary progressive form was more common in the EG (62.5%). Most participants did not consume alcohol or tobacco and engaged in weekly physical activity. The right hand was the operated hand in 81.3% of cases. All participants were right-handed prior to disease onset, and the right hand was the operated hand in 81.3% of cases. The mean disease duration since diagnosis was 12 years (SD = 8.28; range 1–30 years).
From a functional standpoint, the pretest assessment showed moderate impairment in manual dexterity and pinch strength. In the JTHFT, times were elevated, especially in the writing task. Pinch strength was low (M = 4.81 kg, SD = 2.67) and performance on the NHPT reflected slow execution (M = 41,534 ms, SD = 17,405.92). In BBT, the mean number of blocks transferred was 38 (SD = 10.30). The score on the FIM scale indicated partial autonomy (M = 104.69, SD = 18.52). The complete sociodemographic and clinical characteristics are presented in Table 1.

Table 1.
Sociodemographic and clinical characteristics of the participants by group (CG and EG).
3.2. Normality Tests
The normality of the variables was evaluated using the Shapiro–Wilk test. The results indicated that most items in the JTHFT did not follow a normal distribution, except for item 2, which showed normality in the pretest (p = 0.446), posttest (p = 0.154), and follow-up (p = 0.184) and for the total JTHFT score (pretest p = 0.157; posttest p = 0.058; follow-up p = 0.013), which was considered acceptable for parametric tests, given the moderate sample size and the robustness of parametric methods to mild deviations from normality. Non-parametric analyses were chosen for items 1, 3, 4, 5, 6, and 7. The following results were observed for the remaining clinical variables: pinch strength was not normal in the pretest (p = 0.004), but was normal in the posttest (p = 0.729) and follow-up (p = 0.152), so parametric analyses were applied; NHPT were normal in the pretest (p = 0.101), but not in the posttest (p = 0.036) or the follow-up (p = 0.014), although it was considered valid for parametric analysis because skewness and kurtosis remained within acceptable levels (±2) [23]; FIM met normality in all its measurements—pretest (p = 0.952), posttest (p = 0.313), and follow-up (p = 0.834); BBT also showed a normal distribution in the pretest (p = 0.151), posttest (p = 0.621), and follow-up (p = 0.872); finally, (Appendix A).
3.3. Baseline Analysis
Baseline comparability between groups was assessed for demographic and clinical characteristics, including age, disease duration, MS subtype, and dominant hand. To verify the equivalence between the CG and the EG prior to the intervention, independent samples Student’s t-tests were applied to the variables that met the normality assumption. The NHPT was the only one that showed differences, with the EG being faster than the CG in the pretest (CG: M = 50.37, SD = 19.86; EG: M = 32.69, SD = 8.73). To minimize the effect of this difference, ANCOVA-type analyses were conducted (Table 2). For the JTHFT variables (items 1, 3, 4, 5, 6, and 7) that did not meet the normality assumption, Mann–Whitney U tests were performed, with no significant differences found between groups: item 1 (p = 0.574), item 3 (p = 0.505), item 4 (p = 0.878), item 5 (p = 0.442), item 6 (p = 0.878), and item 7 (p = 1.000).

Table 2.
Intergroup comparisons in the pretest evaluation for clinical and functional variables with normal distribution.
