Abstract
Trace concentrations of dyes are often present in textile wastewater streams and present a serious environmental problem. Thus, these dyes must be removed from wastewater either by degradation or sequestration prior to discharge of the wastewater into the environment. Existing processes to remove these wastewater contaminants include the use of solid sorbents to sequester dyes or the use of biochemical or chemical methods of dye degradation. However, these processes typically generate their own waste products, are not necessarily rapid because of the low dye concentration, and often use expensive or non-recyclable sequestrants or reagents. This paper describes a simple, recyclable, liquid–liquid extraction scheme where ionic dyes can be sequestered into poly(α-olefin) (PAO) solvent systems. The partitioning of anionic and cationic dyes from water into PAOs is facilitated by ionic PAO-phase anchored sequestering agents that are readily prepared from commercially available vinyl-terminated polyisobutylene (PIB). This is accomplished by a sequence of reactions involving hydroboration/oxidation, conversion of an alcohol into an iodide, and conversion of the resulting primary alkyl iodide into a cationic nitrogen derivative. The products of this synthetic sequence are cationic nitrogen iodide salts which serve as anionic sequestrants that are soluble in PAO. These studies showed that the resulting series of cationic PIB-bound cationic sequestering agents facilitated efficient extraction of anionic, azo, phthalein, and sulfonephthalein dyes from water into a hydrocarbon PAO phase. Since the hydrocarbon PAO phase is completely immiscible with water and the PIB derivatives are also insoluble in water, neither the sequestration solvent nor the sequestrants contaminate wastewater. The effectiveness and efficiency of these sequestrations were assayed by UV–visible spectroscopy. These spectroscopic studies showed that extraction efficiencies were in most cases >99%. These studies also involved procedures that allowed for the regeneration and recycling of these PAO sequestration systems. This allowed us to recycle the PAO solvent system for at least 10 sequential batch extractions where we sequestered sodium salts of methyl red and 4′,5′-dichlorofluorescein dyes from water with extraction efficiencies of >99%. These studies also showed that a PIB-bound derivative of the sodium salt of 1,1,1-trifluoromethylpentane-2,4-dione could be prepared from a PIB-bound carboxylic acid ester by a Claisen-like reaction and that the sodium salt of this β-diketone could be used to sequester cationic dyes from water. This PIB-bound anion rapidly and efficiently extracted >99% of methylene blue, malachite green, and safranine O from water based on UV–visible and 1H NMR spectroscopic assays.
1. Introduction
The presence of dyes in industrial wastewater is an important environmental issue. Industries generate 900 million tons of dye-polluted wastewater annually [1,2,3]. The textile industry worldwide uses approximately 200,000 tons of dyes per year and accounts for more than 50% of dye effluents released into the environment [3]. Estimates suggest that this amounts to 60,000 tons of dye pollution into the environment annually. While there are many different dyes, it is estimated that 80% of the pollutant dyes in wastewater belong to a class of dyes called azo dyes [1]. Across all industries, there are about 100,000 different dyes and pigments [3]. Whatever their structure, dyes are typically hazardous towards the environment, causing negative impacts on aquatic and land ecosystems. In an aquatic ecosystem, dye effluent can lower the dissolved oxygen levels, affect salt levels, and affect pH levels [4,5,6,7]. These are some of the more immediate effects of water contamination by dyes. Dyes are also often toxic, and the pollution of dyes into waterways could promote a plethora of longer-range health issues [8].
Current methods for dye removal from water commonly require multiple techniques used in tandem [3,9,10]. For example, dye-contaminated wastewater is first adjusted to an appropriate pH and temperature. Then, primary treatment occurs. This involves flocculation and sedimentation to precipitate less soluble dyes. This step however is not very effective for polar, water-soluble dyes. Second, anaerobic or aerobic degradation can be used to degrade the soluble dyes. While this technique can be effective, the efficacy of this treatment depends on the structures of the dyes and what other oxidizable organic compounds are present. In most cases, a tertiary treatment stage is required to remediate dye-contaminated wastewater. This can involve membrane filtration, advanced oxidative treatment, ion exchange, or adsorption.
Filtration techniques like ultrafiltration or nanofiltration can be applicable in some cases to treat dye-contaminated wastewater. However, the membranes used can be costly. Second, if they become fouled, they are not easily regenerated.
Advanced oxidation is a more common treatment for dye-contaminated wastewater. Alternatives can involve chemical or biological methods. For example, oxidation with Fenton reagents, ozonation, and electrochemical oxidation have all been used to degrade dyes in dye-contaminated wastewater. These techniques effect the degradation of larger dye molecules to smaller molecules [3,9,10]. For example, the ozonation of azo dyes fragments the molecules into two smaller molecules by oxidizing their –N=N– bond [11]. However, while oxidatively degrading dyes effectively decolorizes wastewater, the decolorized water product still contains degradation products of the dyes that too could possess environmental problems [11,12,13], though this is often overlooked.
Sequestration of dye impurities with a solid sorbent is a common procedure for the treatment of dye-contaminated wastewater. Sequestration, unlike oxidation, does not produce any potentially problematic by-products. For example, ion exchange resins can be used to sequester dyes by exchange of sodium cations or chloride anions. The solid spent resin is easily separated from wastewater and can be recycled via ion exchange. Drawbacks for solid ion exchange resins include the cost of the resins themselves, the competition of other soluble anions and cations with dyes, and the cost of materials used to regenerate the spent resin.
A common procedure for the sequestration of dyes from wastewater at this tertiary treatment stage is to use a physical process such as adsorption onto solid sorbents [9,10]. Activated carbon, peat, and silica are among the more common sorbents. However, while these sorbents are relatively inexpensive, they are not always effective. Thus, there has been significant recent work on the sequestration of dyes from water that has focused on solid sorbents. This has included designing tailored sorbents. For example, functionalized β-cyclodextrins have proven to be attractive sorbents that can use the principles of molecular recognition to sequester dyes [14]. Functionalized β-cyclodextrins were capable of removing >90% of methylene blue and safranine O from water at a pH > 4. While β-cyclodextrin has been garnering academic and commercial attention, additional sorbents like surface-functionalized silica, and agricultural waste biomass have also attracted interest and can be similarly effective for sequestration of dyes from contaminated water samples [15,16,17]. For example, when fungal biomasses were studied for the removal of methyl orange or Congo red from water, extraction efficiencies of 53% and 97% were observed, respectively [17]. Activated carbon generated from agricultural biomass has also proven effective for the removal of ionic dyes including methyl red and methyl orange, with reported extraction efficiencies of 95% and 99%, respectively [16].
