Development of a New Prototype Paediatric Central Sleep Apnoea Monitor
Abstract
1. Introduction
- A bubble sensor attached to the baby’s abdomen and connected by wire or tubing to the monitor, e.g., SISS Babycontrol®H (manufactured by Schulte-Elektronik GMBH Medical Technology, Olsberg, Germany).
- A large sensor pad placed under the baby’s mattress, e.g., Nanny (manufactured by Jablotron, Czech Republic, Europe)
- A small monitor that clips onto a nappy, e.g., Snuza HeroMD (developed by Snuza, UK)
- Ensuring child-friendly features. The device is miniaturised and comfortable to wear by children of any age, including neonates and infants.
- Applicability of use to both homes and hospitals.
- Reducing false alarms. A false alarm occurs when the breathing is normal during sleep but the device detects a breathing pause exceeding the preset time and triggers an alarm. False alarms may heighten parents’ anxiety and lead to disturbance of the child’s sleep. These may occur due to the disconnection of the sensor from the base unit, or shallow abdominal movements that are not detected [7].
- Avoidance of the child’s entanglement by wires or tubing. The device is fully contained in a soft elastic band and thus no wiring or tube is needed to connect it to a base unit. Monitors that use wires or tubing to connect their sensors to their base units pose the risk of babies becoming entangled with the sensor leads [8].
- What are the technological challenges in the design and development of a CSA monitor and the manner they could be adapted?
- How well does the developed device perform during testing on healthy adult volunteers?
- The development of a new, rechargeable, cost-effective, and child-friendly prototype CSA monitor (called RespyBelt) integrating respiration signal recording and on-board data storage, respiratory pause detection, and an associated alarm.
