Abstract
A new prototype device to monitor breathing in children diagnosed with central sleep apnoea (CSA) was developed. CSA is caused by the failure of central nervous system signals to the respiratory muscles and results in intermittent breathing pauses during sleep. Children diagnosed with CSA require home respiration monitoring during sleep. Apnoea monitors initiate an audio alarm when the breath-to-breath respiration interval exceeds a preset time. This allows the child’s parents to attend to the child to ensure safety. The article describes the development of the monitor’s hardware, software, and evaluation. Features of the device include the detection of abnormal respiratory pauses and the generation of an associated alarm, the ability to record the respiratory signal and its storage using an on-board disk, miniaturised hardware, child-friendliness, cost-effectiveness, and ease of use. The device was evaluated on 10 healthy adult volunteers with a mean age of 46.6 years (and a standard deviation of 14.4 years). The participants randomly intentionally paused their breathing during the recording. The device detected and provided an alarm when the respiratory pauses exceeded the preset time. The respiration rates determined from the device closely matched the values from a commercial respiration monitor. The study indicated the peak-detection method of the respiration rate measurement is more robust than the zero-crossing method.
1. Introduction
Central sleep apnoea (CSA) is temporary cessation of breathing caused by the failure of either the respiratory control centre in the brainstem or peripheral chemoreceptor responses to trigger a breath [1]. The condition is common in premature babies but persists into infancy and early childhood in around 1% of cases [2]. The clinical impact of CSA is potentially life-threatening, and associated with sudden unexplained death in infants (SUDI). In severe cases, babies are found unresponsive, accounting for 7.5% of calls to emergency medical services for infants [3], requiring resuscitation and hospital admission [4]; in less extreme cases, long-term sleep fragmentation for babies and children leads to persistent insomnia, educational difficulties, and cognitive and behavioural problems; repeated hypoxia leads to pulmonary hypertension and heart failure in later life. CSA is currently diagnosed and monitored using a cardio–respiratory sleep study (CRSS), requiring in-patient admission and intensive, costly, clinician interpretation. Apnoea monitors provide constant monitoring for those at risk [5], producing an alarm during sleep when the respiratory pauses exceed a preset time that is typically between 10 and 20 s. Currently, there are three main types of medical devices to monitor CSA. These are based on the following:
- A bubble sensor attached to the baby’s abdomen and connected by wire or tubing to the monitor, e.g., SISS Babycontrol®H (manufactured by Schulte-Elektronik GMBH Medical Technology, Olsberg, Germany).
- A large sensor pad placed under the baby’s mattress, e.g., Nanny (manufactured by Jablotron, Czech Republic, Europe)
- A small monitor that clips onto a nappy, e.g., Snuza HeroMD (developed by Snuza, UK)
Table 1 provides an overview of some commercial apnoea monitors.

Table 1.
Examples of some commercial apnoea monitors.
There are also some commercial movement monitors available to parents as ‘reassurance’ monitors but which are not certified as medical devices. There is a risk associated with these devices, as parents may rely upon the monitor for assurance that the baby is well and not look out for other signs of illness [6].
The CSA device developed in this study aims to improve on existing devices by accomplishing the following:
- Ensuring child-friendly features. The device is miniaturised and comfortable to wear by children of any age, including neonates and infants.
- Applicability of use to both homes and hospitals.
- Reducing false alarms. A false alarm occurs when the breathing is normal during sleep but the device detects a breathing pause exceeding the preset time and triggers an alarm. False alarms may heighten parents’ anxiety and lead to disturbance of the child’s sleep. These may occur due to the disconnection of the sensor from the base unit, or shallow abdominal movements that are not detected [7].
- Avoidance of the child’s entanglement by wires or tubing. The device is fully contained in a soft elastic band and thus no wiring or tube is needed to connect it to a base unit. Monitors that use wires or tubing to connect their sensors to their base units pose the risk of babies becoming entangled with the sensor leads [8].
Apnoea monitors are issued via health services (e.g., the UK’s National Health Service, NHS) for parental reassurance after an acute unresponsive episode, premature birth, or for the CONI (Care Of the Next Infant) scheme after a family has suffered a SUDI. Concerned parents may buy non-medical apnoea monitors for peace of mind, even though their baby may not be in a risk category. Although there is no research evidence that apnoea monitors prevent sudden infant death syndrome (SIDS) [9,10], a monitor can help parents to look after their baby with more confidence, and 91% of the parents using monitors provided by CONI said the monitor was helpful or very helpful [11].
A review of the existing literature revealed a range of potential technological solutions. These were either adapted in the current prototype design or have been identified to be implemented in its future models. Special consideration was given to the fragile skin and chest dimensions of infants [12]. Pressure and resistive sensors are very sensitive [13] and informed our sensor laboratory testing. Other sensors, such as impedance or humidity [14] incorporated into a facemask sensor [15]; acoustic sensors [16] in a microphone near the nose, mouth, and suprasternal notch; accelerometers [17]; respiratory inductance plethysmography [18]; and optical light sources [19] were discounted on the grounds of reported discomfort [14,15], ambient noise and movement artefacts [16,17,19], and low sensitivity [18]. Infrared light-emitting diodes and electrocardiogram signals have also been used but do not directly detect respiratory signals [18,20]. The ultrasound [21] and infrared thermal imaging [22,23] methods of respiratory monitoring are non-contact and require the subject to remain in the field of view of the monitoring device thus large movements become an issue. The aim of the study was to develop and evaluate a new cost-effective and child-friendly prototype CSA monitor. The research questions were as follows:
- What are the technological challenges in the design and development of a CSA monitor and the manner they could be adapted?
- How well does the developed device perform during testing on healthy adult volunteers?
The contributions of this study include the following:
- The development of a new, rechargeable, cost-effective, and child-friendly prototype CSA monitor (called RespyBelt) integrating respiration signal recording and on-board data storage, respiratory pause detection, and an associated alarm.
- Evaluation of the device in a research laboratory environment on 10 healthy adult volunteers. This included comparing the respiration rate values obtained from the device with the values obtained from a commercial respiratory monitor.
- Comparison of effectiveness of peak-detection and zero-crossing detection methods for breath-to-breath respiration rate measurement.
- Identification of advanced features to be adapted in the device’s future models.
In the following sections, the methodology employed is outlined, the results are discussed, and the study’s conclusion is provided.
2. Materials and Methods
This section outlines the development of RespyBelt and its evaluation on 10 healthy adult volunteers. The device’s development followed a V-shaped process model where, at different stages, an associated validation was undertaken to ensure correct operation. This applied to both the hardware and software. Life-cycle assessment (LCA) was carried out to ensure the device’s sustainable production, use, and environmental conformation. Initially, the hardware development will be described, and then its software, followed by a discussion of the methodology for the testing on 10 heathy adult volunteers. The statistical analysis to interpret the operation of the device is also described.
2.1. Hardware Development
The specifications of RespyBelt were established by examining the requirements of a modern and effective CSA monitor. These included the following:
- A 16-bit data capture, providing an accurate analogue-to-digital conversion of the sensor signal.
- A respiratory signal sample rate of 32 samples per second. Respiration rate does not typically exceed 60 breaths per minute (bpm). This corresponds to 1 breath per second, or 1 Hz. The sample rate was therefore sufficiently high.
- On-board (integrated), fast secure digital (SD) synchronous data storage.
- Electromagnetic compatibility (EMC) and high-frequency compatibility.
- Velcro attachment for easy fitting/removal of the device and being trap-proof, i.e., the device’s release from the child’s body when sharply pulled or trapped.
- A washable and reusable cover for the device and its sensor.
- Ease of use and comfort for children of any age.
- Safe to use on patients for a long-duration recording.
- Rechargeable power supply.
- Flexibility to adapt new features and technologies in future models.
- Modular design allowing ease of fault finding and repair.