An alternative to using solid sorbents to sequester dyes is to use liquid/liquid extraction. In the context of dye sequestration from wastewater, this has been accomplished using ionic liquids (ILs) or ionic liquid coacervates (Figure 1) that are designed to form biphasic mixtures with an aqueous dye solution [18,19,20,21,22,23]. Alkylated guanidinium, imidazolium, and pyrrolidinium ILs have been explored as solvents [18,19,20,23] for the extraction of anionic azo dyes. At least a C6–C10 alkyl group is required on the IL in order for the IL to be sufficiently hydrophobic. Such chains allow the IL to form a separate phase and minimize any back-contamination of the aqueous phase by the ionic liquid phase [19]. IL coacervates too have been used as a liquid phase for the sequestration of water contaminated with ionic contaminants [21,22]. In this case, the IL surfactants are designed to form complex emulsions when mixed with water. In either case, charged organic contaminants like azo dyes partition into these IL phases or droplets and can then be separated by density from the cleaned aqueous solution. Sequestration efficiencies ranging from 95 to 99% have been reported for the removal of dyes like methyl orange from water by liquid–liquid extraction using guanidinium-based ionic liquids [18]. Alkylimidazolium-based ionic liquids were similarly effective for the removal of anionic dyes such as methyl red from water, with extraction efficiencies of >99% [19].
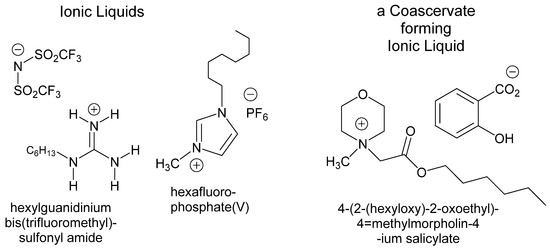
Figure 1.
Ionic liquids or ionic liquid surfactants used to effect liquid/liquid dye sequestration.
Our group has a long history of using biphasic systems to separate catalysts from products using phase-selectively soluble polymers [24]. This same chemistry can be used for the separation of trace metals from polar solvents [25,26]. In more recent work, we showed that fully recyclable poly(α-olefin) (PAO) solvents that were completely insoluble in water were also effective as green solvents for recycling catalysts [27,28,29,30]. PAOs are fully hydrogenated alkanes derived from 1-decene and 1-dodecene oligomers. They are commercially available, relatively nonflammable, and nonvolatile materials [31,32,33,34] that can quantitatively serve as solvents to separate functionalized polyisobutylene (PIB) oligomer-bound catalysts from polar phases.
Our work recently showed that PIB/PAO systems were alternatives to other liquid/liquid and liquid/solid approaches for separating trace organics from water. While PAOs alone effectively remove nonpolar organics like benzene, toluene, ethylbenzene, and xylene (BTEX) from water [35,36], the sequestration of polar trace organic contaminants like phenols required adding a PIB H-bonding agent to form a PIB/PAO system that rapidly and quantitatively removed phenols from water [37].
Based on our prior work, we thought that PIB/PAO systems containing cationic or anionic end-functionalized PIB derivatives could also be a viable alternative to tailored solid sorbents and ionic liquids for the sequestration of cationic and anionic dyes from aqueous solutions. The results below show that this hypothesis was correct.
2. Materials and Methods
2.1. General Information
Poly(α-olefin)s (PAOs), including a PAO with a molecular weight of 432 Da, were provided by Exxon Mobil (Houston, Texas, USA). Alkene-terminated polyisobutylenes (PIBs) with reported molecular weights (Mn) of 450, 1000, and 2300 Da were obtained from Texas Polymer Corporation (TPC) (Baytown, TX, USA) and/or BASF [38,39]. All other reagents and solvents were purchased from commercial sources. 1H NMR spectra were recorded on Bruker NMR spectrometers operating at 400 MHz, and chemical shifts were reported in ppm with reference to CDCl3 at 7.26 ppm. 13C NMR spectra were recorded on Bruker NMR spectrometers operating at 100 MHz. Chemical shifts are reported in ppm with reference to CDCl3 at 77.00 ppm. UV–visible spectra were obtained on a Shimadzu UV-2700 spectrophotometer.
2.2. General Procedure for the Extraction of Dyes from Water
Solutions of a PIB oligomer with a terminal ammonium or imidazolium iodide group (1–3) in PAO432 were prepared at various concentrations. Then, 5 mL of a pH 10 aqueous phase containing the dye of interest was added to a centrifuge tube along with 0.5 g of the PAO solution. The mixture was vortexed for either 2, 5, or 10 min at 3000 rpm and then centrifuged for 15 min at 3000 rpm. Initial experiments used visual observation to determine the success of dye removal by observing if the water phase became colorless. Subsequently, we used quantitative UV–visible spectroscopy to analyze the lower aqueous phase. The absorbance value at the λmax for the dye of interest in the ‘cleaned’ aqueous solution was then compared to the absorbance of the initial aqueous virgin dye solution to determine the extraction efficiency (Equation (1)). Starting dye concentrations were determined gravimetrically. If the virgin dye solution’s absorbance was >1.5, 1H NMR
spectroscopic analysis was also used to determine dye concentration before and after extraction [40]. In this 1H NMR spectroscopy experiment, a solution of a methoxy-terminated polyethylene glycol (Mn = 2000) standard was prepared in D2O at a concentration of 1000 mg/L, and 0.5 g of this solution was combined with 0.5 g of the extracted aqueous phase. This D2O/H2O mixture solution was then analyzed by 1H NMR spectroscopy using the WATERGATE protocol. The amount of dye contaminant was quantified by integrating the dye contaminant peaks with respect to the integral of the 13C satellite peaks for the methoxy−CH3 singlet and/or the –CH2CH2O– satellite peak [40].