- Evaluation of the device in a research laboratory environment on 10 healthy adult volunteers. This included comparing the respiration rate values obtained from the device with the values obtained from a commercial respiratory monitor.
- Comparison of effectiveness of peak-detection and zero-crossing detection methods for breath-to-breath respiration rate measurement.
- Identification of advanced features to be adapted in the device’s future models.
2. Materials and Methods
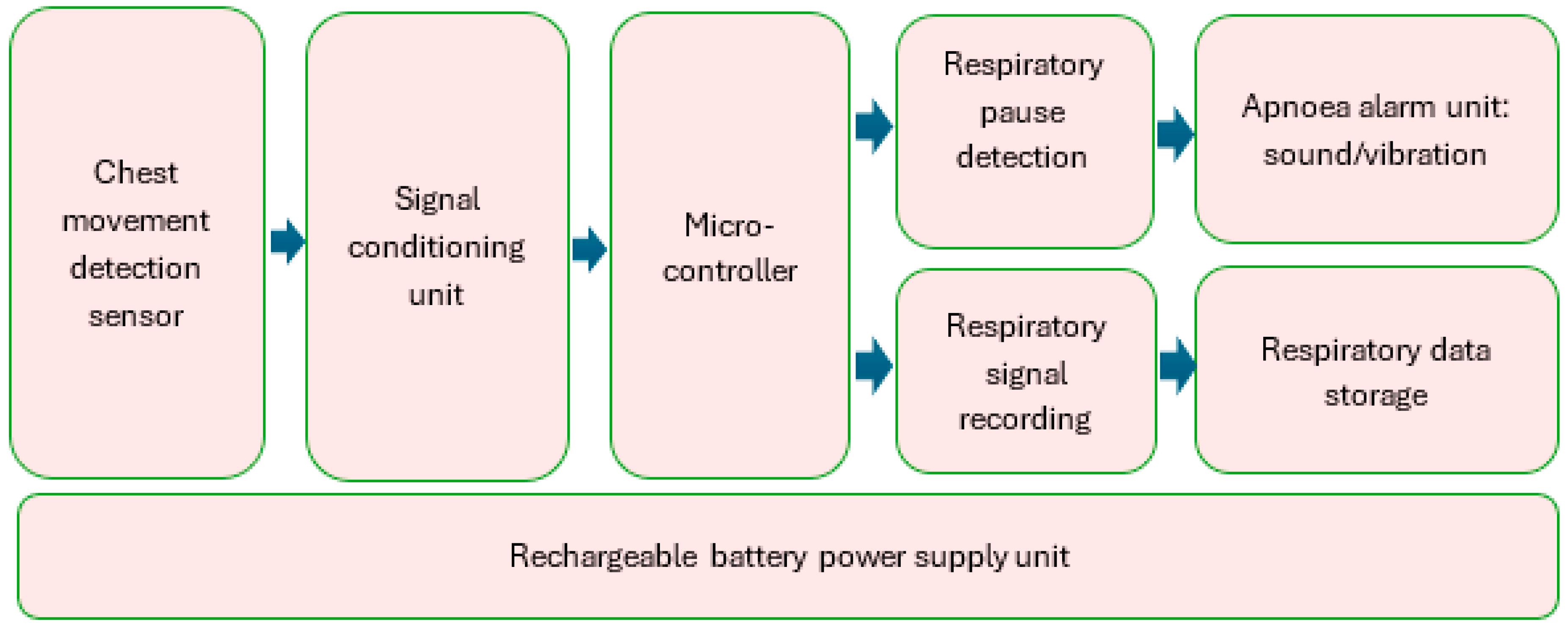
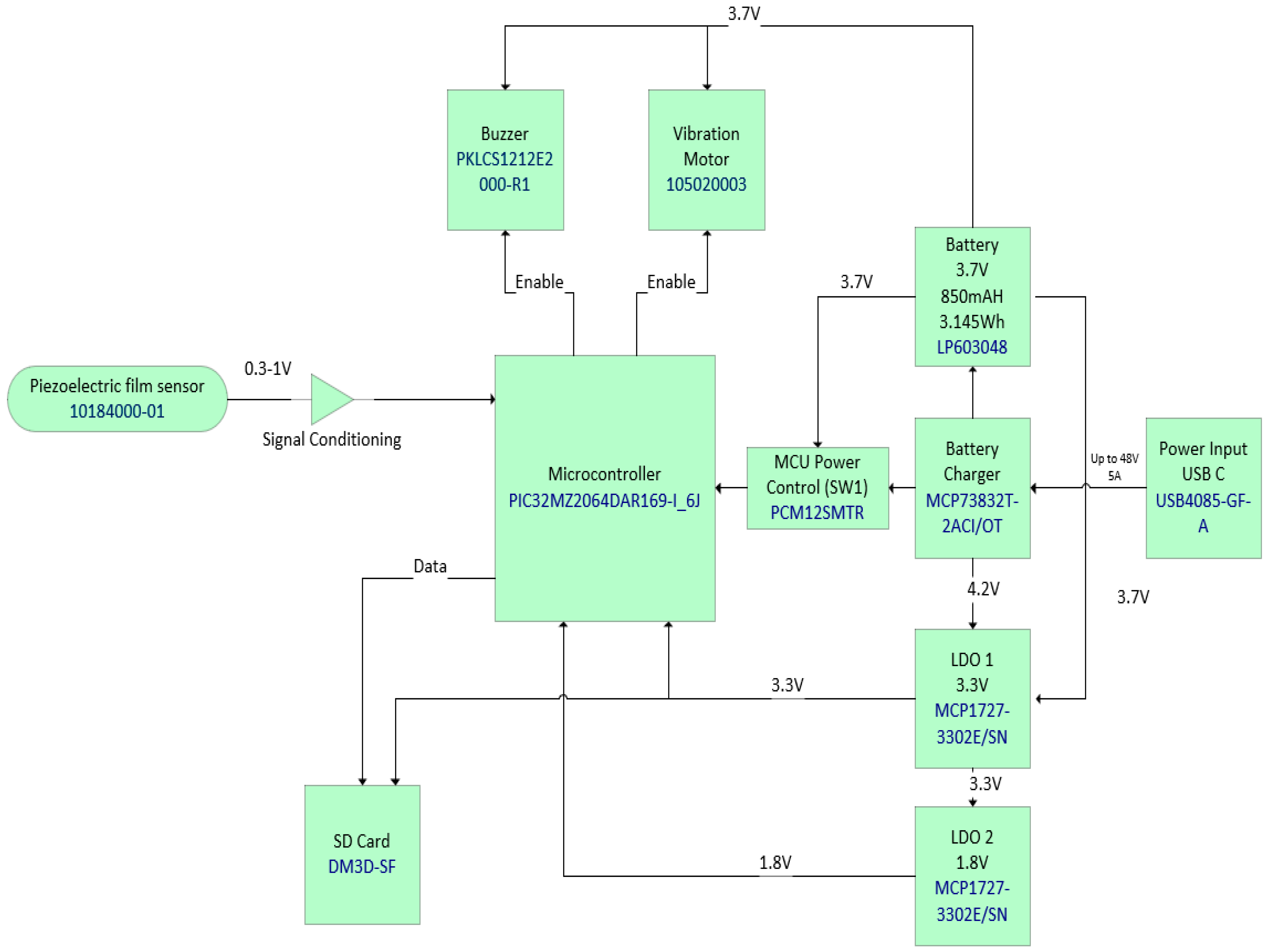
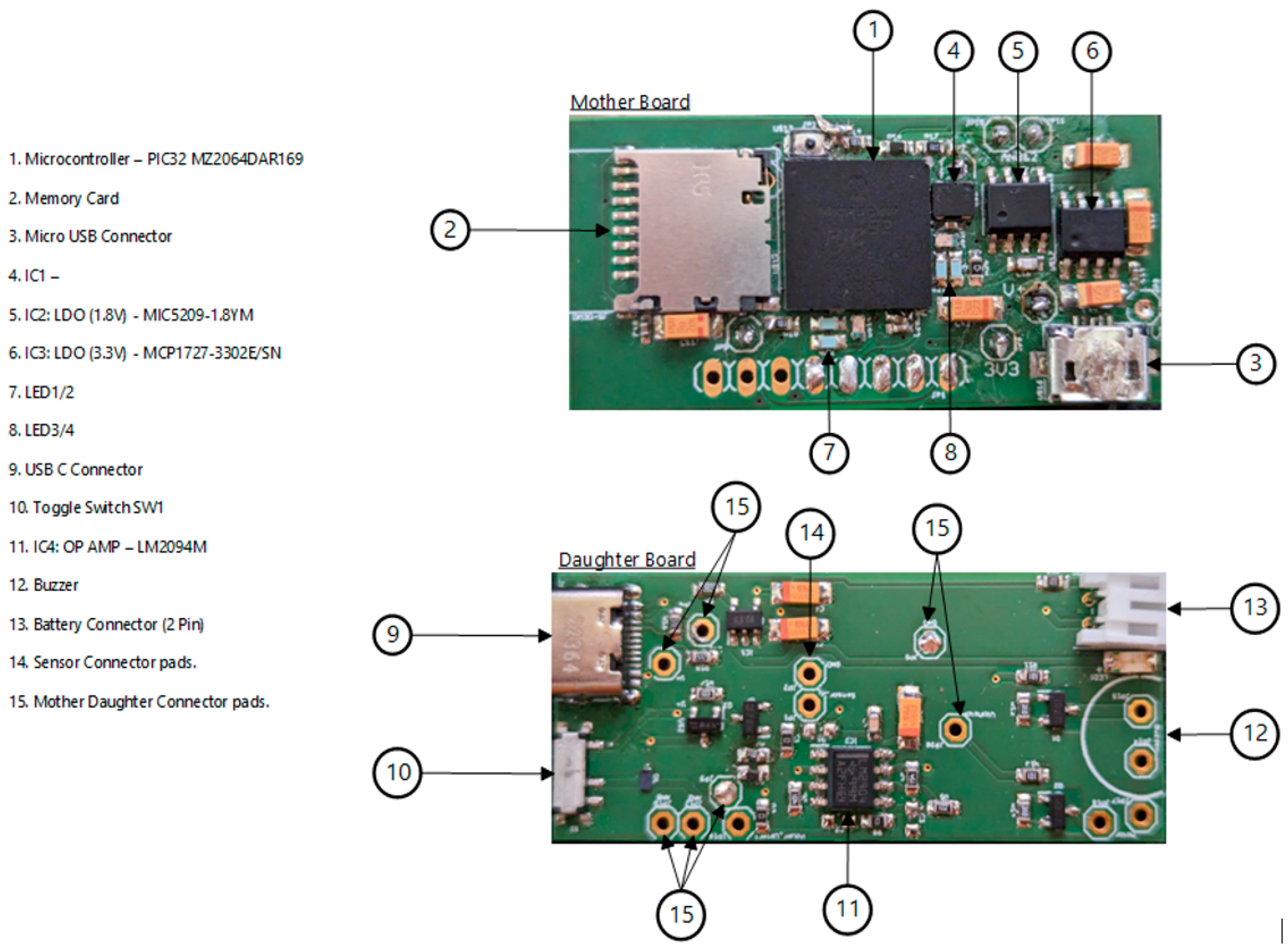
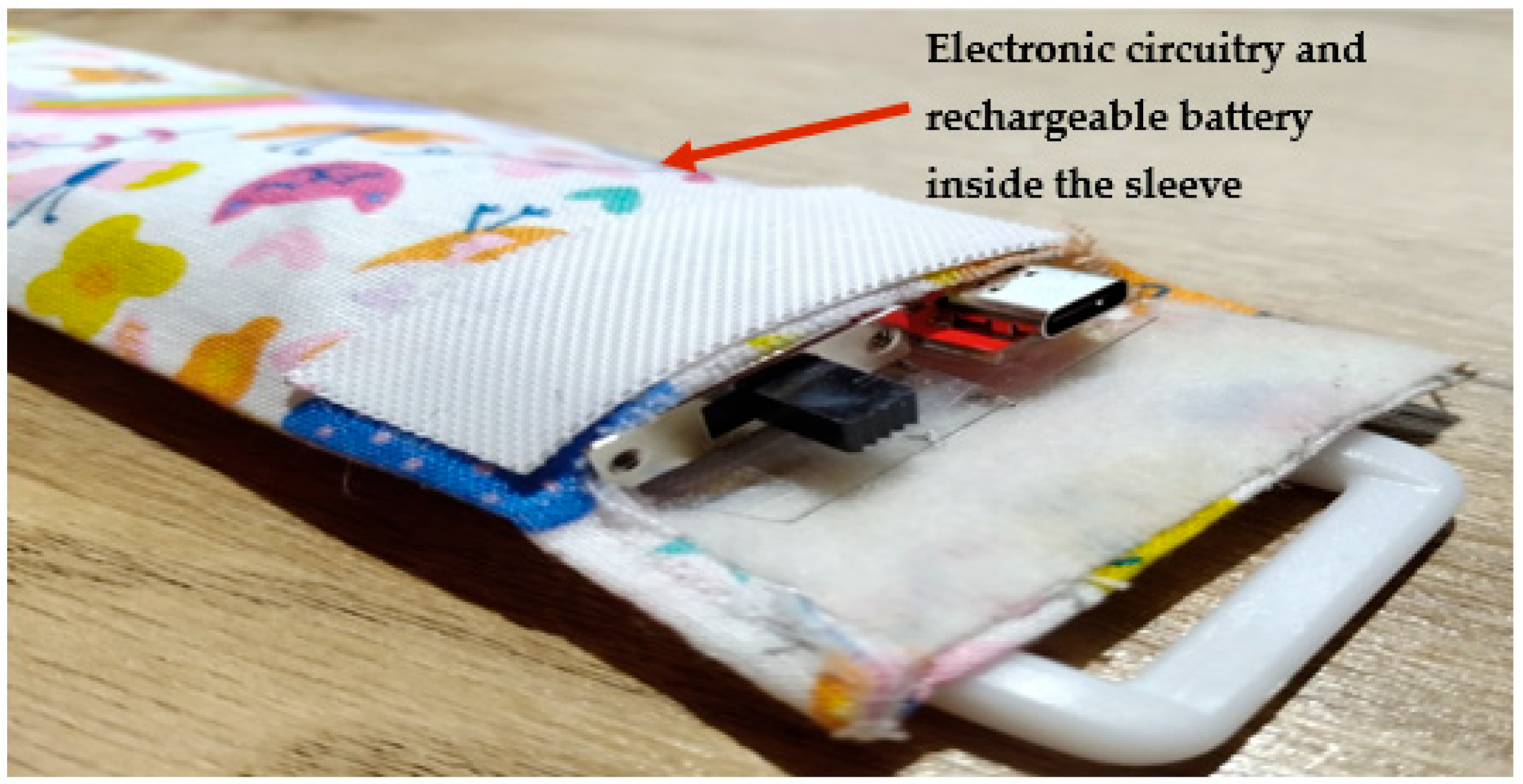
2.1. Hardware Development
- A 16-bit data capture, providing an accurate analogue-to-digital conversion of the sensor signal.