3.4. Inferential Between-Subjects Analysis
To analyze the differences in progression between the EG and CG, between-subject analyses (one-way ANOVA and ANCOVA for NHPT) were performed on the difference scores for posttest–pretest (DS1) and follow-up–posttest (DS2) (Table 3). In JTHFT item 2, no significant differences were found between groups in either DS1 (F(1,14) = 0.413, p = 0.531, η2ₚ = 0.029) or DS2 (F(1,14) = 0.413, p = 0.531, η2ₚ = 0.029). For pinch strength, the EG improved more than the CG in DS1 (1.17 vs. 0.26), but this was not significant (F(1,14) = 1.437, p = 0.251, η2ₚ = 0.093), and no differences were observed in DS2 (F(1,14) = 0.039, p = 0.846, η2ₚ = 0.003). In terms of functionality (FIM), the EG showed improvement (DS1: 1.63; DS2: 1.63) compared to a decline in the CG (DS1: −6.38; DS2: −4.71), though significance was not reached in any case (DS1: F(1,14) = 1.501, p = 0.241, η2ₚ = 0.097; DS2: F(1,14) = 1.627, p = 0.223, η2ₚ = 0.104). For BBT, the EG showed improvement while the CG worsened in DS1 (5.75 vs. −1.38), but this did not reach significance (F(1,14) = 2.909, p = 0.110, η2ₚ = 0.172); however, significant differences were found in DS2 (F(1,13) = 9.774, p = 0.008, η2ₚ = 0.429), indicating sustained improvement in the EG compared to a slight decline in the CG. In the total score of the JTHFT, the EG showed improvements in both comparisons (DS1: −13,710 ms; DS2: −21,090 ms), but these did not reach significance (DS1: F(1,14) = 1.980, p = 0.181, η2ₚ = 0.124; DS2: F(1,13) = 1.566, p = 0.234, η2ₚ = 0.107). Finally, for NHPT, an analysis of covariance was applied using the pretest as a covariate, without finding significant differences between groups (DS1: F(1,13) = 1.168, p = 0.299, η2ₚ = 0.082; DS2: F(1,12) = 3.216, p = 0.098, η2ₚ = 0.379). The covariate was not significant in any case (DS1: F(1,13) = 0.926, p = 0.354). In summary, only the BBT showed a statistically significant effect at follow-up, indicating a sustained benefit in the experimental group (p = 0.008).

Table 3.
Intersubject comparisons of the differences in progression between groups (post-pre and follow-up-post).
To compare the groups on the items of the JTHFT, non-parametric analyses (Kruskal–Wallis tests) were conducted due to the lack of normality (Table 4). In the differential scores DS1 (posttest–pretest), no significant differences were found between groups in items 1, 3, 4, 5, and 7 (p = 0.462, 0.674, 0.248, 0.600, and 0.141, respectively), while item 6 did show a significant difference (p = 0.036), indicating a specific improvement in the experimental group. In DS2 (follow-up–post), no significant differences were found in any of the items (p between 0.152 and 0.867). Overall, the results indicate that only item 6 in DS1 reflected a differential effect of intervention between groups.

Table 4.
Between-group comparison of difference scores on the Jebsen–Taylor test items (Kruskal–Wallis test).
3.5. Intragroup Analysis
In the intragroup analysis conducted using paired samples t-tests, significant differences were observed only in the experimental group (Table 5). Specifically, for the variable “page-turning time (JTHFT Item 2),” participants in the experimental group showed a significant improvement after the intervention, with a mean difference of 2332.5 milliseconds (t = 2.419; df = 7; p = 0.046). The other variables did not reach statistical significance: pinch strength (t = −1.880; p = 0.102), NHPT (t = 0.280; p = 0.787), BBT (t = −1.627; p = 0.148), and level of functionality (FIM) (t = −0.634; p = 0.546). In the CG, no significant differences were found in any of the variables, with the following results highlighted: page-turning time (JTHFT Item 2) (t = 0.751; p = 0.477), pinch strength (t = −0.589; p = 0.574), NHPT (t = −0.619; p = 0.556), BBT (t = 0.617; p = 0.557), and FIM (t = 1.061; p = 0.324). These data indicate that the positive effects of the intervention are mainly evident in the experimental group and, more specifically, in the improvement of page-turning time (JTHFT Item 2).

Table 5.
Within-group comparison of clinical and functional variables (paired-samples t-tests).
With regard to the items of the JTHFT (Wilcoxon analysis), (Table 6) the EG showed significant improvements in items 4 (simulated feeding, p = 0.012) and 6 (lifting large/light objects, p = 0.036), and a trend toward improvement in item 5 (stacking checkers, p = 0.069), whereas the CG did not show significant differences in any of the items (all p > 0.20). Overall, the intragroup results reflect positive effects of the intervention primarily in the EG, with significant improvements in specific functional tasks.

Table 6.
Wilcoxon analysis.