2.3. Regeneration and Recycling of the PAO Phase
Solutions were prepared that contained 5 mM of PIB2300 which contained as a terminal group an imidazolium salt (3) that was analogous to the imidazolium salt Coates previously described [41]. Then, 2 mL of this PAO432 solution was combined with 5 mL of a pH 10 aqueous phase which contained 0.1 mM of either p-methyl red or dichlorofluorescein. A greater volume of PAO was used in this experiment to facilitate the transfer of PAO from container to container. A larger volume of PAO432 was used in this experiment since physical losses in experiments with 0.5 g of PAO would have been problematic. The mixture was then vortexed for 10 min at 3000 rpm, then centrifuged for 15 min at 3000 rpm. The lower aqueous phase was analyzed by UV–visible spectroscopy to determine the concentration of dye after extraction. The upper PAO phase was stirred with 2 drops of CH3–I to convert the dye anion into either a methyl ester or ether. This regenerated the iodide anion of 3. At this point, the PAO solution of 3 could be reused, and this cycle could be repeated up to 10 times. After the 5th extraction, the PAO solution became cloudy. This solid in the PAO was analyzed and was determined to be the methyl ester of p-methyl red. After the 5th cycle, care was taken after centrifugation to separate as much of the insoluble solid from the PAO phase as possible before proceeding with the subsequent extraction. A total of 10 sequential extractions were performed using the same, regenerated PAO phase containing 3 as a sequestering agent.
3. Results and Discussion
A typical batch extraction of dye from water was performed using the following protocol. First, we combined 5 mL of an aqueous phase with 0.5 g of a PAO phase that contained an ionic sequestering agent. In the case of anionic dyes, the aqueous-phase pH was adjusted to pH 10 using Na2CO3. Cationic dyes were sequestered from distilled water and the pH was not adjusted. Typical concentrations of dye in the water used in these experiments ranged from 0.02 mm to 1.6 mm. The aqueous phase was then mixed with the PAO phase using a vortex mixer, then centrifuged at 3000 rpm for 15 min. At this point, the denser aqueous phase was separated from the PAO phase using a pipette, then analyzed by UV–visible spectroscopy. In most cases, extraction efficiencies were obtained for extractions where the virgin dye solution’s absorbance was <1. The concentration of the dye in the virgin dye solutions could then be compared with the aqueous phases that had been treated by liquid–liquid extraction with a PAO solution containing a given sequestering agent in order to obtain an extraction efficiency (see Equation (1) in Section 2 above). Where necessary, serial dilution was used to ensure that dye concentrations were linearly related to absorbance.
The names and corresponding structures of anionic dyes that were sequestered are shown in Figure 2 below.
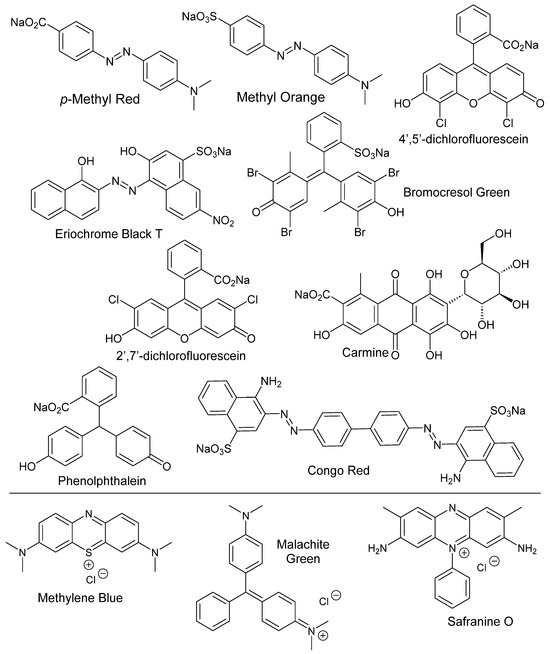
Figure 2.
Structures of anionic and cationic dyes that were extracted in this project.
The anionic dyes we selected had carboxylate and sulfonate moieties on their aromatic rings. These included azo dyes like methyl red, methyl orange, Eriochrome Black T, and Congo red—dyes that are commonly used in the textile industry [3,4,5,6]. We also studied the feasibility of using PIB/PAO systems for the sequestration of phthalein dyes like phenolphthalein and dichlorofluorescein from water. Bromocresol green (a sulfonephthalein dye) and carmine (cochineal extract) were also tested. The cationic dyes that we sequestered using anionic sequestering agents are also shown in Figure 2 below. These three dyes were malachite green, methylene blue, and safranine O.
3.1. Sequestration of Anionic Dyes
Prior to analyzing the efficiency of various sequestering agents for the removal of dyes from solution, we tested whether PAO alone could sequester these anionic dyes. Given our prior results where PAO itself was only an effective sequestrant for nonpolar trace impurities in water [35,37], we expected that dyes would not be sequestered by PAO432 alone because charged species partition poorly into alkane phases. This hypothesis was confirmed. The extraction efficiencies for the sequestration of each dye by PAO432 alone are given in Table 1 below. While we saw small decreases in absorbance at λmax for the dye solutions after extracting with PAO432 alone, in all but one case (Eriochrome Black T), this effect was small. Thus, since PAO432 alone is ineffective as a sequestrant, we moved on to testing cationic PIB-bound sequestering agents for the removal of anionic dyes from water.

Table 1.
Extraction efficiencies for the removal of anionic dyes from water using PAO432 alone. The extraction efficiencies were calculated using Equation (1) above. In general, the initial solutions were prepared by serial dilution of a stock solution to an absorbance <1.
A series of cationic PIB oligomers containing cationic nitrogen functionality as terminal functional groups were synthesized using the chemistry shown in Scheme 1. These included a PIB2300-diethylmethyl ammonium iodide (1), a PIB2300-butyl imidazolium iodide (2), and a PIB2300-pentasubstituted imidazolium iodide (3). The PIB2300-pentasubstituted imidazolium iodide 3 is an analog of an imidazolium cation prepared by Coates’ research group.