- A respiratory signal sample rate of 32 samples per second. Respiration rate does not typically exceed 60 breaths per minute (bpm). This corresponds to 1 breath per second, or 1 Hz. The sample rate was therefore sufficiently high.
- On-board (integrated), fast secure digital (SD) synchronous data storage.
- Electromagnetic compatibility (EMC) and high-frequency compatibility.
- Velcro attachment for easy fitting/removal of the device and being trap-proof, i.e., the device’s release from the child’s body when sharply pulled or trapped.
- A washable and reusable cover for the device and its sensor.
- Ease of use and comfort for children of any age.
- Safe to use on patients for a long-duration recording.
- Rechargeable power supply.
- Flexibility to adapt new features and technologies in future models.
- Modular design allowing ease of fault finding and repair.
- Integrated circuit (IC) microcontroller (model: PIC32MZ2064DAR169-I_6J, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Secure digital (SD) card (model: DM3D-SF, manufacturer: Hirose Electric Co., Ltd., Kanagawa, Japan), a dedicated high-speed, secure digital high-capacity (SDHC) controller.
- Lithium polymer (LiPo) charger built in for a 5 Volt supply (model: MCP73832T-2ACI/OT, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Microcontroller power switch, ON/OFF (model: PCM12SMTR, manufacturer: C&K Switches, Waltham, MA, USA).
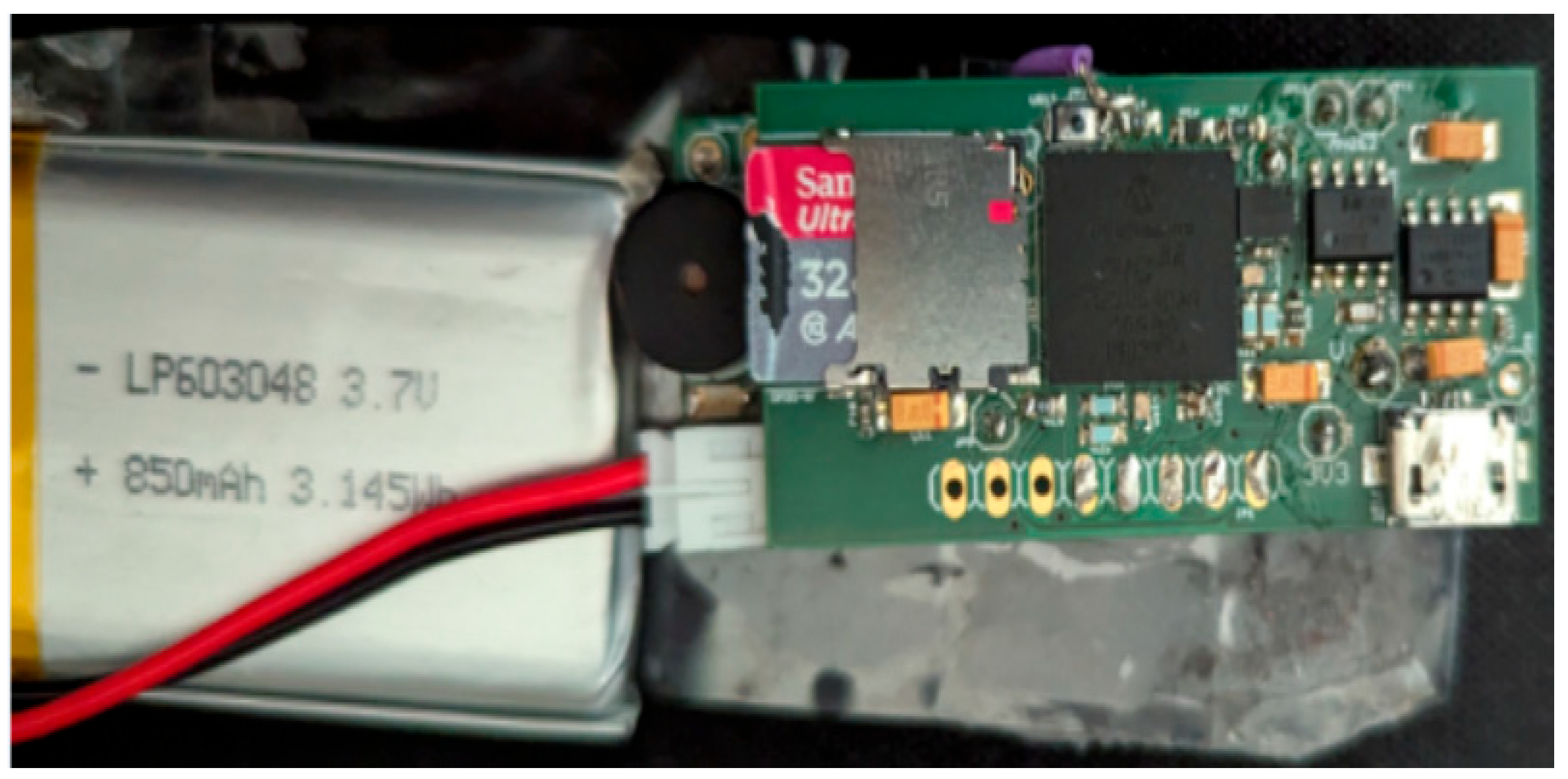
- Rechargeable battery, 850 mAH, 3.7 Volts, and 3.145 WH (model: LP603048).