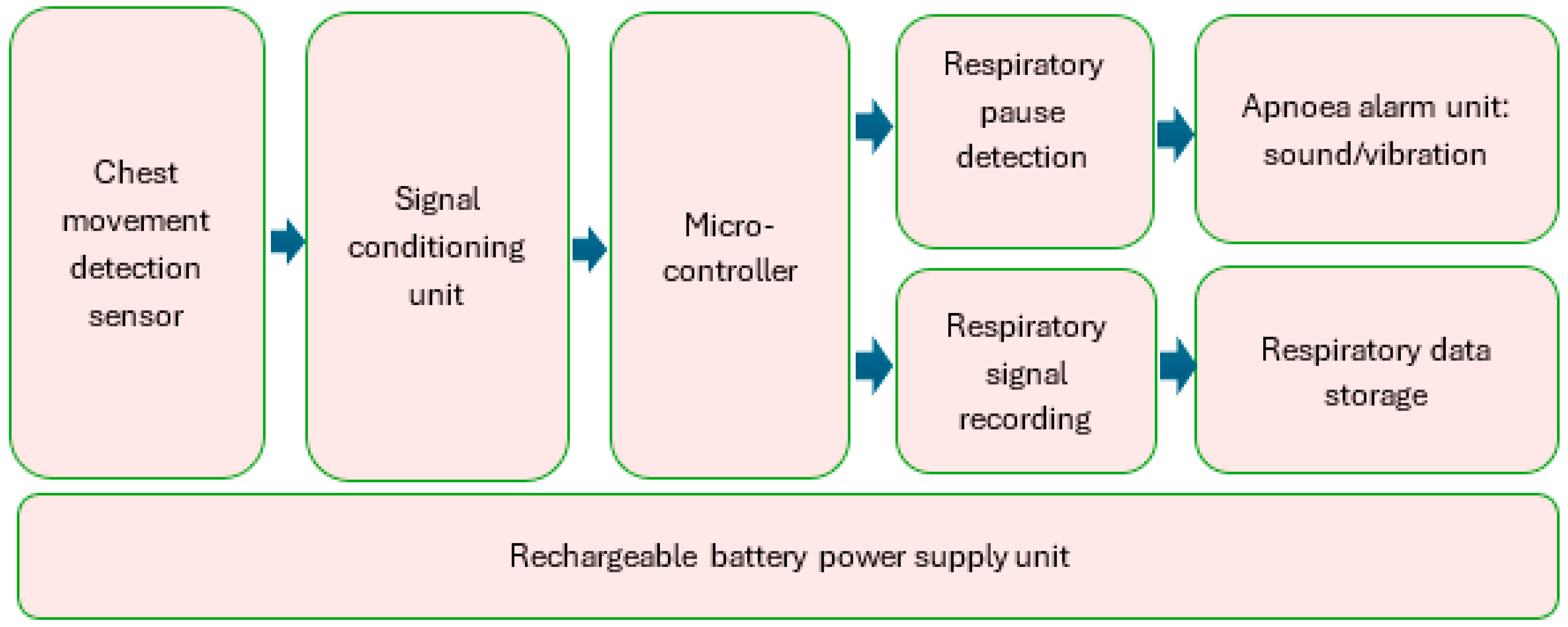
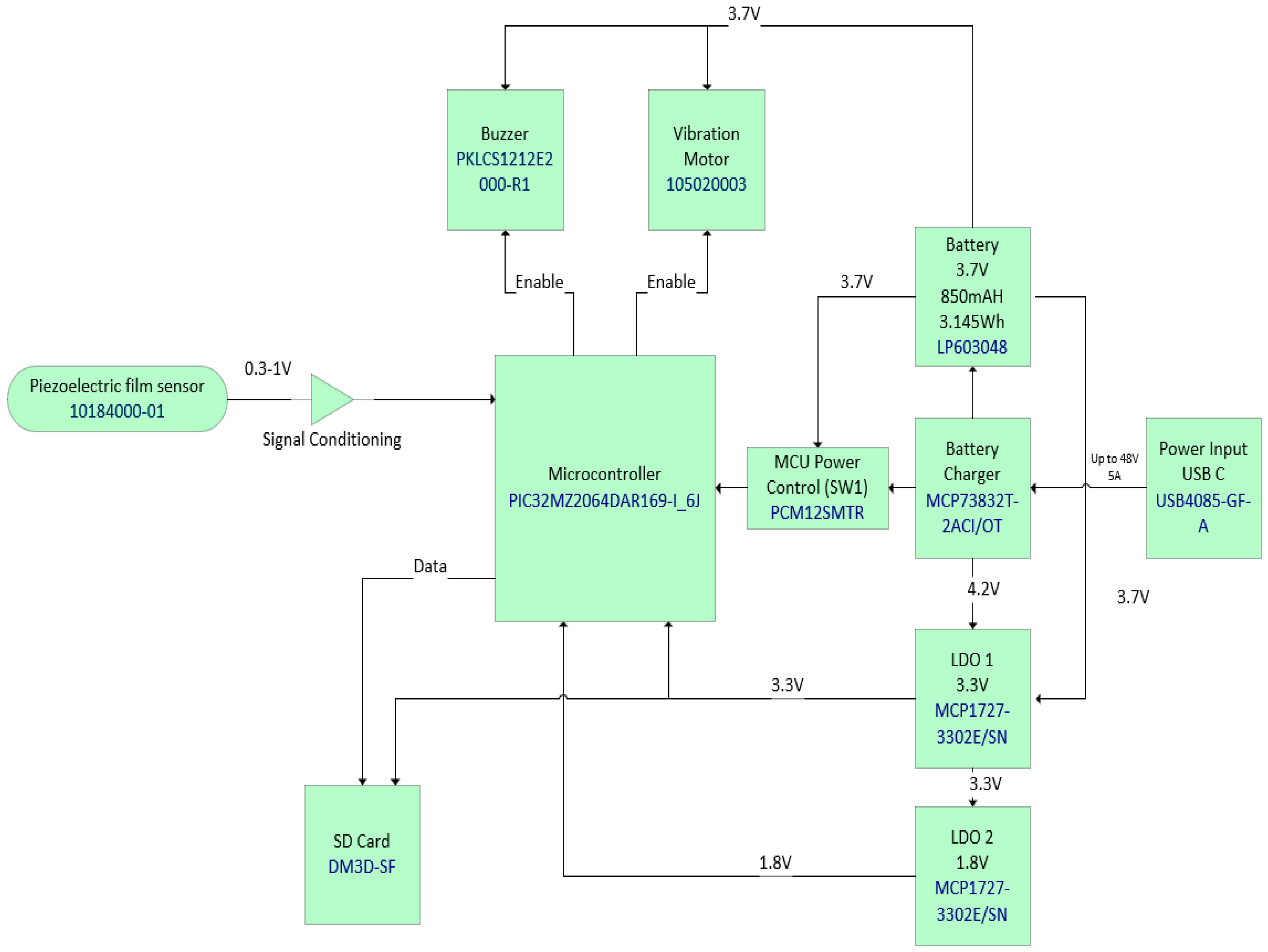
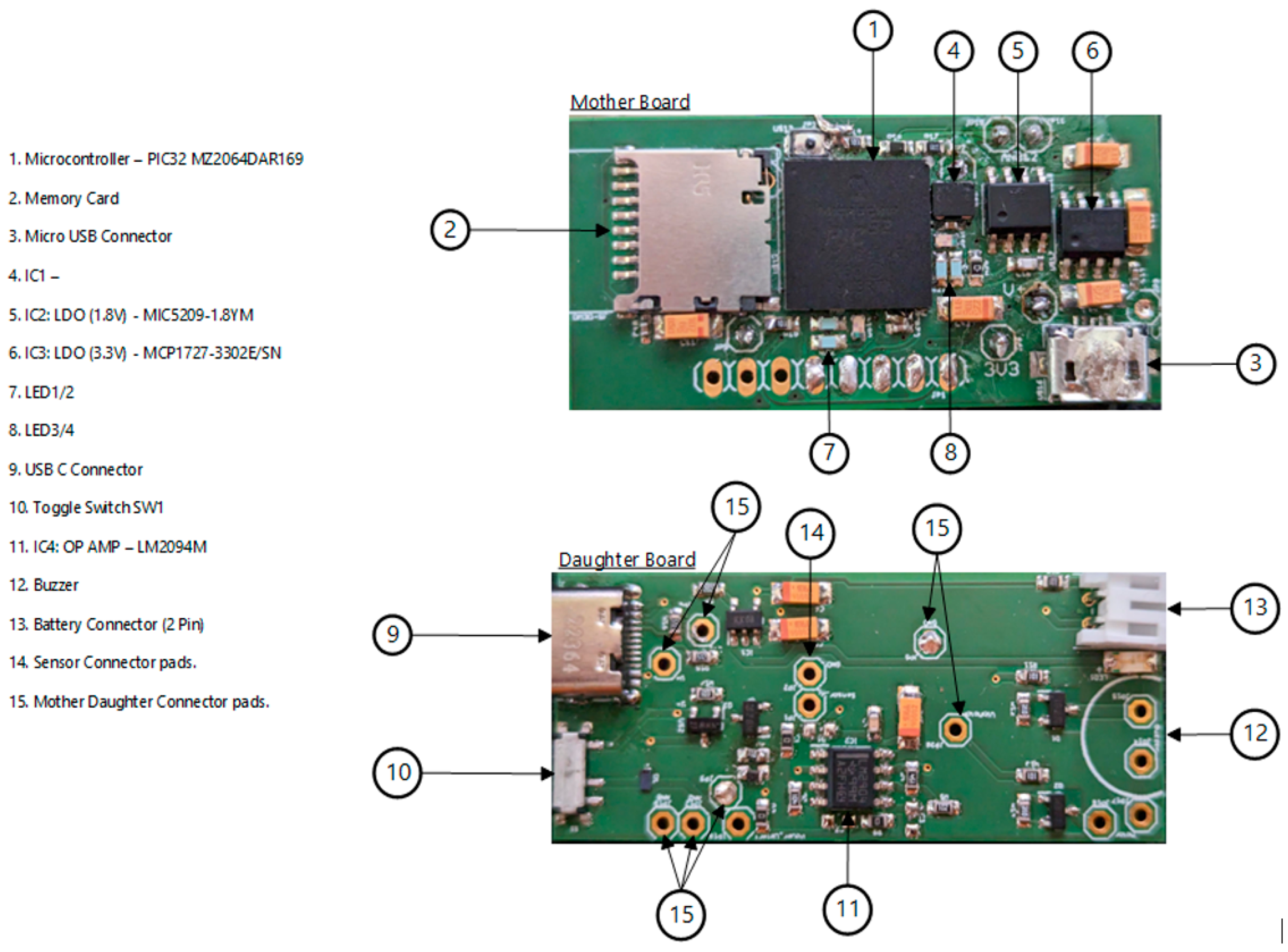
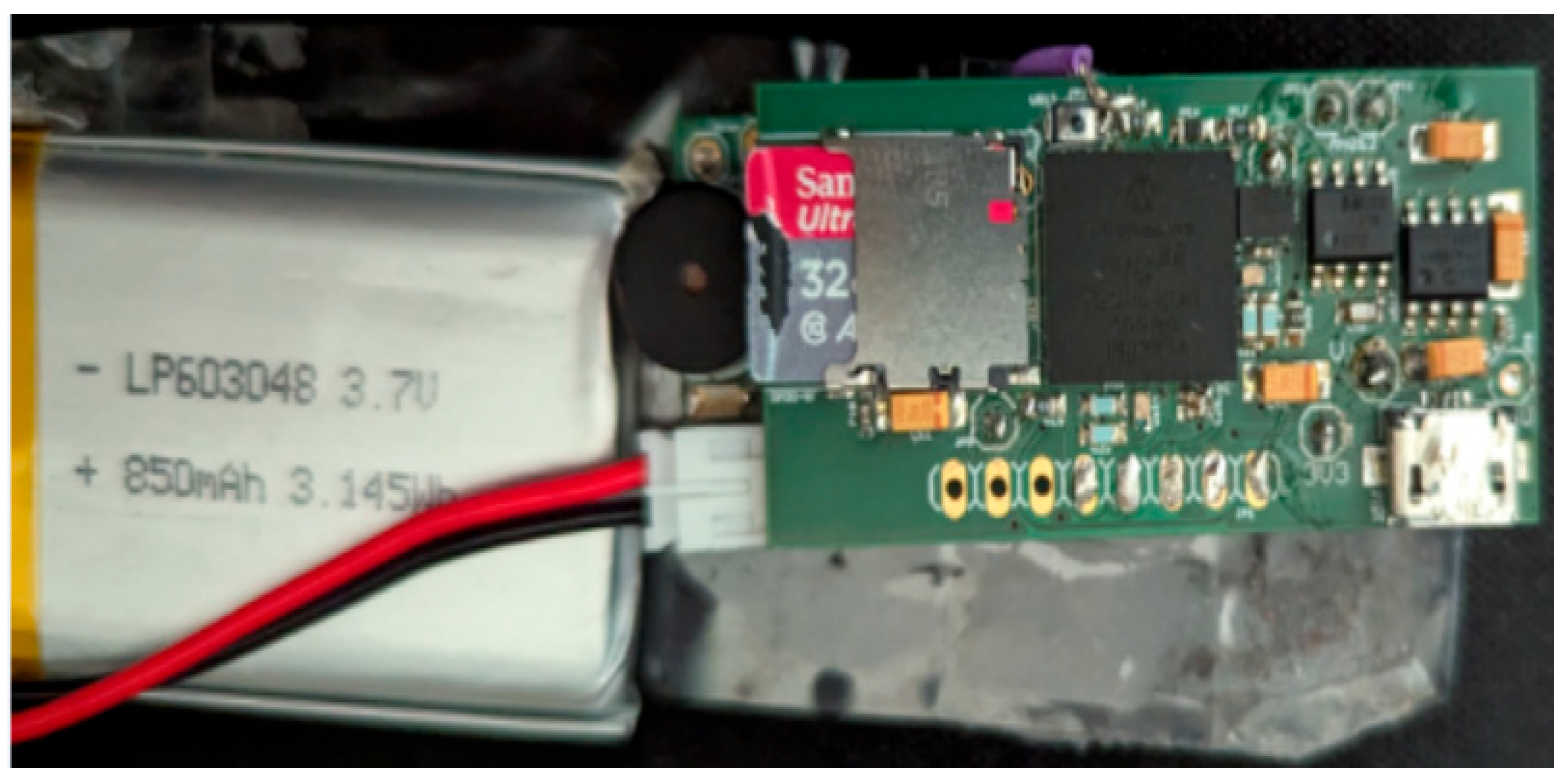
The block diagram of RespyBelt is shown in Figure 1. Its hardware interconnections are shown in Figure 2. Its pictures in different settings are provided in Figure 3, Figure 4, Figure 5 and Figure 6.