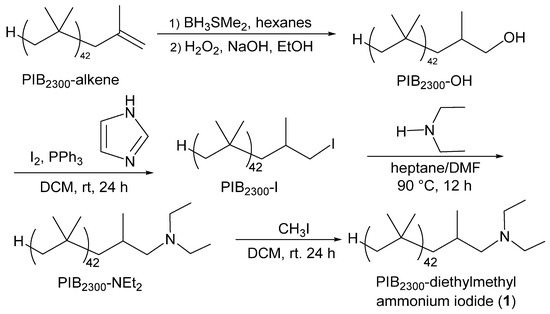
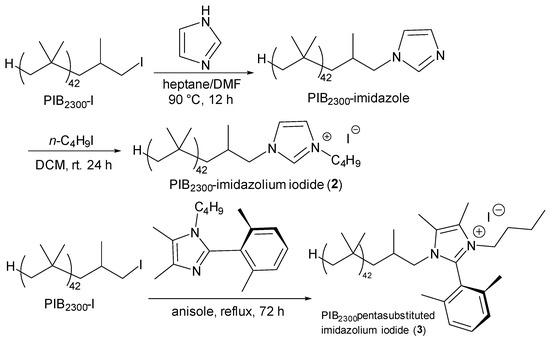
Scheme 1.
Preparation of PIB-bound ammonium and imidazolium salts 1, 2, and 3. Imidazolium salt 3 was prepared using the synthesis scheme described in [41].
It is reported to be significantly more stable to an alkaline solution than typical imidazolium salts [41].
To test the capability of each cationic sequestering agent to remove dyes from solution, 0.05 M solutions of each cationic PIB derivative in PAO432 were prepared. These solutions were stirred over solid Na2CO3 overnight to remove any acidic contaminants because in one case, our prior work detected trace acidic impurities in the commercial PAO432 [27]. 13C NMR spectroscopic analysis determined that the exchange of iodide for carbonate counteranions did not occur during this process. After this step, 0.5 g of each PAO solution was vortexed for 2 min with aqueous solutions of methyl red or methyl orange at pH 10. After vortex mixing, the mixtures were centrifuged at 3000 rpm for 15 min, then separated, and the aqueous phases were analyzed visually and by UV–visible spectroscopy. From visual studies after 2 min of mixing, 3 was the most efficient sequestering agent for carboxylate and sulfonate anions (Figure 3), though other cationic PIB derivatives were comparably efficient with longer mixing times.
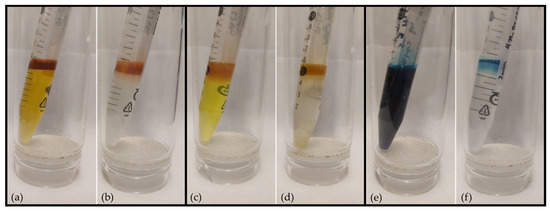
Figure 3.
(a,b) show the removal of p-methyl red from an aqueous phase into a 0.05 M solution of 3 in PAO432 (respectively). (c,d) show the extraction of 4′,5′-dichlorofluorescein from water into this PIB/PAO solution. (e,f) show the extraction of bromocresol green from water into this PIB/PAO solution.
The aqueous phase’s absorbance due to residual dye after extraction by a PAO solution of 3 was near zero after 2 min of mixing, whereas the aqueous phase’s absorbance after extraction by a PAO solution of 1 or 2 still showed some dye was still present (Figure 4a,b). Further mixing of the PAO solutions of 1 or 2 with the aqueous solution of the dye for a total of 5 min decreased the aqueous phase’s absorbance about as efficiently as 3 had in 2 min. While longer mixing times could be used with 1 or 2, further sequestration experiments focused on the use of cation 3 as a PAO soluble sequestrant.
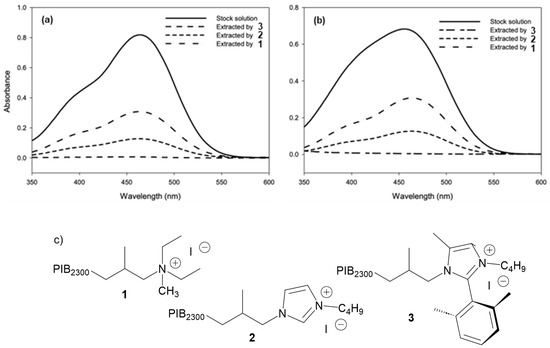
Figure 4.
(a) An aqueous stock solution of methyl red extracted by 0.05 M PAO solutions of 1, 2, and 3 by liquid–liquid extraction (2 min of vortex mixing). (b) An aqueous stock solution of methyl orange extracted by 0.05 M PAO solutions of 1, 2, and 3 by liquid–liquid extraction (2 min of vortex mixing). (c) Structures of PIB2300-bound salts 1, 2, and 3 used in the above experiment.
To test the generality of this process, dye sequestration for a series of anionic dyes from water using 0.05 M PAO solutions of 3 was studied. The initial studies used dye solutions with absorbances in the range of 0.4 to 1.3. These extractions were carried out in the same manner as described above—brief vortex mixing of the PAO solutions with 5 mL of aqueous dye solutions at pH 10 for 2 min, centrifugation, phase separation, and analysis of the aqueous phase by UV–visible spectroscopy. The results in Table 2 show that 3 in PAO432 was an effective way to remove a broad spectrum of dyes from water.

Table 2.
Extraction efficiencies for the removal of ionic dyes (ca. 2 × 10−5 M) from water using 0.05 M solution of 3 in PAO432 after 2 min of vortex mixing. The extraction efficiencies were calculated using Equation (1) above. Where necessary, solutions for analysis were prepared by serial dilution of the dye solution so that the absorbance of the resultant dye solution was between 0.4 and 1.3.
All of these experiments involved dye solutions with dye concentrations on the order of 2 × 10−5 M. The PIB-imidazolium salt was present in excess during these experiments. These initial experiments showed that even a brief mixing of the aqueous dye solution with the PAO solution of the sequestrant 3 was sufficient for near quantitative removal of the dye of interest. These conclusions were confirmed by a second set of experiments using 10 min of mixing of 0.5 g of a 0.05 M solution of 3 in PAO432 with 6 × 10−5 M solutions of methyl red, bromocresol green, phenolphthalein, and 4,5-dichlorofluorescein in pH 10 water. Modest differences were seen in this second set of experiments, notably with phenolphthalein. This suggests that mixing efficiency moderated by mixing time is a factor in how effective PAO solutions of 3 are in dye sequestration.