- Power input USB (model: USB4085-GF-A, manufacturer: Global Connector Technology Limited, Hertfordshire, UK).
- Low-dropout voltage regulator (LDO1), 3.3 Volts and 1.5 A (model: MCP1727-3302E/SN, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Low-dropout voltage regulator (LDO2), 3.3 Volts and 1.5 A (model: MCP1727-3302E/SN, manufacturer: Microchip Technology, Chandler, AZ, USA).
- Audio piezo transducer (buzzer), frequency = 2 kHz and sound pressure level of 76 dB (typical) (model: PKLCS1212E2000-R1, manufacturer: Murata Electronics, Munich, Germany).
- Seeed Grove—vibration ‘coin’-type vibration motor (model: 105020003, manufacturer: Seeed Technology Co., Ltd., Fremont, CA, USA).
2.2. Software Development
- The software is integrated into the RespyBelt microcontroller. This is operated in real-time and is described in Section 2.2.1
- The software that performs the signal processing and statistical analysis. This operated off-line and is described in Section 2.2.2.
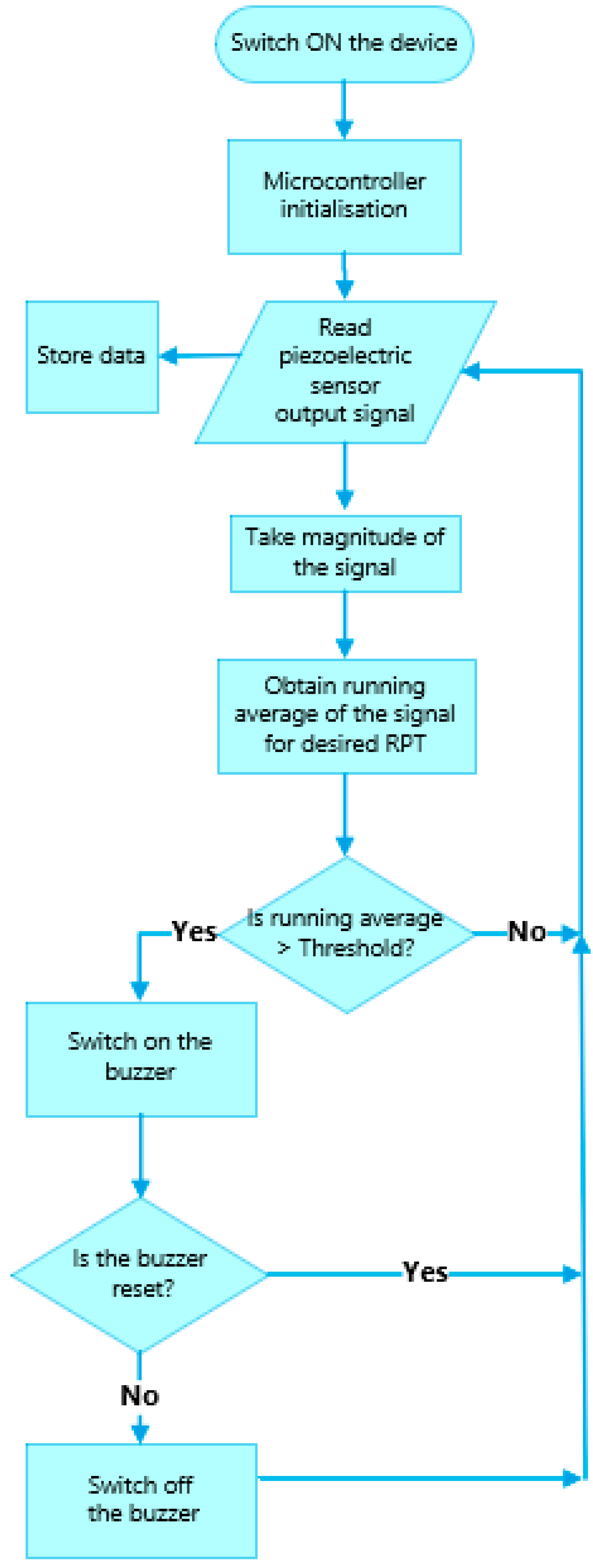
2.2.1. Real-Time Processing Software
2.2.2. Off-Line Processing Software
- signal: The respiratory signal.
- sample_rate: The signal sample rate. This was 32 samples per second for both the RespyBelt and SOMNOtouch devices.
- ‘MinPeakDistance’: This indicates the next minimum peak distance (in seconds). The minimum peak distance provides a mechanism to avoid the false detection of spurious peaks caused by noise. It identifies the peaks separated by more than the minimum peak distance value (specified by MinPeakDistance_value). In this study, the MinPeakDistance_value was 1 (i.e., 1 s). A time of 1 s was chosen for this parameter, as the respiration rate does not typically exceed 60 bpm, which corresponds to 1 Hz (a period of 1 s).