4. Discussion
This randomized controlled pilot study investigated the effects of a 4-week, task-oriented intervention using a multi-field electrode-based FES device on hand function in people with MS. Participants were randomly assigned to an EG, receiving the FES-enhanced training, or to a CG continuing with usual care. The intervention focused on functional, goal-directed tasks designed to improve manual dexterity through repetitive, task-specific stimulation. Outcome measures were selected to assess multiple aspects of hand function (including gross and fine motor skills, strength, and functional independence) and were evaluated at baseline, post-intervention, and at 1-month follow-up to examine both immediate effects and short-term retention. The main finding was a sustained between-group improvement in gross manual dexterity, measured by the BBT, at 1-month follow-up. Secondary analyses showed task-specific gains in the EG, with significant intragroup improvements in JTHFT items related to simulated feeding and lifting light and large objects, a trend toward better performance in stacking checkers, and a decrease in page-turning time after the intervention. Other outcomes (including pinch strength, NHPT, total JTHFT score, and FIM) showed non-significant changes favoring the EG in several comparisons but did not reach statistical significance.
The significant and sustained improvement in BBT observed in our cohort suggests a clinically meaningful enhancement of gross manual dexterity that persisted at least one-month post-treatment. The moderate-large effect size for BBT (η2ₚ = 0.429) supports the clinical relevance of this between-group difference despite the small sample. The pattern of task-specific gains on several JTHFT subtests (simulated feeding, lifting large/light objects and faster page-turning) could indicate that the intervention preferentially benefited functional tasks that rely on coordinated gross grasp–release rather than on rapid, fine peg manipulation; this is consistent with the greater sensitivity of the BBT to changes in gross dexterity [18] compared with measures of fine dexterity such as the NHPT [24]. Short-term increases in pinch strength and trends in FIM favored the experimental group but did not reach statistical significance, and the baseline imbalance in NHPT performance (EG faster at baseline) together with modest sample size, mean the study was likely underpowered to detect more subtle effects.
Compared with other neurological conditions, the clinical literature on FES in people with MS is comparatively limited. Systematic and narrative reviews highlight a much larger evidence base for FES applied to stroke and spinal cord injury than to MS [25,26]. In MS specifically, the majority of published FES research has focused on lower-limb applications (footdrop and gait [27]), whereas controlled studies addressing upper-limb rehabilitation are relatively scarce [28]. Moreover, when attention is restricted to multi-field electrode-based FES systems, the published literature is even more limited: only a few studies in any neurological population have gone beyond proof-of-concept or technical evaluations. Most available reports focus on aspects such as stimulation parameters [29,30], movement generation [10], or device usability [31], with only isolated clinical trials in other pathologies [32] and, to date, no peer-reviewed randomized clinical trials in people with MS.
Despite the limited number of trials, the available MS literature points to meaningful benefits of FES for selected outcomes. Notably, studies of FES for gait in MS have reported improvements in walking speed [27], reductions in the physiological cost of walking [33] or improvements in the central fatigue [34], illustrating that FES can positively affect fatigue-related and energy expenditure outcomes in this population.
Multi-field electrode technologies, which allow sequential or spatially distributed activation of several small pads over a muscle group, offer some clinical advantages that could be especially relevant for people with MS. Experimental and clinical works have shown that spatially distributed/sequential stimulation strategies can delay the onset of electrically induced muscle fatigue [35] and may reduce stimulation discomfort [36] compared with conventional single-electrode approaches. These properties could be particularly beneficial in MS, where central and peripheral fatigue and altered somatosensation are common [37]; multi-field stimulation may therefore both prolong effective stimulation during therapy and improve tolerability/sensory experience, potentially enhancing adherence and functional gains.
Strengths of this study include its rigorous randomized controlled design, which reduces bias and enhances the internal validity of the findings. Additionally, outcome assessments were conducted in a blind manner, minimizing the risk of assessor-related bias. The study employed validated, multidimensional outcome measures that comprehensively covered multiple domains relevant to patients’ functional status, including fine and gross dexterity, muscle strength, and overall functional independence. This thorough evaluation approach strengthens the relevance and applicability of the results. Moreover, the inclusion of a 1-month follow-up period allowed for the assessment of short-term retention of treatment effects, providing insight into the persistence of benefits beyond the immediate post-intervention phase.