The extraction efficiencies in Table 2 are equal to the extraction efficiencies previously reported for the removal of methyl red and methyl orange from water using alkylguanidinium- or alkylimidazolium-based ionic liquids (ILs) [18,19]. Bouchal previously reported that >99% of methyl orange could be removed from water using an array of alkyl-guanidinium ionic liquids. Fan reported that >99% of methyl red could be removed from water using various alkylimidazolium ionic liquids.
While the use of PIB/PAO systems for removing dyes from water proved successful, a key to making this scheme efficient and sustainable was the regeneration of the sequestrant in the PAO phase. Given that the initial sequestrants like 3 had an iodide counterion, we explored ion exchange of the sequestered carboxylate or sulfonate dye counterions for halides. First, we tried simply stirring PAO solutions of 3 that had been used to sequester dyes such as p-methyl red or 4′,5′-dichlorofluorescein in the presence of solid halide salts. This included using KBr, NaI, KI, or CsI. However, the absorbance of the PAO solution did not change after stirring in the presence of these salts, indicating that ion exchange did not occur. Next, either a THF solution of tetrabutylammonium bromide or tetrabutylammonium iodide was added to a colored PAO solution containing dye sequestered by 3. However, when this mixed solvent system was perturbed to be biphasic, the absorbance of the PAO solution did not change. Stirring with carbonate salts such as K2CO3, Na2CO3, and Cs2CO3 also did not affect the absorbance of the PAO phase. Stirring our PAO solution of 3 with solid toluene sulfonic acid hydrate was likewise unsuccessful at affecting the absorbance of the PAO solution.
Further experiments included attempts to back-extracting the dyes into aqueous solutions of NaI, KBr, and dilute acids such as trifluoroacetic acid, HCl, HBr, and HI or mixing the PAO solutions with saturated aqueous solutions of NaI and NaBr. While treatments with NaI or NaBr were unsuccessful, mixing with aqueous acid did have some effect. Specifically, we observed that the dye was protonating based on the visible observation that the color of the PAO phase was changing. However, the protonated dye still could not be fully extracted into the aqueous solutions. We briefly explored extracting the protonated dyes into alcoholic phases in solvents such as ethanol, isopropanol, and n-butanol. However, we discovered that 3 leached significantly into all the alcohol solvents tested. In the case of ethanol, we observed that ca. 50% of 3 had leached from a 0.05 M PAO432 solution into ethanol. This precluded using any back-extractions of our PAO solutions into polar organic solvents. Ultimately, these experiments led us to the conclusion that simple ion exchange would be very difficult without the use of a significant volume of volatile organic solvents to aid in the processing of the PAO phase.
We next turned our attention to recycling PAO solutions of 3 that had sequestered a carboxylate-containing dye by adding methyl iodide (MeI) to the PAO phase. The hypothesis here was that the carboxylate salt would undergo an SN2 reaction with MeI if stirred for a brief period. This chemistry has precedence in prior work where we used PIB-bound imidazolium cations as nonpolar phase solubilizing agents for carboxylate salts which were reactive as nucleophiles in SN2 reactions in heptane and PAO solutions [42]. These experiments did successfully regenerate 3 in the PAO phase.
The regeneration and recycling experiments using MeI were carried out with a larger amount of the PAO sequestrant phase to facilitate the recycling of the PAO phase since processing and transferring 0.5 g of the PAO phase through 10 sequential batch extractions was experimentally difficult. These regeneration and recycling experiments were also modified in that a lower ratio of sequestrant/dye was used to show if lower amounts of 3 relative to dye would be as effective as the ca. 200/1 ratio of sequestrant to dye used earlier. This experiment used 2 mL of a 5 mM PAO solution of 3 and 5 mL of a 0.1 mM solution of either p-methyl red or 4′,5′-dichlorofluorescein. These aqueous stock solutions had absorbances of 2.5 and 2.0, respectively. In the event, this mixture was vortexed for 10 min, then centrifuged for 15 min at 3000 rpm. As described above, the dye was quantitatively removed from the aqueous phase. Then, the upper PAO phase was removed and stirred with two drops of MeI for 2 h and a second aqueous phase containing either methyl red or methyl orange was added. After mixing, the lower aqueous phase was analyzed by UV–visible spectroscopy. The results in Table 3 show that both p-methyl red and 4′,5′-dichlorofluorescein were efficiently extracted in each cycle. The absorbance of either dye decreased by >99.9% in each cycle. This PAO solution of 3 could be recycled >10 times in the extractive removal of methyl red and 4′,5′-dichlorofluorescein from water. The efficiency of the extraction of the aqueous phase after the 10th cycle was still at >99% efficiency.

Table 3.
Extraction efficiencies for the removal of methyl red and 4′,5′-dichlorofluorescein over 10 extraction cycles. A PAO solution of 45 was regenerated in between each cycle.
As noted above, the presumption was that the carboxylate group of the methyl red and the carboxylate or phenolate group on 4′,5′-dichlorofluorescein were being converted into methyl esters or ethers. We were able to confirm this was the case for both the sodium salt of methyl red and the sodium salt of methyl orange. In the first case, we noted that the PAO phase in the regeneration and recycling experiment where methyl red dyes were sequestered became cloudy after the 5th extraction cycle. We confirmed that this precipitate was the methyl ester of methyl red by adding CH3CN to this PAO suspension. PAO and CH3CN are immiscible. While the dye sequestered by 3 does not partition into the CH3CN phase, the methyl ester of methyl red as a polar species is phase-selectively soluble in MeCN. UV–visible spectroscopic analysis of this CH3CN phase showed that the ester of methyl red formed (λmax of 409 nm). While regeneration/recycling experiments with 4′,5′-dichlorofluorescein did not form a cloudy PAO phase, in this case, there was a change in the UV–visible spectrum of the PAO phase. The PAO phase containing the carboxylate anion form of 4′,5′-dichlorofluorescein had a λmax at 530 nm. After treatment of the PAO phase with MeI, this λmax changed to 370 nm, showing that the sodium salt of 4′,5′-dichlorofluorescein had been converted into the methyl ether of 4′,5′-dichlorofluorescein.