- ‘MinPeakHeight’: This provides a means to select the peaks that are greater than the specified highlight (specified by MinPeakHeight_value). This was a further feature to avoid spurious small peaks introduced by noise. In this study, to determine the MinPeakHeight_value, the absolute value of the respiratory signal was initially obtained (which ensured the negative part of the signal reverted to positive values). The average value of the resulting signal was obtained and then the value was divided by 3. This meant that those peaks with amplitudes of less than 1/3 of the average height of the signal were excluded as a part of the respiration rate measurement. Using a smaller height increased the risk of selecting spurious peaks and a larger height increased the risk of missing relevant peaks.
- peaks: This contains the amplitudes of the identified peaks. However, this array was not required in this study, as only the signal frequency was related to the respiration rate.
- locations: This contains the time locations (indices) of the identified peaks. The breath-by-breath respiration rate in bpm was determined by the following:
2.3. Particupant Recruitment, Validation, and Statistical Analysis
3. Results
4. Discussion
- The current version of the device does not have a wireless data transmission feature. The justifications for not integrating a wireless feature in this version were to reduce the power consumption, cost, weight, size, and complexity of its use. Wireless transmission of medical data also requires appropriate data security considerations. The integrated SD card has the capacity to store the data recordings continuously for multiple nights. However, in future models, a wireless feature could be adapted as an alternative means of data download and to remotely alert parents that their child is having an apnoeic episode. The wireless data download feature reduces the risk of the SD card being lost or being accessed by an unauthorised person.
- The main purpose of the device was for the home monitoring of children already diagnosed with CSA. The child may sleep in a separate room from the parents and thus the parents may not hear the device’s alarm. The inclusion of a remote alarm would overcome this limitation. For the current version, the parent could be alerted by the device’s alarm using a separate commercially available baby monitor. These monitors can detect any sound from the baby’s room and alert the parents in their room. In a future model, the integration of a remote alert feature will be considered in the device’s design.
- The current design does not have a user interface. The user information, such as the respiratory pause time to trigger an alarm, needs to be adapted in the device’s software. Inclusion of an easy-to-use interactive user interface will be a feature of the next model of the device.
- The respiration rate measurement was currently performed off-line following the data recording. This operation in a future design could become real-time.
- The possibility of predicting the onset of CSA had been explored in an earlier study [24]. If the onset of CSA episodes could be predicted, then it may be possible to take remedial action to prevent respiratory pauses that can pose risk to the child’s health. This is an on-going study but may provide an opportunity for RespyBelt to become a preventative device in addition to its current function as an alarm. The recent innovations in artificial intelligence in healthcare could increase the likelihood of this possibility [25,26].
- The current device can record and store respiratory signals during sleep. However, the device currently does not perform any real-time analysis of sleep patterns. In future models, the software will be amended to register the onset times of CSA pauses and their durations.