Nonetheless, several important limitations should be acknowledged when interpreting the findings. First, the small sample size (n = 16) inherently limits the statistical power of the study, increasing the likelihood of type II errors, whereby true effects may go undetected. This is reflected in several measures showing moderate effect sizes that did not reach statistical significance, suggesting potential underestimation of intervention effects. Second, the study sample was heterogeneous with respect to MS subtype and baseline functional performance, factors that may have influenced individual responsiveness to the intervention despite statistical adjustment using ANCOVA. Such variability could have introduced confounding effects that complicate the interpretation of results. Third, the control group continued with usual care and did not receive a shame or attention matched intervention, which raises concerns about non-specific factors such as placebo effects, participant expectations, or differential attention influencing outcomes. Fourth, the study was conducted at a single center and involved a relatively short follow-up duration, which constrains the external validity and limits the ability to draw conclusions about the long-term maintenance of treatment gains. Fourth, the study was conducted at a single center and involved a relatively short follow-up duration, which constrains the external validity and limits the ability to draw conclusions about the long-term maintenance of treatment gains. Furthermore, another limitation is the absence of detailed information on disease severity (e.g., EDSS scores), which could act as a potential confounder. Future studies should include these clinical variables to better account for baseline heterogeneity and ensure group comparability. Finally, the use of multiple outcome measures and various subtests increases the risk of type I error, whereby statistically significant findings might occur by chance. Therefore, any significant results should be interpreted with caution and ideally confirmed in larger, multi-center trials.
5. Conclusions
A 4-week program of task-oriented training using multi-field FES led to specific improvements in hand function in people with MS, with a statistically significant and sustained gain in gross manual dexterity observed at 1-month follow-up. These preliminary findings suggest that FES-based interventions may be a valuable complement to conventional upper limb rehabilitation in this population. However, the small sample size and short follow-up period limit the generalizability of these results. Larger multicenter trials with longer follow-up are warranted to confirm these findings and determine their clinical applicability.
Author Contributions
Conceptualization, M.S.-V., A.M.-O. and H.O.-H.; methodology M.S.-V. and H.O.-H.; formal analysis, O.S.-V. and M.S.-V.; investigation, T.M.-P. and M.S.-V.; resources, H.O.-H. and T.M.-P.; data curation, O.S.-V.; writing—original draft preparation, T.M.-P., O.S.-V., A.M.-O. and M.S.-V.; writing—review and editing, O.S.-V., M.S.-V. and A.M.-O.; project administration, H.O.-H.; funding acquisition, H.O.-H. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by COLEGIO PROFESIONAL DE TERAPEUTAS OCUPACIONALES DE CASTILLA Y LEÓN (COPTOCYL).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Burgos (protocol code IO/6/2024; approval date: 29 January 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original data presented in the study are openly available in riubu.ubu.es at [https://doi.org/10.71486/ptp9-7f36].
Acknowledgments
The authors would like to thank the company Fesia Technology for temporarily lending the device to carry out the interventions. We would also like to thank the Multiple Sclerosis Association of Burgos (AFAEM) for their collaboration in recruiting the sample. And finally, we would like to thank COPTOCYL for the funding provided. During the preparation of this manuscript/study, the author(s) used PAPERPAL V5.11.2. for the purposes of translation. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
A.M.-O. is an employee of Fesia Technology, from which he receives financial compensation. Fesia Technology contributed to the development of the device employed in this study. The rest of the authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BBT | Box And Block Test |
| CG | Control Group |
| CNS | Central Nervous System |
| DS1 | Difference Score Posttest—Pretest |
| DS2 | Difference Score Follow Up-Posttest |
| EG | Experimental Group |
| FES | Functional Electrical Stimulation |
| FIM | Functional Independence Measure |
| JTHFT | Jebsen–Taylor Hand Function Test |
| LLIC | Lower Limit Interval Confidence |
| M | Mean |
| MS | Multiple Sclerosis |
| NHPT | Nine-Hole Peg Test |
| PPMS | Primary Progressive Multiple Sclerosis |
| SD | Standard Deviation |
| SPMS | Secondary Progressive Multiple Sclerosis |
| RCT | Randomized Controlled Trial |
| RRMS | Relapsing–Remitting Multiple Sclerosis |
| ULIC | Upper Limit Interval Confidence |
Appendix A

Table A1.
Results of the normality test (Shapiro-Wilk) for the main clinical variables.
Table A1.