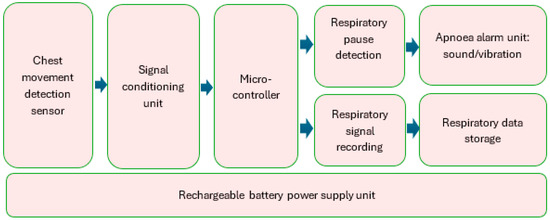
Figure 1.
Block diagram of RespyBelt.
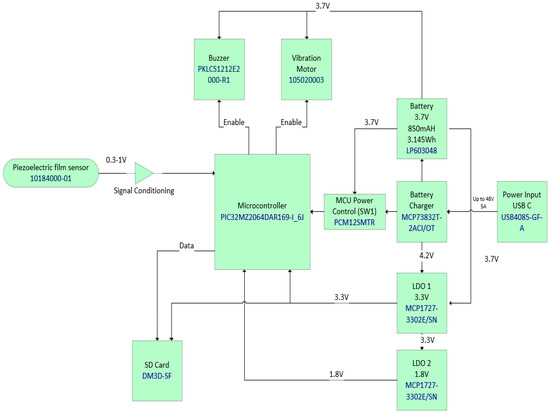
Figure 2.
RespyBelt hardware components interconnections and their types.
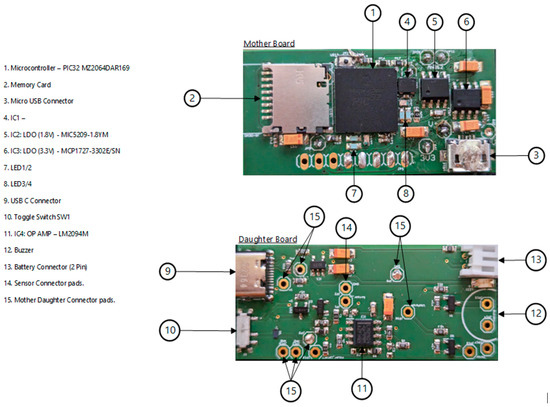
Figure 3.
The mother and daughter boards of RespyBelt.
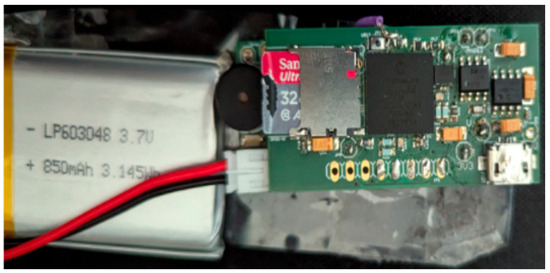
Figure 4.
RespyBelt mother board connected to its rechargeable battery.
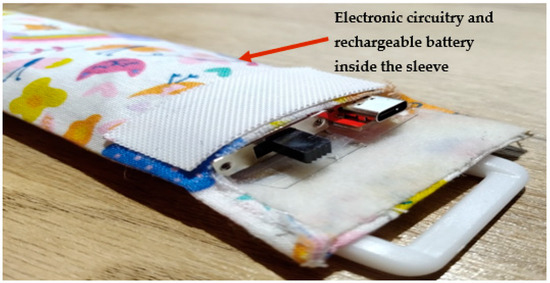
Figure 5.
RespyBelt inside its cover showing its ON/OFF power supply switch and USB port.
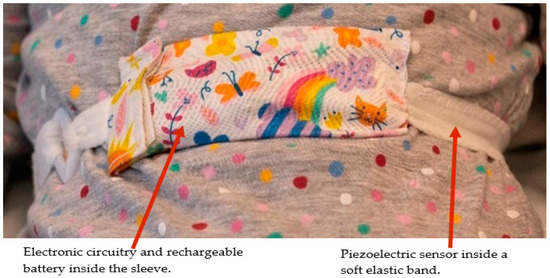
Figure 6.
RespyBelt worn by a child.
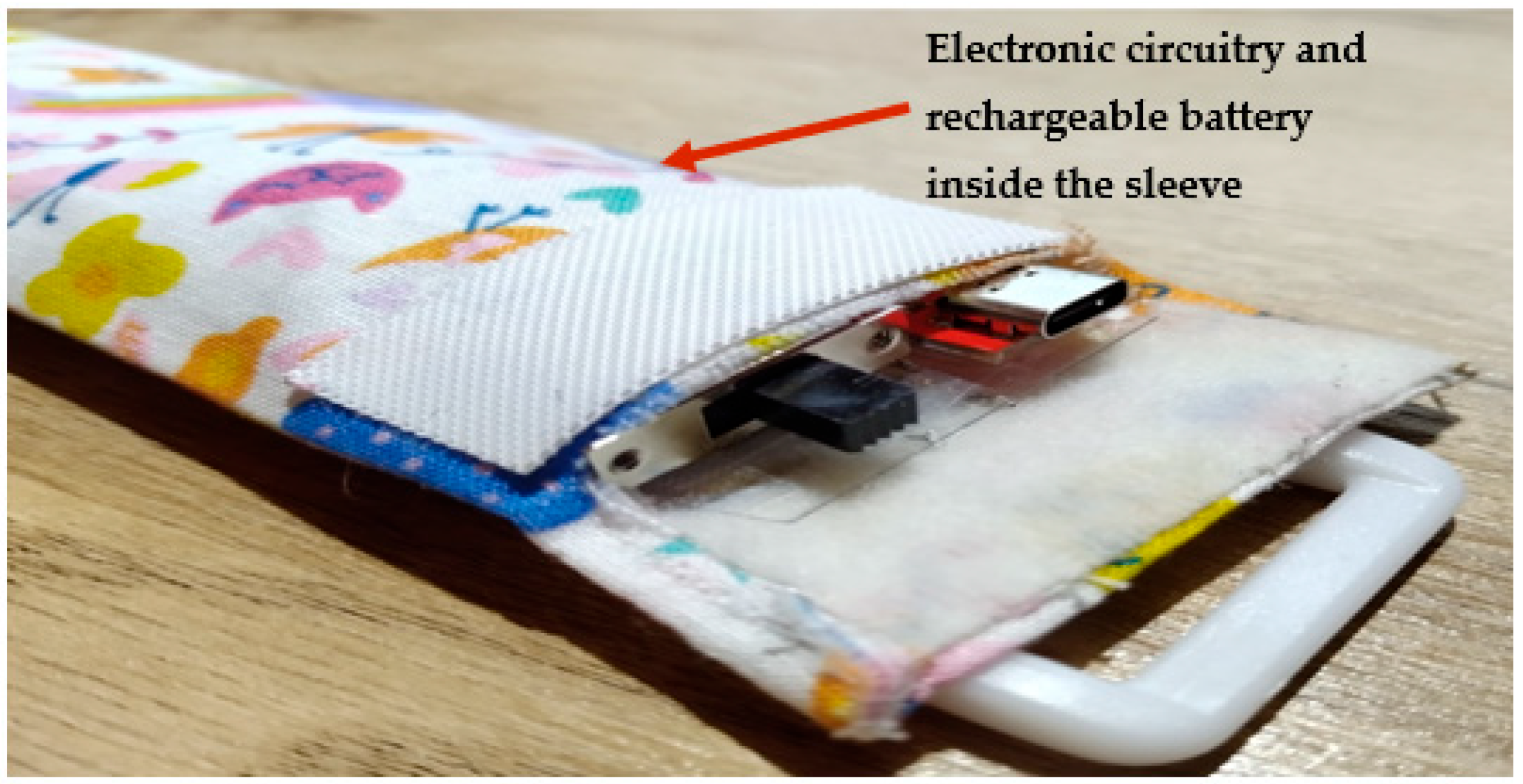
The device consists of a 60 cm piezoelectric film sensor (model: 10184000–01, manufacturer: Measurement Specialities, Hampton, Virginia, USA). It converts the chest movements due to respiratory efforts into an electrical signal. The sensor is covered with a removable soft elastic band that easily wraps around the chest (Figure 6). Its electronic components and rechargeable battery are housed in a washable sleeve, as shown in Figure 6.
Its hardware components consist of the following:
- Integrated circuit (IC) microcontroller (model: PIC32MZ2064DAR169-I_6J, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Secure digital (SD) card (model: DM3D-SF, manufacturer: Hirose Electric Co., Ltd., Kanagawa, Japan), a dedicated high-speed, secure digital high-capacity (SDHC) controller.
- Lithium polymer (LiPo) charger built in for a 5 Volt supply (model: MCP73832T-2ACI/OT, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Microcontroller power switch, ON/OFF (model: PCM12SMTR, manufacturer: C&K Switches, Waltham, MA, USA).
- Rechargeable battery, 850 mAH, 3.7 Volts, and 3.145 WH (model: LP603048).