The success of regenerated PAO solutions of 3 for dye sequestration is described in Table 3 and favorably compares to the recycling of ILs that sequester dyes. In the case of Bouchal’s work [18], IL regeneration was described for ILs that sorbed chromate anions but was not described for ILs containing dyes. In that example, regeneration was possible by ion exchange, though the regeneration process required a large excess of diethyl ether as a solvent. The recycling of ILs in other cases proceeds with varying efficiencies. For example, in Kamenicka’s study [23], the most successful processes involving chemical decomposition of azo dyes in IL by a reduction process showed good sequestration efficiency for the regenerated IL for up to five cycles. However, the recycled IL’s efficiency for dye removal decreased significantly after the third cycle in most of the regeneration experiments.
3.2. Sequestration of Cationic Dyes
Extraction of three cationic dyes from water using PAO solutions alone and with an anionic sequestering agent (4) was also briefly studied. In this case, we modified the terminus of a PIB oligomer by forming a chelating anion derived from a β-diketone. This sequestering agent (4) was prepared using the synthetic route shown in Scheme 2.
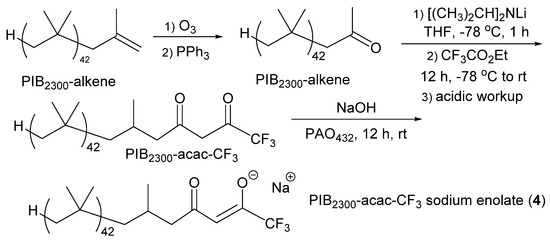
Scheme 2.
Preparation of PIB2300acac-CF3 and its enolate (4).
To explore the use of 4 as a sequestration agent for cationic dyes, stock solutions of three cationic dyes were first prepared in deionized water using methylene blue, safranine O, and malachite green. In these cases, the concentrations of the cationic dyes in the aqueous phases were higher than the anionic dyes studied above. Specifically, we used concentrations of 1.6, 1.4, and 1.4 mM for methylene blue, safranine O, and malachite green, respectively. These concentrations represented ca. 400 mg/L of dye in water. The dye concentrations in these solutions were determined gravimetrically and were consistent with concentrations determined by 1H NMR spectroscopic analysis with an mPEG2000 internal standard, and they are listed in Table 4 and in Table 5 below. These aqueous cationic dye stock solutions were initially extracted by PAO432 alone to test PAO’s ability to sequester these cationic dyes from aqueous solutions. As expected, the PAO432 phase was unsuccessful at removing the cationic dyes from water based on analysis of the aqueous phase by UV–visible spectroscopy (see Table 4 below). That conclusion was confirmed by the analysis of the extracted aqueous solutions by 1H NMR spectroscopy using mPEG2000 as an internal standard. Both spectroscopic analyses showed that the cationic dyes were not significantly sequestered from water by PAOs alone.

Table 4.
Extraction efficiencies for the removal of cationic dyes from water using PAOs alone. The extraction efficiencies were calculated using Equation (S1) in the Supporting Information. Extraction of the initial 400 ppm solutions by PAO432 alone could not be analyzed using Beer’s law because the absorbances were too high.

Table 5.
Extraction efficiencies for cationic dye removal from water using a 0.1 M 4 in PAO432.
PAO432 solutions of sequestering agent 4 were prepared by first dissolving 1.86 g of PIB2300-acac-CF3 in 5 g of PAO432 to prepare a 0.1 M solution in PAO432. This solution was then stirred over 0.5 g of crushed NaOH (s) for 12 h to form the enolate salt (4) if this PIB-bound β-diketone. Then, 0.5 g of this 0.1 M solution of 4 was vortexed with 5 mL of an aqueous solution of malachite green, safranine O, or methylene blue for 5 min. The mixture was then centrifuged at 3000 rpm for 10 min. The aqueous phase was analyzed by UV–visible spectroscopy (see Table 5 below). In addition, we analyzed these aqueous solutions by 1H NMR spectroscopy using an mPEG2000 internal standard. Both spectroscopic analyses showed that the cationic dyes were sequestered from water. Safranine O and methylene blue could not be detected by either UV–visible or 1H NMR spectroscopic analysis after extraction by 4, indicating that the extraction efficiencies were >99.9%. Malachite green was extracted with 90% efficiency under these conditions. Recycling of the extractant 4 was not examined in these brief studies, though separate studies have shown that PIB bound bases can readily be protonated in biphasic reactions with acetonitrile, methanol, or aqueous solutions containing sulfonic acids.
3.3. Future Directions
This approach, from the removal of anionic and cationic dyes, could be expanded to include cationic and anionic trace contaminants other than dyes. While we were successful in devising a protocol for recycling sequestration phases that extracted anionic dyes, we did not investigate that for cationic dyes. In the context of green chemistry, recycling is, we think, especially important. Thus, while the solvent used in these sequestration processes, PAO432, is fully recyclable and while the sequestrants used in the sequestration of anionic dyes were recyclable, developing better protocols that use safer reagents than methyl iodide or developing a protocol for recycling an anionic sequestrant warrants future attention. Likewise, we only explored a limited number of cationic and anionic PIB derivatives. Other species could be even more effective. Finally, while we did not have problems in these experiments, we have noted in the past that some PIB derivatives like a PIB-bound sulfonic acid when mixed with water form emulsions that could be a problem with other anionic PIB derivatives.
4. Conclusions
Analyses of a series of PIB-bound cationic and anionic sequestering agents demonstrated that liquid–liquid extraction may be a viable alternative for removing ionic dyes from contaminated water. PIB-bound imidazolium 3 proved to be the most effective PIB-bound cation we prepared for the sequestration of anionic dyes. It required the shortest mixing time of all three PAO solutions tested to effect the quantitative partitioning of p-methyl red and methyl orange from pH 10 aqueous phases into PAO432. While PAO432 alone was ineffective at sequestering ionic dyes from pH 10 aqueous phases, a 0.05 M solution of 3 in PAO432 proved to be extremely effective for sequestering a series of carboxylate- and sulfonate-containing dyes from pH 10 water at concentrations ca. 2 × 10−4 to 2 × 10−5 M. Moreover, PAO solutions of 3 that had sequestered dyes could be regenerated upon mixing these PAO soluble salts with MeI. This produced a neutral dye and regenerated an iodide counteranion on 3. Recycling was studied for the sequestration of p-methyl red and 4′,5′-dichlorofluorescein, and the PAO phase could be regenerated and recycled up to 10 times in each case.