Results of the normality test (Shapiro-Wilk) for the main clinical variables.
| Variable/Item | Time | p (Shapiro-Wilk) | p | Skewness | Kurtosis | Test Type |
|---|---|---|---|---|---|---|
| JTHFT Item 1 | Pretest | 0.849 | 0.017 | 1.209 | 1.209 | Non parametric |
| Postest | 0.885 | 0.057 | 1.097 | 0.530 | ||
| Follow-up | 0.839 | 0.012 | 1.013 | −0.279 | ||
| JTHFT Item 2 | Pretest | 0.945 | 0.446 | 0.193 | 0.193 | Parametric |
| Postest | 0.914 | 0.154 | 0.934 | 0.258 | ||
| Follow-up | 0.919 | 0.184 | 1.048 | 0.953 | ||
| JTHFT Item 3 | Pretest | 0.902 | 0.101 | 1.064 | 1.064 | Non parametric |
| Postest | 0.927 | 0.215 | 0.438 | −0.872 | ||
| Follow-up | 0.783 | 0.002 | 2.217 | 6.248 | ||
| JTHFT Item 4 | Pretest | 0.891 | 0.069 | 0.595 | 0.595 | Non parametric |
| Postest | 0.860 | 0.024 | 1.477 | 3.300 | ||
| Follow-up | 0.674 | 0.000 | 2.804 | 9.095 | ||
| JTHFT Item 5 | Pretest | 0.760 | 0.001 | 1.046 | 1.046 | Non parametric |
| Postest | 0.737 | 0.001 | 1.701 | 2.095 | ||
| Follow-up | 0.808 | 0.005 | 1.872 | 3.959 | ||
| JTHFT Item 6 | Pretest | 0.875 | 0.041 | 1.244 | 1.244 | Non parametric |
| Postest | 0.677 | 0.000 | 2.077 | 3.461 | ||
| Follow-up | 0.460 | 0.000 | 3.532 | 12.957 | ||
| JTHFT Item 7 | Pretest | 0.917 | 0.174 | 1.027 | 1.027 | Non parametric |
| Postest | 0.872 | 0.036 | 1.336 | 1.751 | ||
| Follow-up | 0.553 | 0.000 | 3.152 | 10.610 | ||
| Pinch strength | Pretest | 0.802 | 0.004 | 1.530 | 1.515 | Parametric |
| Postest | 0.962 | 0.729 | 0.488 | 0.664 | ||
| Follow-up | 0.913 | 0.152 | 0.991 | 0.856 | ||
| NHPT | Pretest | 0.902 | 0.101 | 1.046 | 0.442 | Parametric |
| Postest | 0.872 | 0.036 | 0.588 | –1.236 | ||
| Follow-up | 0.843 | 0.014 | 0.830 | –0.874 | ||
| FIM | Pretest | 0.978 | 0.952 | 0.064 | −0.469 | Parametric |
| Postest | 0.934 | 0.313 | 0.138 | −1.314 | ||
| Follow-up | 0.968 | 0.834 | −0.107 | −0.646 | ||
| BBT | Pretest | 0.913 | 0.151 | 1.051 | 0.744 | Parametric |
| Postest | 0.956 | 0.621 | 0.161 | −1.140 | ||
| Follow-up | 0.971 | 0.872 | −0.061 | −0.711 | ||
| JTHFT Total | Pretest | 0.914 | 0.157 | 0.787 | −0.110 | Parametric |
| Postest | 0.886 | 0.058 | 1.273 | 1.382 | ||
| Follow-up | 0.841 | 0.013 | 1.652 | 3.709 |
p: significance value of the Shapiro-Wilk test; normal distribution was considered when p > 0.05. For variables with non-normal values or borderline values, skewness and kurtosis were evaluated before deciding on the type of statistical analysis to be applied.