- The manner the sensor is integrated into the flexible band could be further improved. If the child pulls the band hard, the sensor could become detached. This would trigger an alarm after the user-predefined respiratory pause. Future designs will improve the design to allow for more robustness.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urquhart, D.S.; Tan, H.L. Sleep disordered breathing at the extremes of age: Infancy. Breathe 2016, 12, e1–e11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orr, J.E.; Mlhotra, A.; Sands, S.A. Pathogenesis of central and complex sleep apnoea. Clin. J. Asian Pac. Soc. Respirol. 2017, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kondamudi, N.P.; Virji, M. Brief Resolved Unexplained Event (BRUE). National Library of Medicine. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441897/ (accessed on 12 April 2024).
- McLaren, A.T.; Bin-Hasan, S.; Narang, I. Diagnosis, management and pathophysiology of central sleep apnea in children. Paediatr. Respir. Rev. 2019, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, R.M. The fundamentals of … apnoea monitors. Biomed. Instrum. Technol. 2011, 45, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.L.; Keens, T.G.; Chan, L.S.; Chipps, B.E.; Carson, S.H.; Deming, D.D.; Krishna, V.; MacDonald, H.M. Sudden infant death syndrome in infants evaluated by apnoea programs in California. Pediatrics 1986, 77, 451–458. [Google Scholar] [CrossRef]
- Milner, A.D. Apnoea monitors and sudden infant death. A report from the Foundation for the Study of Infant Deaths and the British Paediatric Respiratory Group. Arch. Dis. Child. 1985, 60, 76–80. [Google Scholar]
- Emery, J.L.; Taylor, E.M.; Carpenter, R.G.; Waite, A.J. Apnoea monitors and accidental strangulation(letter). Br. Med. J. 1992, 304, 385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleming, P.J.; Blair, P.S.P.; Bacon, C.; Berry, P.J. Sudden Unexpected Deaths in Infancy: The CESDI SUDI Studies 1993–1996; The Stationary Office: London, UK, 2000; ISBN 0113222998. [Google Scholar]
- Samuels, M.P.; Noyes, J.P.; Poets, C.F.; Southall, D.P.; Stebbens, V.A. Deaths on infant apnoea monitors (abstract). Pediatr. Pulmonol. 1992, 14, 258. [Google Scholar]
- Waite, A.; McKenzie, A.; Daman-Willems, C. CONI: Confirmation of continuing relevance after 20 years. Community Pract. 2011, 84, 25–29. [Google Scholar]
- Pullano, S.A.; Mahbub, I.; Bianco, M.G.; Shamsir, S.; Islam, S.K.; Gaylord, M.S.; Lorch, V.; Fiorillo, A.S. Medical devices for pediatric apnea monitoring and therapy: Past and new trends. IEEE Rev. Biomed. Eng. 2017, 10, 199–212. [Google Scholar] [CrossRef]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllylä, R. Measurement of respiratory rate with high-resolution accelerometer and EMFit pressure sensor. In Proceedings of the IEEE Sensors Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Ionescu, C.M.; Copot, D. Monitoring respiratory impedance by wearable sensor device: Protocol and methodology. Biomed. Signal Process. Control 2017, 36, 57–62. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Z.; Wang, H. A novel sleep respiratory rate detection method for obstructive sleep apnea based on characteristic moment waveform. J. Healthc. Eng. 2018, 2018, 1902176. [Google Scholar] [CrossRef]
- Pang, Y.; Jian, J.; Tu, T.; Yang, Z.; Ling, J.; Li, Y.; Wang, X.; Qiao, Y.; Tian, H.; Yang, Y.; et al. Wearable humidity sensor based on porous graphene network for respiration monitoring. Biosens. Bioelectron. 2018, 116, 123–129. [Google Scholar] [CrossRef]
- Jortberg, E.; Silva, I.; Bhatkar, V.; McGinnis, R.S.; Sen-Gupta, E.; Morey, B.; Wright, J.A., Jr.; Pindado, J.; Bianch, M.T. A novel adhesive biosensor system for detecting respiration, cardiac, and limb movement signals during sleep. Nat. Sci. Sleep 2018, 10, 397–408. [Google Scholar] [CrossRef]
- Wu, D.; Wang, L.; Zhang, Y.T.; Huang, B.-Y.; Wang, B.; Lin, S.-J.; Xu, X.-W. A wearable respiration monitoring system based on digital respiratory inductive plethysmography. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 4844–4847. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, J.; Sun, Y.; Yu, H.; Zhou, N.; Zhang, H.; Jia, D. Wearable respiration monitoring using an in-line few-mode fiber Mach-Zehnder interferometric sensor. Biomed. Opt. Express 2020, 11, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Helfenbein, E.; Firoozabadi, R.; Chien, S.; Carlson, E.; Babaeizadeh, S. Development of three methods for extracting respiration from the surface ECG: A review. J. Electrocardiol. 2014, 47, 819–825. [Google Scholar] [CrossRef]