- Power input USB (model: USB4085-GF-A, manufacturer: Global Connector Technology Limited, Hertfordshire, UK).
- Low-dropout voltage regulator (LDO1), 3.3 Volts and 1.5 A (model: MCP1727-3302E/SN, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Low-dropout voltage regulator (LDO2), 3.3 Volts and 1.5 A (model: MCP1727-3302E/SN, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Audio piezo transducer (buzzer), frequency = 2 kHz and sound pressure level of 76 dB (typical) (model: PKLCS1212E2000-R1, manufacturer: Murata Electronics, Munich, Germany).
- Seeed Grove—vibration ‘coin’-type vibration motor (model: 105020003, manufacturer: Seeed Technology Co., Ltd., Fremont, CA, USA).
The signal from the sensor is filtered and amplified as a part of the signal conditioning. The filter type is a 4th-order bandpass active filter with a low cutoff frequency of 0.1 Hz and a high cutoff frequency of 1 Hz. The device is controlled by a microcontroller (model: PIC32MZ2064DAR169-I_6J, manufacturer: Microchip Technology, Arizona, United States). It provides a microprocessor unit performance, 2 MB flash and 640 kB of static random memory (SRAM), 12-bits, and has an 18 mega samples per second (MSPS) analogue-to-digital convertor (ADC) module capability. The pressure variations exerted by the chest on the piezoelectric sensor while the device is worn convert the respiration efforts into a respiratory signal that is then stored on a secure digital (SD) card (model: DM3D-SF, manufacturer: Hirose Electric Co Ltd, Kanagawa, Japan). The respiratory signal can be downloaded by removing the SD card from the device’s port and inserting it into a computer. Some computers have a dedicated SD card port, but otherwise an SD card adaptor can be used for this purpose.
The device uses a rechargeable battery of 850 mAH, 3.7 Volts, and 3.145 WH (model: LP603048). Its charging is facilitated by a Lithium polymer (LiPo) charger built in for a 5 Volt supply (model: MCP73832T-2ACI/OT manufacturer: Microchip Technology, Arizona, United States). The alarm is generated by an audio piezo transducer (buzzer) with a frequency = 2 kHz and a sound pressure level of 76 dB (typical) (model: PKLCS1212E2000-R1, manufacturer: Murata Electronics, Munich, Germany). A Seeed Grove—vibration ‘coin’-type vibration motor (model: 105020003) is also included for any subject that may have difficulty hearing sounds. It vibrates upon detecting respiratory pauses. The device has an on-board data storage facility capable of storing respiration signals for multiple nights. This is based on a secure digital (SD) card (model: DM3D-SF) that uses a dedicated secure digital high-capacity (SDHC) controller.
The microcontroller communicates with the SD card over I2C. The components are mounted on a printed circuit board (PCB). The PCB was constructed using a 4-layer board incorporating a mixed signal design, separating the analogue and digital circuitry to maximise the signal integrity whilst also using surface-mounted device (SMD) components wherever possible to keep the design compact.
2.2. Software Development
The software elements associated with the operation of RespyBelt (version 0.1.1) are as follows:
- The software is integrated into the RespyBelt microcontroller. This is operated in real-time and is described in Section 2.2.1
- The software that performs the signal processing and statistical analysis. This operated off-line and is described in Section 2.2.2.
2.2.1. Real-Time Processing Software
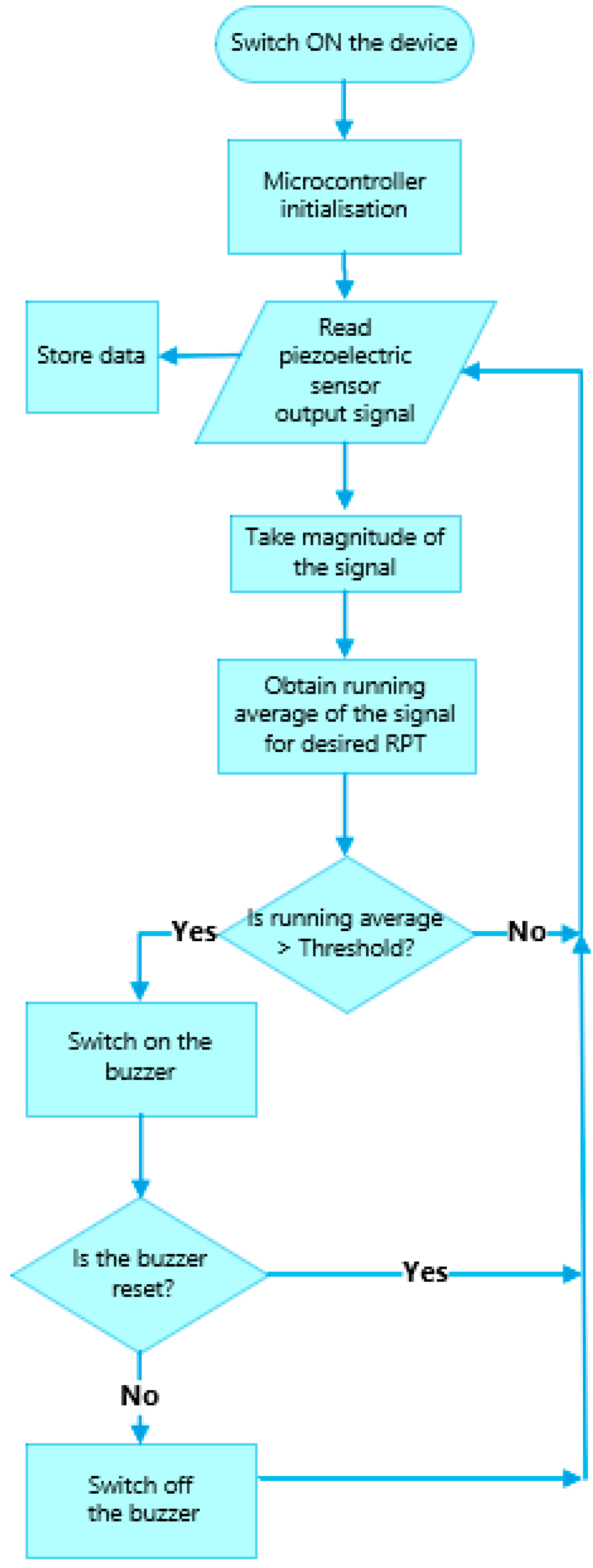
The flowchart of the software integrated into the RespyBelt’s microcontroller is provided in Figure 7.
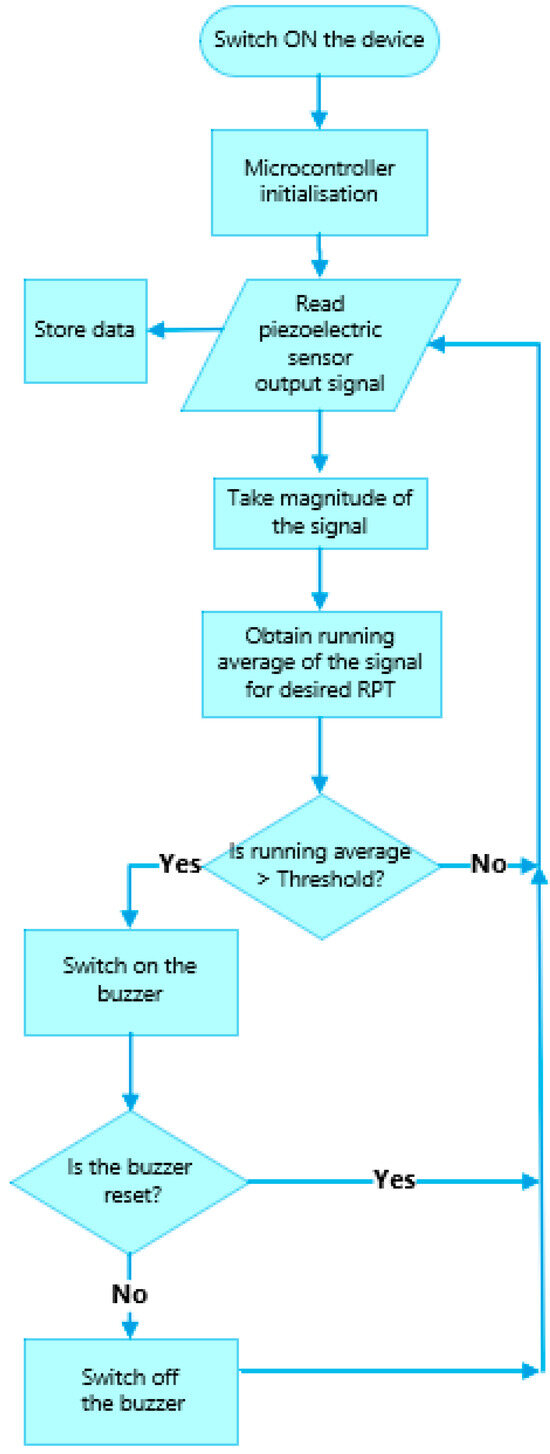
Figure 7.
Flowchart of the RespyBelt software operating in real-time (RPT: respiratory pause time), indicating the manner respiratory pauses are detected.
The microcontroller is initialised once the device is turned on. The piezoelectric film sensor converts the chest movements into an electrical signal. The microcontroller keeps a running average of the signal’s magnitude (i.e., the absolute value of the signal was obtained with the negative cycle reverting to positive) for the stated respiratory pause time (RPT) requiring an alarm. Typically, the RPT is a value chosen between 10 s and 20 s, based on the interval known to lead to significant clinical consequences, e.g., bradycardia or oxygen desaturation. When the running average exceeded this predefined threshold value, the breathing was considered to conform to RPT and an alarm was triggered. The threshold value was determined experimentally as 0.3 Volts. The alarm would remain on until the parent attended to the child to ensure their safety and reset the alarm.
2.2.2. Off-Line Processing Software
To evaluate the operation of the RespyBelt, the breath-by-breath respiration rate in breaths per minute (bpm) was determined off-line using Matlab© (MathWorks®, Natick, MA, USA) from its recorded respiratory signals. The measurements were compared with the respiration rates obtained from respiration signals recorded using a commercial respiratory measurement device called SOMNOtouchTM RESP (SOMNOmedics AG, Randersacker, Germany). The respiratory signal was initially filtered using a 4th-order lowpass Butterworth filter with a cutoff frequency of 1 Hz. This further removed unwanted noise. The Butterworth filter provided a flat passband in its magnitude frequency response, while the 4th-order filter ensured a sufficiently steep transition from the passband to the stopband. The chosen cutoff frequency of 1 Hz corresponded to 1 respiratory cycle per second or a respiration rate of 60 breaths per minute (bpm). The respiratory signal was then normalised to its magnitude by dividing its sample values by the absolute of the maximum sample value present in the signal. This resulted in the signal amplitude in a range between −1 and 1 (no unit). This operation was performed with respect to the desired information related to the signal’s frequency, not its amplitude.