The sequestration of cationic dyes was also briefly examined. The cationic dyes studied did not partition into PAO432. That was expected given their polarity and given that PAO432 is simply an alkane. However cationic dyes did partition into a 0.1 M solution of 4 in PAO432 after mixing for 5 min. In these experiments, 99% of 1.6 and 1.4 mM methylene blue and safranine O solutions, respectively, and 90% of a 1.4 mM malachite green solution could be extracted into PAOs in this fashion. Though we did not carry out extensive studies on cationic dye extraction, we speculate that we could have recycled the PAO solution of 4 that had sequestered cationic dyes by protonating the PIB enolate to dissociate the cationic dye from 4.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/technologies12080138/s1. Reference [43] is cited in Supplementary Materials.
Author Contributions
Conceptualization, D.E.B. and N.R.; data curation, N.R.; formal analysis, D.E.B. and N.R.; funding acquisition, D.E.B.; investigation, N.R.; methodology, D.E.B. and N.R.; project administration, D.E.B. and N.R.; resources, D.E.B.; supervision, D.E.B.; validation, M.P.A. and C.H.; visualization, N.R.; writing—original draft, N.R.; writing—review and editing, D.E.B. and N.R. All authors have read and agreed to the published version of the manuscript.
Funding
Mara P. Alonso was funded by the NSF REU program, NSF CHE 1851936.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations were used in this manuscript:
| MeI | Methyl iodide |
| MeCN | Acetonitrile |
| NMR | Nuclear magnetic resonance |
| PAO | Poly(α-olefin) |
| PDI | Polydispersity index |
| PIB | Polyisobutylene |
| rpmrt | Revolutions per minuteRoom temperature |
| SN2 | Bimolecular nucleophilic substitution |
| UV–visible | Ultraviolet–visible |
References
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxiol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.S.; Jeon, B.H.; Park, Y.K. A Review on Recent Advances in the Treatment of Dye-Polluted Wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Trivedi, A.; Desireddy, S.; Chacko, S.P. Effect of pH, Salinity, Dye, and Biomass Concentration on Decolourization of Azo Dye Methyl Orange in Denitrifying Conditions. Water 2022, 14, 3747. [Google Scholar] [CrossRef]
- Majeed, F.; Razzaq, A.; Rehmat, S.; Azhar, I.; Mohyuddin, A.; Rizvi, N.B. Enhanced dye sequestration with natural polysaccharides-based hydrogels: A review. Carbohydr. Polym. 2024, 330, 121820. [Google Scholar]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Bruggen, B.V.D. Environmental Impacts and Remediation of Dye-Containing Wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Li, M.; Yao, Y.; Zhang, W.; Zheng, J.; Zhang, X.; Wang, L. Fractionation and Concentration of High-Salinity Textile Wastewater using an Ultra-Permeable Sulfonated Thin-film Composite. Environ. Sci. Technol. 2017, 51, 9252–9260. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; De Vito, S.C. Predicting Azo Dye Toxicity. Crit. Rev. Env. Sci. Tec. 1993, 23, 249–324. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A Critical Review on Advances in the Practices and Perspectives for the Treatment of Dye Industry Wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Aziz, A.A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.D. Performance Evaluation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Miller, R.E. Ozonation of Azo and Azomethine Double Bonds. J. Org. Chem. 1961, 26, 2327–2330. [Google Scholar] [CrossRef]
- Farooq, U.; Phul, R.; Alshehri, S.M.; Ahmed, J.; Ahmad, T. Electrocatalytic and Enhanced Photocatalytic Applications of Sodium Niobate Nanoparticles Developed by Citrate Precursor Route. Sci. Rep. 2019, 9, 4488. [Google Scholar] [CrossRef] [PubMed]
- Routoula, E.; Patwardhan, S.V. Degradation of Anthraquinone Dyes from Effluents: A Review Focusing on Enzymatic Dye Degradation with Industrial Potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpää, M. EDTA-Cross-Linked β-Cyclodextrin: An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes. Environ. Sci. Technol. 2015, 49, 10570–10580. [Google Scholar] [CrossRef]
- Farrukh, A.; Akram, A.; Ghaffar, A.; Tuncel, E.; Oluz, Z.; Duran, H.; Rehman, H.; Yameen, B. Surface-Functionalized Silica Gel Adsorbents for Efficient Remediation of Cationic Dyes. Pure Appl. Chem. 2014, 86, 1177–1188. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. Dyes Removal from Textile Wastewater by Agricultural Waste as an Absorbent—A Review. Clean. Waste Syst. 2022, 3, 100051. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-Based Adsorbents for Removal of Dyes from Wastewater: A Review. Front. Environ. Sci. 2021, 9, 764958. [Google Scholar] [CrossRef]
- Bouchal, R.; Prelot, B.; Hesemann, P. Alkylguanidinium Based Ionic Liquids in a Screening Study for the Removal of Anionic Pollutants from Aqueous Solution. RSC Adv. 2016, 6, 39125–39130. [Google Scholar] [CrossRef]
- Fan, J.; Fan, Y.; Zhang, S.; Wang, J. Extraction of Azo Dyes from Aqueous Solutions with Room Temperature Ionic Liquids. Sep. Sci. Technol. 2011, 46, 1172–1177. [Google Scholar] [CrossRef]