References
- Martinez-Altarriba, M.C.; Ramos-Campoy, O.; Luna-Calcaño, I.M.; Arrieta-Antón, E. Revisión de la Esclerosis Múltiple (1). A propósito de un caso. SEMERGEN-Med. Fam. 2015, 41, 261–265. [Google Scholar] [CrossRef]
- Jiménez-Pérez, J.P.; Camacho-Castillo, E.Z.; del Toro Chávez, D.Á.; Buenrostro-Villanueva, D.M.; Luevanos-Villalpando, G.; Lomelí-Gómez, A.L.; Acuña-Vaca, S.; Delgado-Lara, D.L. Esclerosis Múltiple, un panorama actual sobre esta entidad eeurodegenerativa Qué sabíamos y qué sabemos ahora? Metodol. Instrumentación Lógica Estadística Evid. Epistemol. Salud 2024, 1, 34–50. [Google Scholar]
- Hocking, D.R.; Bradshaw, J.L.; Fielding, J. Degenerative Disorders of the Brain; Routledge: London, UK, 2019. [Google Scholar]
- Alcoriza Rodríguez, S.; Morais Vargas, C. Esclerosis Múltiple. Bachelor’s Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2015. [Google Scholar]
- Vázquez Sánchez, F.; García López, B. Manual de Neurología para Terapia Ocupacional; Editorial Médica Panamericana: Madrid, Spain, 2023. [Google Scholar]
- Abdullahi, A.; Wong, T.W.; Ng, S.S. Variation in the rate of recovery in motor function between the upper and lower limbs in patients with stroke: Some proposed hypotheses and their implications for research and practice. Front. Neurol. 2023, 14, 1225924. [Google Scholar] [CrossRef] [PubMed]
- Offner, F.F.; Liberson, W.T. Method of Muscular Stimulation in Human Beings to Aid in Walking. U.S. Patent No. 3,344,792, 3 October 1967. [Google Scholar]
- Sabut, S.K.; Lenka, P.K.; Kumar, R.; Mahadevappa, M. Effect of functional electrical stimulation on the effort and walking speed, surface electromyography activity, and metabolic responses in stroke subjects. J. Electromyogr. Kinesiol. 2010, 20, 1170–1177. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Song, J.-Y.; Park, S.-H.; Lee, M.-M. Effect of functional electrical stimulation-based mirror therapy using gesture recognition biofeedback on upper extremity function in patients with chronic stroke: A randomized controlled trial. Medicine 2023, 102, e36546. [Google Scholar] [CrossRef] [PubMed]
- Martín-Odriozola, A.; Rodríguez-de-Pablo, C.; Caceres-Salegi, A.; García-Calleja, A.; Marín-Ojea, J.I.; Hernández, E.; Imatz-Ojanguren, E.; Keller, T.; Zabaleta-Rekondo, H. Analysis of the movements generated by a multi-field functional electrical stimulation device for upper extremity rehabilitation. Artif. Organs 2022, 46, 2027–2033. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Popovic, M.R. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed. Eng. Online 2020, 19, 34. [Google Scholar] [CrossRef]
- Sears, E.D.; Chung, K.C. Validity and responsiveness of the Jebsen–Taylor hand function test. J. Hand Surg. 2010, 35, 30–37. [Google Scholar] [CrossRef]
- Fabbri, B.; Berardi, A.; Tofani, M.; Panuccio, F.; Ruotolo, I.; Sellitto, G.; Galeoto, G. A systematic review of the psychometric properties of the Jebsen–Taylor Hand Function Test (JTHFT). Hand Surg. Rehabil. 2021, 40, 560–567. [Google Scholar] [CrossRef]
- Santamaría-Vázquez, M.; Ortiz-Huerta, J.H.; Martín-Odriozola, A.; Saiz-Vazquez, O. Improvement of Motor Imagination and Manual Ability Through Virtual Reality and Selective and Nonselective Functional Electrical Stimulation: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2024, 13, e63329. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Valdes, K. Reliability of pinch strength testing in elderly subjects with unilateral thumb carpometacarpal osteoarthritis. J. Phys. Ther. Sci. 2014, 26, 993–995. [Google Scholar] [CrossRef]
- Feys, P.; Lamers, I.; Francis, G.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R. Multiple Sclerosis Outcome Assessments Consortium the Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult. Scler. J. 2017, 23, 711–720. [Google Scholar] [CrossRef]
- Figueiredo, I.; Sampaio, R.; Mancini, M.; Silva, F.; Souza, M. Test of grip strength using the Jamar dynamometer. Acta Fisiátrica 2007, 14, 104–110. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, É.; Mercier, L. Validation of the Box and Block Test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar]
- Mathiowetz, V.; Rennells, C.; Donahoe, L. Effect of elbow position on grip and key pinch strength. J. Hand Surg. 1985, 10, 694–697. [Google Scholar] [CrossRef]
- Rozo, A.L.; Juliao, A.J. Medida de la independencia funcional con escala FIM en los pacientes con evento cerebro vascular del Hospital Militar Central de Bogotá en el periodo octubre 2010–mayo 2011. Revista Med. 2013, 21, 72–82. [Google Scholar]
- Dodds, T.A.; Martin, D.P.; Stolov, W.C.; Deyo, R.A. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993, 74, 531–536. [Google Scholar] [PubMed]
- I.B.M Corporation. IBM SPSS Statistics, Version 28.0; I.B.M Corporation: Armonk, NY, USA, 2021.