Two approaches to determine the respiration rate were explored. These were the peak detection and zero-crossing detection. Both approaches allowed for a breath-by-breath (i.e., individual respiratory cycle) respiration rate measurement and had a low computational requirement, making them suitable for real-time operations. For the peak detection, the Matlab© (version 2024) function ‘findpeaks’ was used. This function identified the signal’s local peaks as defined by the instantaneous amplitude of the signal (sample value) being larger than the two neighbouring sample values. Its operation could be defined by multiple parameters. The Matlab© instruction used for this purpose was as follows:
[peaks, locations] = findpeaks(signal, sample_rate, ‘MinPeakDistance’, MinPeakDistance_value, ‘MinPeakHeight’, MinPeakHeight_value)
The definitions of the parameters in the above instruction are as follows:
- signal: The respiratory signal.
- sample_rate: The signal sample rate. This was 32 samples per second for both the RespyBelt and SOMNOtouch devices.
- ‘MinPeakDistance’: This indicates the next minimum peak distance (in seconds). The minimum peak distance provides a mechanism to avoid the false detection of spurious peaks caused by noise. It identifies the peaks separated by more than the minimum peak distance value (specified by MinPeakDistance_value). In this study, the MinPeakDistance_value was 1 (i.e., 1 s). A time of 1 s was chosen for this parameter, as the respiration rate does not typically exceed 60 bpm, which corresponds to 1 Hz (a period of 1 s).
- ‘MinPeakHeight’: This provides a means to select the peaks that are greater than the specified highlight (specified by MinPeakHeight_value). This was a further feature to avoid spurious small peaks introduced by noise. In this study, to determine the MinPeakHeight_value, the absolute value of the respiratory signal was initially obtained (which ensured the negative part of the signal reverted to positive values). The average value of the resulting signal was obtained and then the value was divided by 3. This meant that those peaks with amplitudes of less than 1/3 of the average height of the signal were excluded as a part of the respiration rate measurement. Using a smaller height increased the risk of selecting spurious peaks and a larger height increased the risk of missing relevant peaks.
The function returned two arrays. These included the following:
- peaks: This contains the amplitudes of the identified peaks. However, this array was not required in this study, as only the signal frequency was related to the respiration rate.
- locations: This contains the time locations (indices) of the identified peaks. The breath-by-breath respiration rate in bpm was determined by the following:
The second method to determine the respiration rate was by identifying the time points that the signal crossed the horizontal axis (i.e., the amplitude changing from a positive to a negative value) on the falling edge of the signal. Although this study considered the falling edge, the rising edge would have been equally valid. Once these zero-crossing time locations were identified, Equation (1) was used to determine the respiration rate.
2.3. Particupant Recruitment, Validation, and Statistical Analysis
As the device was a proof-of-concept prototype, it was evaluated on 10 healthy adult volunteers in a research laboratory. The mean and standard deviation of their age were 46.6 years and 14.4 years, respectively. The participants were recruited from the School of Engineering and Built Environment of Sheffield Hallam University. These were all male students and staff from the school. They consented to take part after reading the provided study’s information sheet. The study had Sheffield Hallam University ethical approval. Female participants were not deliberately excluded.
The device was attached to the participant’s chest and the recording was undertaken while they laid down. The duration of the recording was about two minutes. The participants were asked to intentionally hold their breath at random intervals during the recording for a few seconds to simulate CSA episodes. These data were initially stored on the device’s SD card; for processing, they were transferred to a PC. The processing was performed off-line using Matlab© (MathWorks®, USA). As the adult participants' respiration rate was about 20 bpm, the recording of two minutes provided around 40 breath-to-breath respiration rate readings per participant that were considered sufficient for statistical analysis. Statistical tests were used to compare the respiration rates measured using RespyBelt and SOMNOtouch. The Shapiro–Wilk test was used to determine whether the difference between the respiration rates across the 10 participants were from a normal distribution. For this test, the null hypothesis was that the difference was from a normal distribution. The hypothesis was rejected when the statistical probability (p-value) was less than 0.05 for a 95% confidence interval.
As the difference was from a normal distribution, a paired t-test was used to determine whether there existed a significant difference between the means of the respiration rates obtained from the two devices. The null hypothesis for this test was that the difference came from a normal distribution, with a mean equal to zero and an unknown variance. For probability (p) values greater than 0.05 (i.e., 95% confidence level), the null hypothesis was not rejected.
3. Results
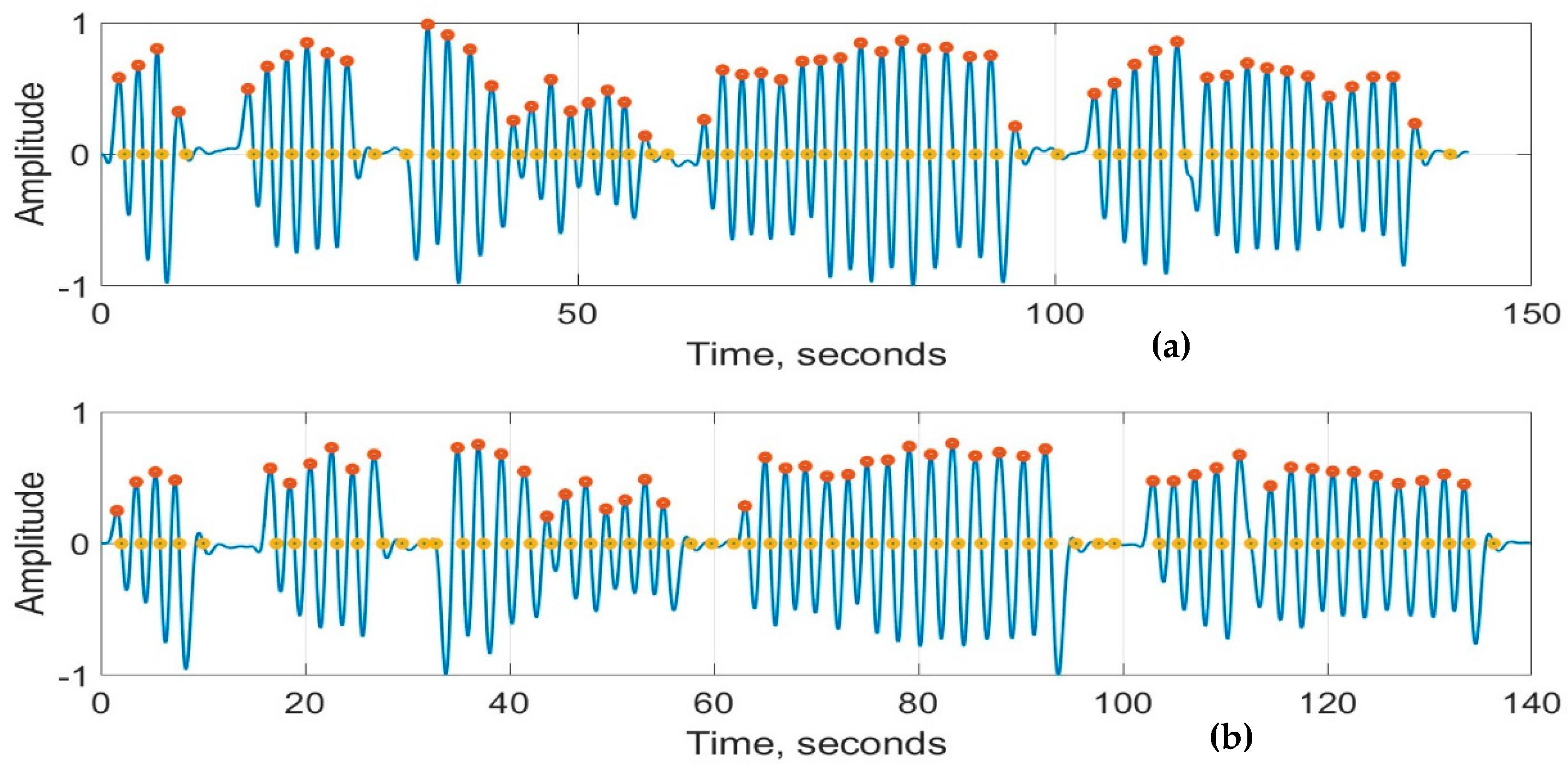
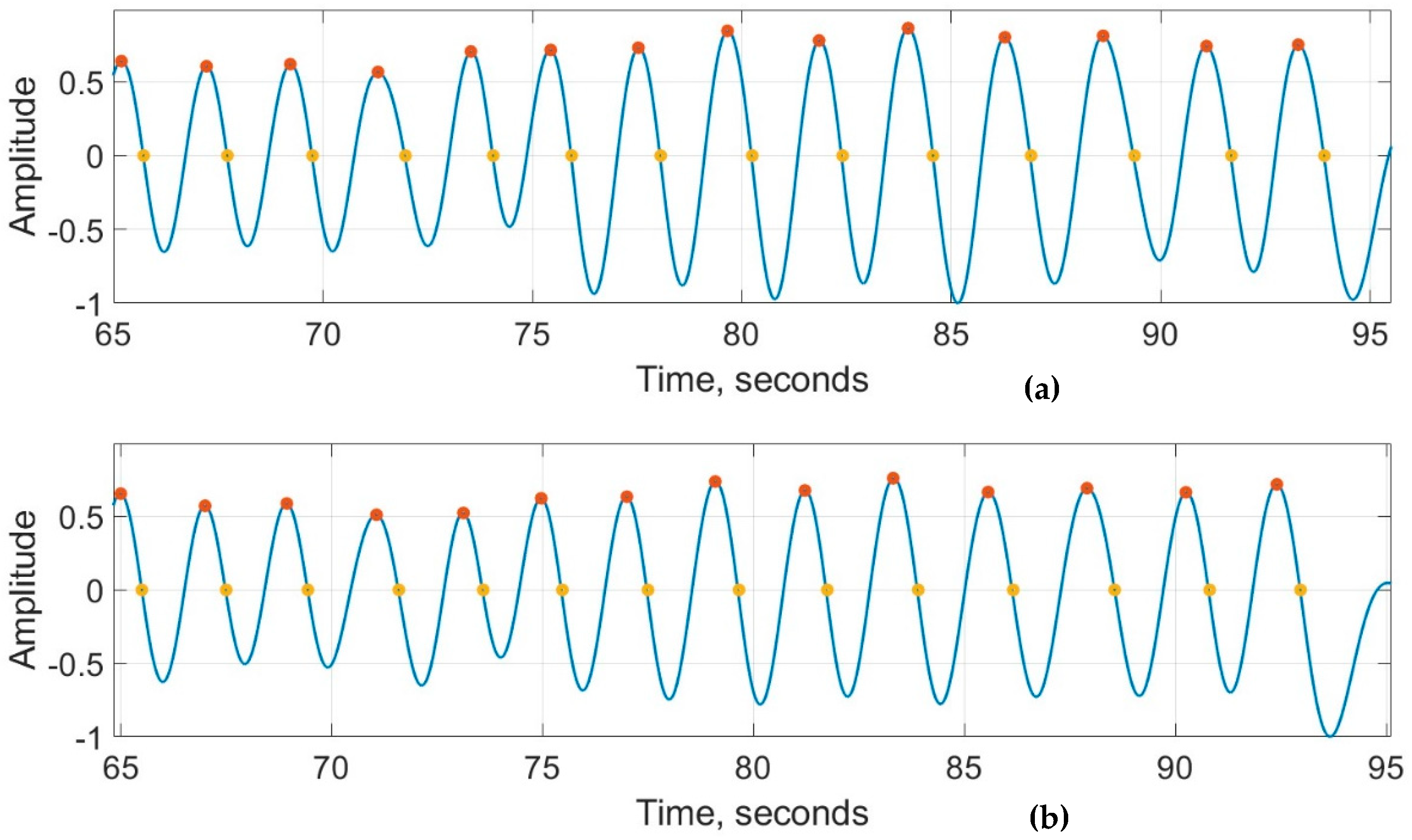
Figure 8a,b show the recordings from the RespyBelt and SOMNOtouch devices, respectively. The signals from both devices were filtered using a fourth-order digital Butterworth filter with a cutoff frequency of 1 Hz. The detected peaks and zero crossings to determine the respiration rate are indicated on the signals. A zoomed-in section of this signal is shown in Figure 9 for further clarity.