- Makrygianni, M.; Lada, Z.G.; Manousou, A.; Aggelopoulos, C.A.; Deimede, V. Removal of Anionic Dyes from Aqueous Solution by Novel Pyrrolidinium-Based Polymeric Ionic Liquid (PIL) as Adsorbent: Investigation of the Adsorption Kinetics, Equilibrium Isotherms and the Adsorption Mechanisms Involved. J. Environ. Chem. Eng. 2019, 7, 103163. [Google Scholar] [CrossRef]
- Shah, A.; Kuddushi, M.; Rajput, S.; El Seoud, O.A.; Malek, N.I. Ionic Liquid-Based Catanionic Coacervates: Novel Microreactors for Membrane-Free Sequestration of Dyes and Curcumin. ACS Omega 2018, 3, 17751–17761. [Google Scholar] [CrossRef]
- Shah, A.; Kuddushi, M.; Ray, D.; Aswal, V.K.; Malek, N.I. Sodium Salicylate Mediated Ionic Liquid Based Catanionic Coacervates as Membrane-Free Microreactors for the Selective Sequestration of Dyes and Curcumin. ChemSystemsChem 2019, 2, e1900029. [Google Scholar] [CrossRef]
- Kamenicka, B.; Svec, P.; Weidlich, T. Separation of Anionic Chlorinated Dyes from Polluted Aqueous Streams Using Ionic Liquids and their Subsequent Recycling. Int. J. Mol. Sci. 2023, 24, 12235. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E. Soluble Polymers as Tools in Catalysis. ACS Macro Lett. 2014, 3, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E.; Koshti, N.; Franchina, J.G.; Frels, J.D. Thermal Sequestration of metals from aqueous solutions using poly(N-isopropylacrylamide) Copolymers. Angew. Chem. Int. Ed. 2000, 39, 1039–1042. [Google Scholar] [CrossRef]
- Chouikhi, D.; Kulai, I.; Bergbreiter, D.E.; Al-Hashemi, M.; Bazzi, H.S. Functionalized Polyisobutylene and Liquid/Liquid Separations as a Method for Scavenging Transition Metals from Homogeneously Catalyzed Reactions. Appl. Sci. 2019, 9, 120. [Google Scholar] [CrossRef]
- Harrell, M.L.; Malinski, T.; Torres-Lopez, C.; Gonzalez, K.; Suriboot, J.; Bergbreiter, D.E. Alternatives for Conventional Alkane Solvents. J. Am. Chem. Soc. 2016, 138, 14650–14657. [Google Scholar] [CrossRef] [PubMed]
- Thavornpradit, S.; Killough, J.M.; Bergbreiter, D.E. Minimizing Solvent Waste in Catalytic Reactions in Highly Recyclable Hydrocarbon Solvents. Org. Biomol. Chem. 2020, 18, 4248–4256. [Google Scholar] [CrossRef]
- Fu, Y.-H.; Bergbreiter, D.E. Recyclable Polyisobutylene-Bound HMPA as an Organocatalyst in Recyclable Poly(a-Olefin) Solvents. ChemCatChem 2020, 12, 6050–6058. [Google Scholar] [CrossRef]
- Watson, C.; Kuechle, A.; Bergbreiter, D.E. Fully Recyclable Bronsted Acid Catalyst Systems. Green Chem. 2021, 23, 1266–1273. [Google Scholar] [CrossRef]
- Technical Data Sheets for SpectraSyn Low Viscosity Polyalphaolefins PAO. Available online: https://www.exxonmobilchemical.com/en/resources/product-data-sheets/synthetic-base-stocks/low-viscosity-polyalphaolefins (accessed on 25 July 2024).
- Chevron Phillips Polyalphaolefins Products. Available online: https://www.cpchem.com/what-we-do/solutions/polyalphaolefins/products (accessed on 25 July 2024).
- Malinski, T.J.; Bergbreiter, D.E. Safer Solvents for Reactive Organometallic Reagents. Tetrahedron Lett. 2018, 44, 3926–3929. [Google Scholar] [CrossRef]
- Thavornpradit, S.; Malinski, T.J.; Bergbreiter, D.E. Applications of poly(α-olefin)s as solvents in organometallic chemistry. J. Organomet. Chem. 2022, 962, 122261. [Google Scholar] [CrossRef]
- Malinski, T.J.; Bazzi, H.S.; Bergbreiter, D.E. Sustainable Hydrocarbon Oligomer Solvent Systems for Sequestration of Trace Organics from Water. ChemSusChem 2018, 12, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Edgehouse, K.; Rosenfeld, N.; Bergbreiter, D.E.; Pentzer, E.B. Capsules of the poly(α-olefin) PAO432 for removal of BTEX contaminants from water. Ind. Eng. Chem. Res. 2021, 60, 14455–14463. [Google Scholar] [CrossRef]
- Rosenfeld, N.; Quinn, E.; Malinski, T.J.; Bergbreiter, D.E. Sequestration of Phenols from Water into Poly(α-Olefin)s Facilitated by Hydrogen Bonding Polyisobutylene Additives. ACS ES&T Water 2022, 2, 1391–1401. [Google Scholar]
- TPC Technical Data Sheets. Available online: https://www.tpcgrp.com/products/technical-information (accessed on 25 July 2024).
- BASF Technical Information HR PIB. Available online: https://www.basf-petronas.com.my/sites/default/files/BPC%20-%20HR%20PIB_final_Nov%2017.pdf (accessed on 25 July 2024).
- Harrell, M.J.; Bergbreiter, D.E. Using 1H NMR Spectra of Polymers and Polymer Products to Illustrate Concepts in Organic Chemistry. J. Chem. Educ. 2017, 94, 1668–1673. [Google Scholar] [CrossRef]
- Hugar, K.M.; Kostalik, H.A.; Coates, G.W. Imidazolium Cations with Exceptional Alkaline Stability: A Systematic Study of Structure–Stability Relationships. J. Am. Chem. Soc. 2015, 137, 8730–8737. [Google Scholar] [CrossRef] [PubMed]
- Samunual, P.; Bergbreiter, D.E. SN2 Reactions in Hydrocarbon Solvents Using Ammonium-Terminated Polyisobutylene Oligomers as Phase-Solubilizing Agents and Catalysts. J. Org. Chem. 2018, 83, 11101–11107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sung, S.; Tian, J.; Bergbreiter, D.E. Polyisobutylene Supports—A Nonpolar Hydrocarbon Analog of PEG Supports. Tetrahedron 2005, 61, 12081–12092. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).