- Kim, H.-Y. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013, 38, 52. [Google Scholar] [CrossRef]
- Koch, M.W.; Repovic, P.; Mostert, J.; Bowen, J.D.; Comtois, J.; Strijbis, E.; Uitdehaag, B.; Cutter, G. The nine hole peg test as an outcome measure in progressive MS trials. Mult. Scler. Relat. Disord. 2023, 69, 104433. [Google Scholar]
- Khan, M.A.; Fares, H.; Ghayvat, H.; Brunner, I.C.; Puthusserypady, S.; Razavi, B.; Lansberg, M.; Poon, A.; Meador, K.J. A systematic review on functional electrical stimulation based rehabilitation systems for upper limb post-stroke recovery. Front. Neurol. 2023, 14, 1272992. [Google Scholar] [CrossRef]
- Luo, S.; Xu, H.; Zuo, Y.; Liu, X.; All, A.H. A Review of Functional Electrical Stimulation Treatment in Spinal Cord Injury. Neuromolecular Med. 2020, 22, 447–463. [Google Scholar] [CrossRef]
- Miller, L.; McFadyen, A.; Lord, A.C.; Hunter, R.; Paul, L.; Rafferty, D.; Bowers, R.; Mattison, P. Functional electrical stimulation for foot drop in multiple sclerosis: A systematic review and meta-analysis of the effect on gait speed. Arch. Phys. Med. Rehabil. 2017, 98, 1435–1452. [Google Scholar]
- Sampson, P.; Freeman, C.; Coote, S.; Demain, S.; Feys, P.; Meadmore, K.; Hughes, A.-M. Using functional electrical stimulation mediated by iterative learning control and robotics to improve arm movement for people with multiple sclerosis. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 235–248. [Google Scholar] [CrossRef]
- Malešević, J.; Štrbac, M.; Isaković, M.; Kojić, V.; Konstantinović, L.; Vidaković, A.; Dedijer Dujović, S.; Kostić, M.; Keller, T. Temporal and Spatial Variability of Surface Motor Activation Zones in Hemiplegic Patients During Functional Electrical Stimulation Therapy Sessions. Artif. Organs 2017, 41, E166–E177. [Google Scholar] [CrossRef]
- Hoffmann, U.; Deinhofer, M.; Keller, T. Automatic determination of parameters for multipad functional electrical stimulation: Application to hand opening and closing. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1859–1863. [Google Scholar]
- Imatz-Ojanguren, E.; Sánchez-Márquez, G.; Asiain-Aristu, J.R.; Cueto-Mendo, J.; Jaunarena-Goicoechea, E.; Zabaleta, H.; Keller, T. A foot drop compensation device based on surface multi-field functional electrical stimulation—Usability study in a clinical environment. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319862141. [Google Scholar] [CrossRef] [PubMed]
- Malešević, N.M.; Maneski, L.Z.; Ilić, V.; Jorgovanović, N.; Bijelić, G.; Keller, T.; Popović, D.B. A multi-pad electrode based functional electrical stimulation system for restoration of grasp. J. Neuroeng. Rehabil. 2012, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.J.; Patritti, B.L.; Bowes, R.; Crotty, M.; McLoughlin, J.V. Orthotic and therapeutic effect of functional electrical stimulation on fatigue induced gait patterns in people with multiple sclerosis. Disabil. Rehabil. Assist. Technol. 2017, 12, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Hsu, M.-J.; Chen, S.-M.; Lin, C.-H. Decreased central fatigue in multiple sclerosis patients after 8 weeks of surface functional electrical stimulation. J. Rehabil. Res. Dev. 2011, 48, 555–564. [Google Scholar] [CrossRef]
- Maneski, L.Z.P.; Malešević, N.M.; Savić, A.M.; Keller, T.; Popović, D.B. Surface-distributed low-frequency asynchronous stimulation delays fatigue of stimulated muscles. Muscle Nerve 2013, 48, 930–937. [Google Scholar] [CrossRef]
- Imatz-Ojanguren, E.; Keller, T. Evoked sensations with transcutaneous electrical stimulation with different frequencies, waveforms, and electrode configurations. Artif. Organs 2023, 47, 117–128. [Google Scholar] [CrossRef]
- Marcus, R. What is multiple sclerosis? JAMA 2022, 328, 2078. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).