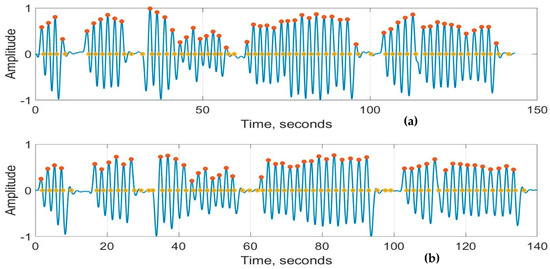
Figure 8.
Respiration signals obtained from (a) RespyBelt and (b) SOMNOtouch (red dots represent detected peaks and yellow dots represent detected zero-crossing time points). The sections with missing respiratory cycles are due to the pauses in breathing. The signal amplitude does not have a unit as it is normalised to .
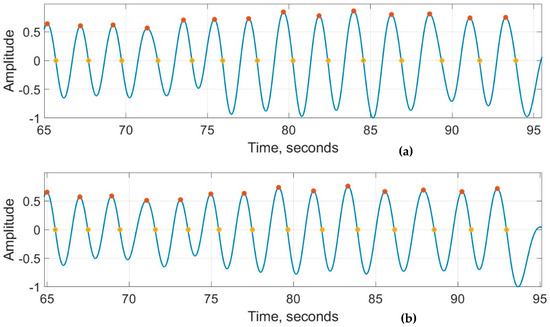
Figure 9.
Zoomed in respiration signals obtained from (a) RespyBelt and (b) SOMNOtouch (red dots represent detected peaks and yellow dots represent detected zero-crossing time points). The signal amplitude does not have a unit as it is normalised to .
The statistics of the respiration rates are provided in Table 2. The mean respiration rate values for RespyBelt and SOMNOtouch are very close. The respiration rates obtained using the zero-crossing method were slightly higher than the values obtained by using the peak-detection method for both devices. The reason for this difference is explained in the Discussion section.

Table 2.
Statistics of the respiration rate determined from the respiratory signals recorded using RespyBelt and SOMNOtouch.
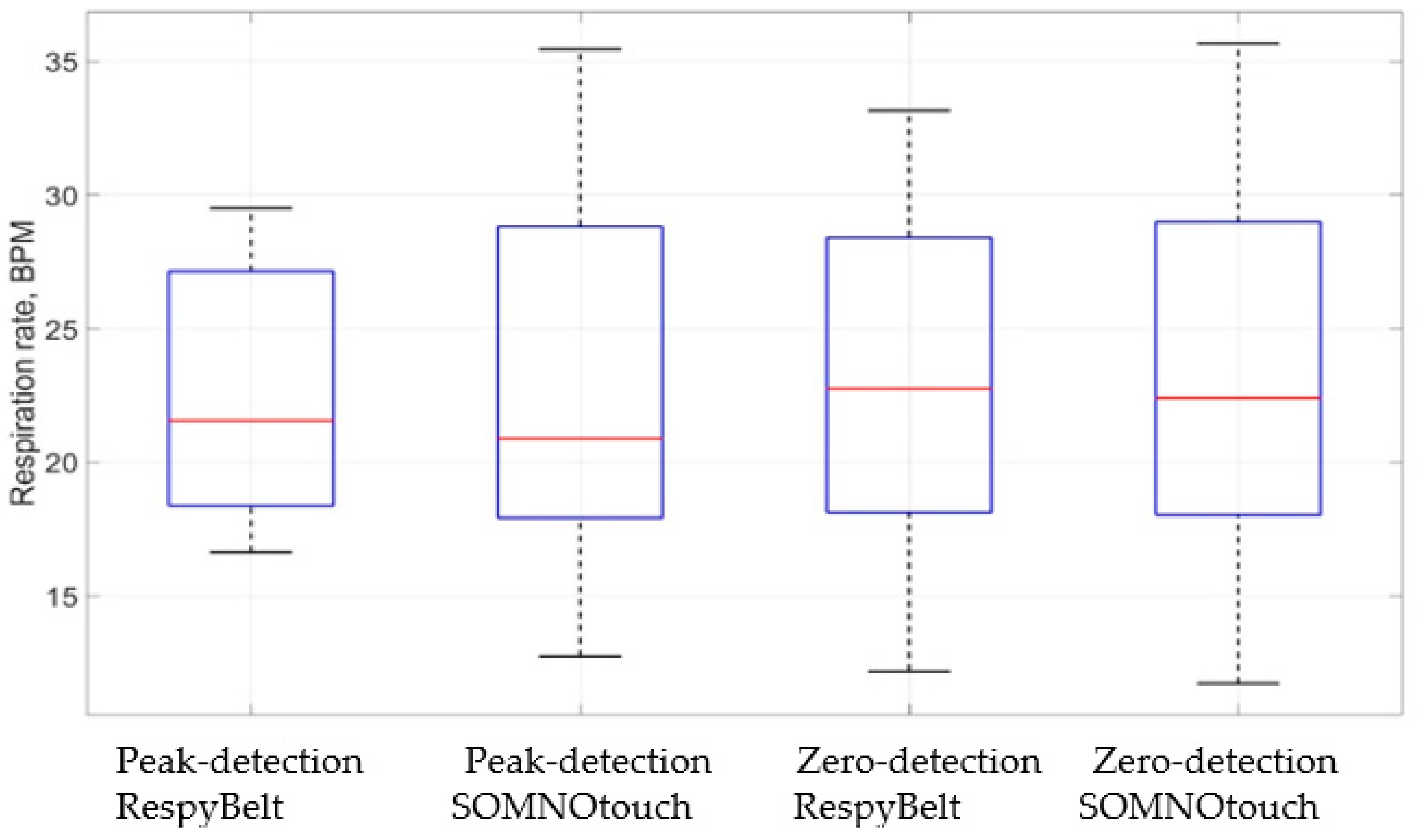
The relationships between the respiration rates determined using the two devices are illustrated by the boxplots of Figure 10. The red line in the boxes is the respiration rate median, highlighting similarity in the respiratory rate measurements for the two devices.
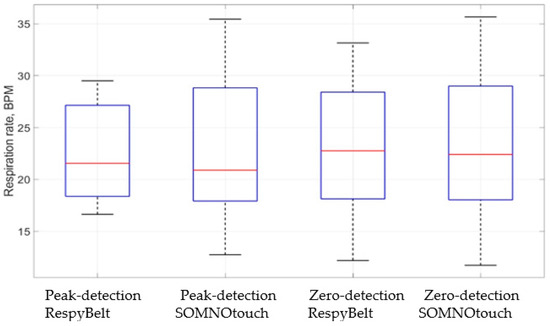
Figure 10.
Boxplots comparing respiration rates measurements using RespyBelt and SOMNOtouch. The plot indicates RespyBelt measurement of the respiration rate closely matches the measurement by SOMNOtouch.
The respiration rates obtained using RespyBelt and SOMNOtouch were examined to determine whether there was a statistically significant difference between them. This was performed for the zero-crossing method only, as the mean respiration rate values for the two devices for the peak detection were identical. The results are summarised in Table 3. Shapiro–Wilk tests indicated the differences in respiration rates determined using the two devices were from a normal distribution. Paired t-tests indicated that the respiration rate values for the two devices were not significantly different. The corresponding probability (p) value was p = 0.727. The confidence level was 95%.

Table 3.
Statistical test of significance to compare mean respiration rates determined from RespyBelt and SOMNOtouch using the zero-crossing method.
4. Discussion
The study outlined the development and evaluation of a new prototype paediatric CSA monitor called RespyBelt. The focus was ensuring the device was child-friendly, safe, reliable, cost-effective, and easy to use. The results were based on recordings from healthy adult volunteers. RespyBelt successfully recorded and stored the respiratory signal, and detected the respiratory pauses. A further evaluation of the device was based on comparing its measured respiration rate values against the measurements from SOMNOtouch. The two devices gave the same mean respiration rate across the 10 participants for the peak-detection method.
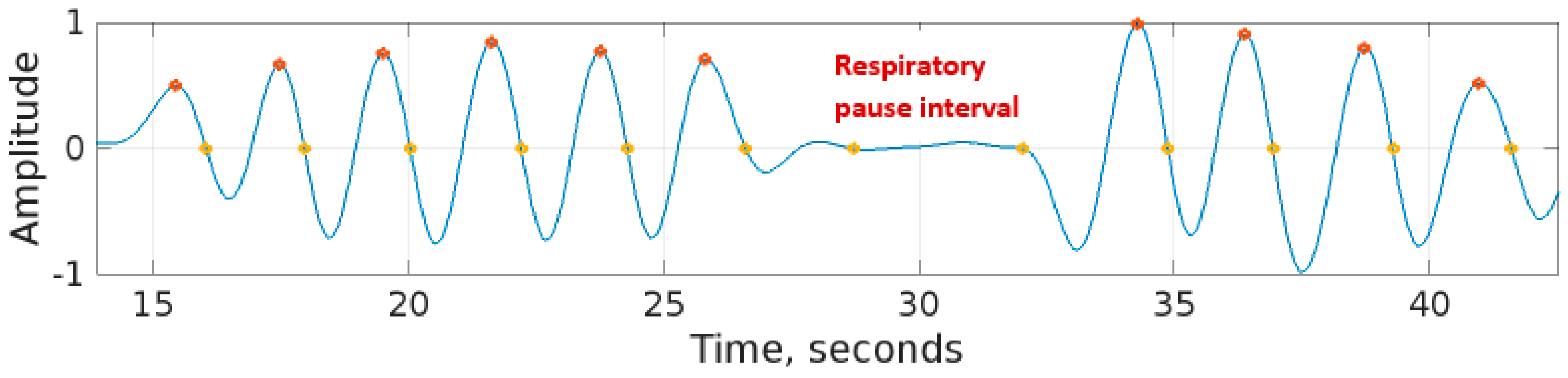
Both peak-detection and zero-crossing detection methods are suitable for real-time breath-to-breath respiration rate measurement. However, the peak-detection method proved to be more robust as compared with the zero-crossing detection method. A limitation of the zero-crossing detection method was that, during respiratory pauses, the signal had relatively low amplitude fluctuations crossing the horizontal axis (zero-level axis). The zero-crossing method counted these fluctuations as part of the respiration rate measurement. This resulted in the respiration rate obtained using the zero-crossing method becoming higher than the peak-detection method. This effect is shown in the respiratory signal of Figure 11 recorded by RespyBelt.
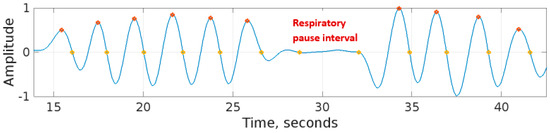
Figure 11.
A respiratory signal recorded by RespyBelt indicating the respiratory pause centred around 30 s. The zero-crossing method identified two spurious respiratory cycles during this interval. The signal amplitude does not have a unit as it is normalised to .
At this stage of development, the device has been kept to minimal complexity to ensure cost-effectiveness and ease of use. However, in future models, the design could become more advanced and adapt further features as needs arise. These include the following:
- The current version of the device does not have a wireless data transmission feature. The justifications for not integrating a wireless feature in this version were to reduce the power consumption, cost, weight, size, and complexity of its use. Wireless transmission of medical data also requires appropriate data security considerations. The integrated SD card has the capacity to store the data recordings continuously for multiple nights. However, in future models, a wireless feature could be adapted as an alternative means of data download and to remotely alert parents that their child is having an apnoeic episode. The wireless data download feature reduces the risk of the SD card being lost or being accessed by an unauthorised person.
- The main purpose of the device was for the home monitoring of children already diagnosed with CSA. The child may sleep in a separate room from the parents and thus the parents may not hear the device’s alarm. The inclusion of a remote alarm would overcome this limitation. For the current version, the parent could be alerted by the device’s alarm using a separate commercially available baby monitor. These monitors can detect any sound from the baby’s room and alert the parents in their room. In a future model, the integration of a remote alert feature will be considered in the device’s design.
- The current design does not have a user interface. The user information, such as the respiratory pause time to trigger an alarm, needs to be adapted in the device’s software. Inclusion of an easy-to-use interactive user interface will be a feature of the next model of the device.
- The respiration rate measurement was currently performed off-line following the data recording. This operation in a future design could become real-time.
- The possibility of predicting the onset of CSA had been explored in an earlier study [24]. If the onset of CSA episodes could be predicted, then it may be possible to take remedial action to prevent respiratory pauses that can pose risk to the child’s health. This is an on-going study but may provide an opportunity for RespyBelt to become a preventative device in addition to its current function as an alarm. The recent innovations in artificial intelligence in healthcare could increase the likelihood of this possibility [25,26].
- The current device can record and store respiratory signals during sleep. However, the device currently does not perform any real-time analysis of sleep patterns. In future models, the software will be amended to register the onset times of CSA pauses and their durations.
- The manner the sensor is integrated into the flexible band could be further improved. If the child pulls the band hard, the sensor could become detached. This would trigger an alarm after the user-predefined respiratory pause. Future designs will improve the design to allow for more robustness.
The study recruited healthy adult volunteers to evaluate the device, but the aim is to take the device further in the follow-up developments for it to be evaluated on children of any age. This will require the recognition that children are not little adults and there are differences in their respiratory characteristics as compared with adults [27]. There are changes in the characteristics of CSA in infants with aging, for example, a decrease in the frequency and duration of CSA over the first 12 months of life [28]. The median respiratory rate in healthy infants during the first 24 h of life was reported to be between 42 bpm to 46 bpm [29]. The median respiration rate in awake, healthy children was measured to be 26 bpm at the age of 2 years [30] and 25.4 bpm at 3 years [31]. A study involving 12 healthy adults (with a mean age of 35.2 years) indicated that their mean respiration rate was 17.7 bpm [23]. These studies indicate that, for infants and children, the device needs to cater to a higher respiration rate and carefully ensure their acceptability.
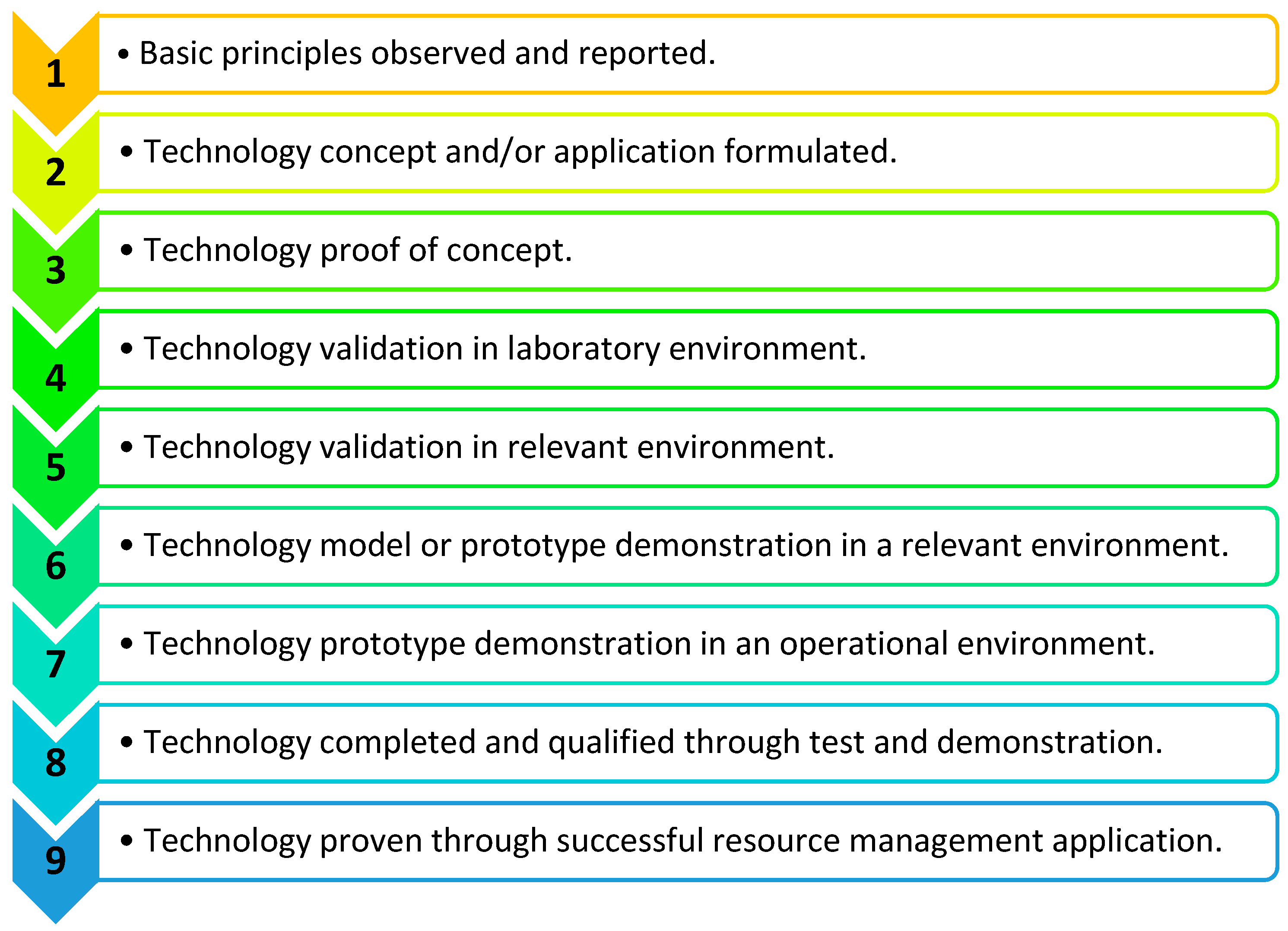
Product development stages are defined by the technology readiness levels (TRLs); usually, these are between one and nine, as defined in Figure 12 [32]. The initial stages relate to the concept development and the later stages move toward manufacturing, validation, and commercialisation. RespyBelt has been validated in the laboratory environment, but it is yet to be clinical trialled. Therefore, its development stage was considered as TRL four. As medical devices can affect health and safety, their commercialisation and use are strictly regulated. According to the Medicines and Healthcare products Regulatory Agency (MHRA) [33], breathing monitors need to conform to strict guidelines once in clinical use. Given the TRL of RespyBelt, and its prototype nature, it currently does not fully conform to the guidelines of the MHRA. However, during its follow-up development stages, these guidelines will be adapted.
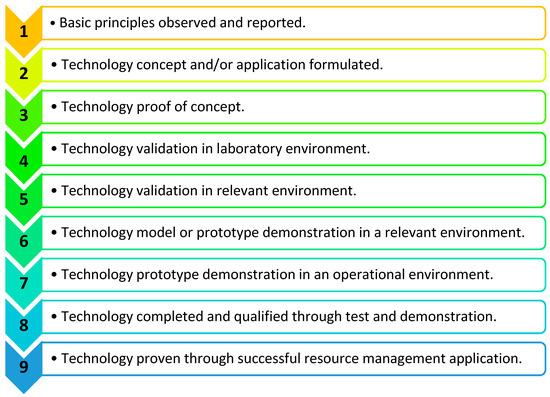
Figure 12.
Product development stages defined by technology readiness levels (TRLs) 1–9 [32]. This is indicative of the stage of the device’s development toward commercialisation and routine use.
Although the current prototype can be further enhanced, as outlined above, the design was robust and could form the basis for clinical use. As part of this, the device will need the adaptation of medical device directives, extensive clinical trials on children of different ages, and the adaptation of extra features.
5. Conclusions
The development of a new prototype paediatric central sleep apnoea home monitoring device was outlined. The device’s main function currently is to detect and provide an alarm when respiratory pauses exceed a preset time and to record the respiratory signal. The monitoring is needed for children and infants diagnosed with central sleep apnoea (CSA). In order ensure cost-effectiveness and ease of use, some advanced features were not integrated in the current model, but their adaptation will be a consideration in future models. The device was successfully evaluated on 10 healthy adult volunteers in a research laboratory. It managed to successfully detect respiratory pauses and provided alarms through a buzzer and a miniature vibration motor. The effectiveness of the peak-detection and zero-crossing methods of the breath-to-breath respiration rate measurement were compared, and the former was found to be more robust in determining the respiration rate. This is an on-going study, and the device’s evaluation results on children in both hospital and home environments will be reported in the future.
Author Contributions
Conceptualisation, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; methodology, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; software, R.S., M.W. and J.M.; validation, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; formal analysis, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; investigation, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; resources, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; data curation, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; writing—original draft preparation, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; writing—review and editing, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; visualisation, R.S., H.E., J.R., M.W., S.S., L.T. and J.M.; supervision, R.S., H.E., and J.R.; project administration, R.S., H.E. and J.R.; funding acquisition R.S., H.E. and J.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical Research Council (United Kingdom) and Sheffield Hallam University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of Sheffield Hallam University (no reference number).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to ethical restrictions, the data are not currently shared.
Acknowledgments
The authors are grateful for the fund received to carry out the study and for all of the participants for taking part in the data recording.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Urquhart, D.S.; Tan, H.L. Sleep disordered breathing at the extremes of age: Infancy. Breathe 2016, 12, e1–e11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orr, J.E.; Mlhotra, A.; Sands, S.A. Pathogenesis of central and complex sleep apnoea. Clin. J. Asian Pac. Soc. Respirol. 2017, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kondamudi, N.P.; Virji, M. Brief Resolved Unexplained Event (BRUE). National Library of Medicine. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441897/ (accessed on 12 April 2024).
- McLaren, A.T.; Bin-Hasan, S.; Narang, I. Diagnosis, management and pathophysiology of central sleep apnea in children. Paediatr. Respir. Rev. 2019, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, R.M. The fundamentals of … apnoea monitors. Biomed. Instrum. Technol. 2011, 45, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.L.; Keens, T.G.; Chan, L.S.; Chipps, B.E.; Carson, S.H.; Deming, D.D.; Krishna, V.; MacDonald, H.M. Sudden infant death syndrome in infants evaluated by apnoea programs in California. Pediatrics 1986, 77, 451–458. [Google Scholar] [CrossRef]
- Milner, A.D. Apnoea monitors and sudden infant death. A report from the Foundation for the Study of Infant Deaths and the British Paediatric Respiratory Group. Arch. Dis. Child. 1985, 60, 76–80. [Google Scholar]
- Emery, J.L.; Taylor, E.M.; Carpenter, R.G.; Waite, A.J. Apnoea monitors and accidental strangulation(letter). Br. Med. J. 1992, 304, 385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleming, P.J.; Blair, P.S.P.; Bacon, C.; Berry, P.J. Sudden Unexpected Deaths in Infancy: The CESDI SUDI Studies 1993–1996; The Stationary Office: London, UK, 2000; ISBN 0113222998. [Google Scholar]
- Samuels, M.P.; Noyes, J.P.; Poets, C.F.; Southall, D.P.; Stebbens, V.A. Deaths on infant apnoea monitors (abstract). Pediatr. Pulmonol. 1992, 14, 258. [Google Scholar]
- Waite, A.; McKenzie, A.; Daman-Willems, C. CONI: Confirmation of continuing relevance after 20 years. Community Pract. 2011, 84, 25–29. [Google Scholar]
- Pullano, S.A.; Mahbub, I.; Bianco, M.G.; Shamsir, S.; Islam, S.K.; Gaylord, M.S.; Lorch, V.; Fiorillo, A.S. Medical devices for pediatric apnea monitoring and therapy: Past and new trends. IEEE Rev. Biomed. Eng. 2017, 10, 199–212. [Google Scholar] [CrossRef]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllylä, R. Measurement of respiratory rate with high-resolution accelerometer and EMFit pressure sensor. In Proceedings of the IEEE Sensors Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Ionescu, C.M.; Copot, D. Monitoring respiratory impedance by wearable sensor device: Protocol and methodology. Biomed. Signal Process. Control 2017, 36, 57–62. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Z.; Wang, H. A novel sleep respiratory rate detection method for obstructive sleep apnea based on characteristic moment waveform. J. Healthc. Eng. 2018, 2018, 1902176. [Google Scholar] [CrossRef]
- Pang, Y.; Jian, J.; Tu, T.; Yang, Z.; Ling, J.; Li, Y.; Wang, X.; Qiao, Y.; Tian, H.; Yang, Y.; et al. Wearable humidity sensor based on porous graphene network for respiration monitoring. Biosens. Bioelectron. 2018, 116, 123–129. [Google Scholar] [CrossRef]
- Jortberg, E.; Silva, I.; Bhatkar, V.; McGinnis, R.S.; Sen-Gupta, E.; Morey, B.; Wright, J.A., Jr.; Pindado, J.; Bianch, M.T. A novel adhesive biosensor system for detecting respiration, cardiac, and limb movement signals during sleep. Nat. Sci. Sleep 2018, 10, 397–408. [Google Scholar] [CrossRef]
- Wu, D.; Wang, L.; Zhang, Y.T.; Huang, B.-Y.; Wang, B.; Lin, S.-J.; Xu, X.-W. A wearable respiration monitoring system based on digital respiratory inductive plethysmography. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 4844–4847. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, J.; Sun, Y.; Yu, H.; Zhou, N.; Zhang, H.; Jia, D. Wearable respiration monitoring using an in-line few-mode fiber Mach-Zehnder interferometric sensor. Biomed. Opt. Express 2020, 11, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Helfenbein, E.; Firoozabadi, R.; Chien, S.; Carlson, E.; Babaeizadeh, S. Development of three methods for extracting respiration from the surface ECG: A review. J. Electrocardiol. 2014, 47, 819–825. [Google Scholar] [CrossRef]
- Abdulqader, T.; Saatchi, R.; Elphick, H. Respiration measurement in a simulated setting incorporating the internet of things. Technologies 2021, 9, 30. [Google Scholar] [CrossRef]
- Muhammad, U.; Evans, R.; Saatchi, R.; Kingshott, R.; Elphick, E. Using non-invasive thermal imaging for apnoea detection. BMJ Open Respir. Res. 2019, 6, A9–A10. [Google Scholar]
- Alkali, A.H.; Saatchi, R.; Elphick, H.; Burke, D. Thermal image processing for real-time noncontact respiration rate monitoring. IET Circuits Devices Syst. 2017, 11, 142–148. [Google Scholar] [CrossRef]
- Abdussalam, A.; Saatchi, R.; Elphick, H.E.; Kingshott, R.N. A plethysmography investigation comparing respiration rate before onset and after the end of central sleep apnoea episodes. In Proceedings of the 19th International Conference on Condition Monitoring and Asset Management, Northampton, UK, 12–14 September 2023. [Google Scholar]
- Ramlakhan, S.L.; Saatchi, R.; Sabir, L.; Ventour, D.; Shobayo, O.; Hughes, R.; Singh, Y. Building artificial intelligence and machine learning models: A primer for emergency physicians. Emerg. Med. J. 2022, 39, 1–8. [Google Scholar] [CrossRef]
- Ramlakhan, S.; Saatchi, R.; Sabir, L.; Singh, Y.; Hughes, R.; Shobayo, O.; Ventour, D. Understanding and interpreting artificial intelligence, machine learning and deep learning in emergency medicine. Emerg. Med. J. 2022, 39, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Haya, S.; Alsubie; BaHammam, A.S. Obstructive sleep apnoea: Children are not little adults. Paediatr. Respir. Rev. 2017, 21, 72–79. [Google Scholar]
- Trachsel, D.; Erb, T.O.; Hammer, J.; von Ungern-Sternberg, B.S. Developmental respiratory physiology. Pediatr. Anesth. 2022, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Tveiten, L.; Diep, L.M.; Halvorsen, T.; Markestad, T. Respiratory rate during the first 24 hours of life in healthy term infants. Pediatrics 2016, 137, e20152326. Available online: https://publications.aap.org/pediatrics/article-abstract/137/4/e20152326/81462/Respiratory-Rate-During-the-First-24-Hours-of-Life?redirectedFrom=fulltext (accessed on 6 July 2024). [CrossRef] [PubMed]
- Fleming, F.; Thompson, M.; Stevens, R.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years: A systematic review of observational studies. Lancet 2011, 19, 1011–1018. [Google Scholar] [CrossRef]
- Herbert, A.; Pearn, J.; Wilson, S. Normal percentiles for respiratory rate in children—Reference ranges determined from an optical sensor. Children 2020, 7, 160. [Google Scholar] [CrossRef]
- Cupp, A.; Fritts, A.; Brey, M.; Woodley, C.; Smith, D.; Cornish, M.; McGovern, A.; Simmonds, R.; Jackson, N. Application of the technology readiness levels framework to natural resource management tools. Fisheries 2023, 48, 474–479. [Google Scholar] [CrossRef]
- Medicines and Healthcare Products Regulatory Agency, Decision Document on Baby Breathing/Movement Monitors. 2021; pp. 1–12. Available online: https://assets.publishing.service.gov.uk/media/60e826b18fa8f50c70bc65a3/Decision_doc_baby_breathing_monitors_Feb-2021__1_.pdf (accessed on 7 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).