- Abdulqader, T.; Saatchi, R.; Elphick, H. Respiration measurement in a simulated setting incorporating the internet of things. Technologies 2021, 9, 30. [Google Scholar] [CrossRef]
- Muhammad, U.; Evans, R.; Saatchi, R.; Kingshott, R.; Elphick, E. Using non-invasive thermal imaging for apnoea detection. BMJ Open Respir. Res. 2019, 6, A9–A10. [Google Scholar]
- Alkali, A.H.; Saatchi, R.; Elphick, H.; Burke, D. Thermal image processing for real-time noncontact respiration rate monitoring. IET Circuits Devices Syst. 2017, 11, 142–148. [Google Scholar] [CrossRef]
- Abdussalam, A.; Saatchi, R.; Elphick, H.E.; Kingshott, R.N. A plethysmography investigation comparing respiration rate before onset and after the end of central sleep apnoea episodes. In Proceedings of the 19th International Conference on Condition Monitoring and Asset Management, Northampton, UK, 12–14 September 2023. [Google Scholar]
- Ramlakhan, S.L.; Saatchi, R.; Sabir, L.; Ventour, D.; Shobayo, O.; Hughes, R.; Singh, Y. Building artificial intelligence and machine learning models: A primer for emergency physicians. Emerg. Med. J. 2022, 39, 1–8. [Google Scholar] [CrossRef]
- Ramlakhan, S.; Saatchi, R.; Sabir, L.; Singh, Y.; Hughes, R.; Shobayo, O.; Ventour, D. Understanding and interpreting artificial intelligence, machine learning and deep learning in emergency medicine. Emerg. Med. J. 2022, 39, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Haya, S.; Alsubie; BaHammam, A.S. Obstructive sleep apnoea: Children are not little adults. Paediatr. Respir. Rev. 2017, 21, 72–79. [Google Scholar]
- Trachsel, D.; Erb, T.O.; Hammer, J.; von Ungern-Sternberg, B.S. Developmental respiratory physiology. Pediatr. Anesth. 2022, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Tveiten, L.; Diep, L.M.; Halvorsen, T.; Markestad, T. Respiratory rate during the first 24 hours of life in healthy term infants. Pediatrics 2016, 137, e20152326. Available online: https://publications.aap.org/pediatrics/article-abstract/137/4/e20152326/81462/Respiratory-Rate-During-the-First-24-Hours-of-Life?redirectedFrom=fulltext (accessed on 6 July 2024). [CrossRef] [PubMed]
- Fleming, F.; Thompson, M.; Stevens, R.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years: A systematic review of observational studies. Lancet 2011, 19, 1011–1018. [Google Scholar] [CrossRef]
- Herbert, A.; Pearn, J.; Wilson, S. Normal percentiles for respiratory rate in children—Reference ranges determined from an optical sensor. Children 2020, 7, 160. [Google Scholar] [CrossRef]
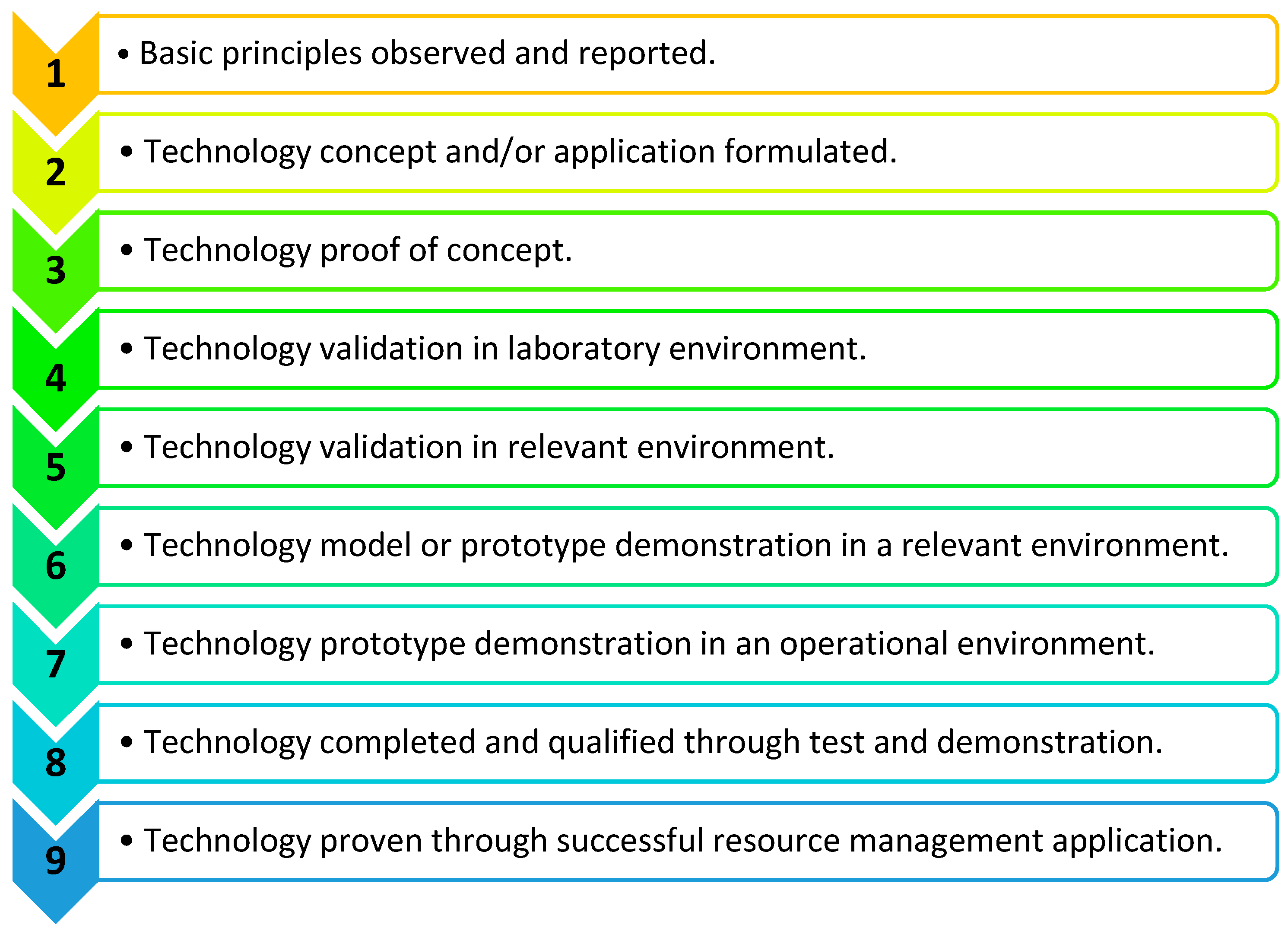
- Cupp, A.; Fritts, A.; Brey, M.; Woodley, C.; Smith, D.; Cornish, M.; McGovern, A.; Simmonds, R.; Jackson, N. Application of the technology readiness levels framework to natural resource management tools. Fisheries 2023, 48, 474–479. [Google Scholar] [CrossRef]
- Medicines and Healthcare Products Regulatory Agency, Decision Document on Baby Breathing/Movement Monitors. 2021; pp. 1–12. Available online: https://assets.publishing.service.gov.uk/media/60e826b18fa8f50c70bc65a3/Decision_doc_baby_breathing_monitors_Feb-2021__1_.pdf (accessed on 7 July 2024).




| Product | Features | Limitations | Related Website |
|---|---|---|---|
| Graseby MR10 infant respiration monitor |
|
| https://www.medicare.ie/product/graseby-mr10-infant-respiration-monitor/ (accessed on 10 July 2024) |
| SISS Babycontrol®H apnoea monitor V2 |
|
| https://schulte-elektronik.de/gb/produkte/atmungs-und-herzmonitore/siss-babycontrol-h/ (accessed on 10 July 2024) |
| Delta apnoea monitor for neonates and infants |
|
| https://deltamedint.com/wp-content/uploads/2022/02/Apnoea-Monitor-Brochure.pdf (accessed on 10 July 2024) |
| Snuza HeroMD baby breathing monitor |
|
| https://www.snuza.com/product/hero-md/ (accessed on 10 July 2024) |
| VitaGaurd® VG 2100 Apnoea and heart rate monitor |
|
| https://www.getemed.de/wp-content/uploads/2022/08/00180-B-_VG_2100_PB_EN_00180_REV_B_20161004.pdf (accessed on 10 July 2024) |
| Nanny movement monitor |
|
| https://www.nanny-monitor.com/ (accessed on 10 July 2024) |
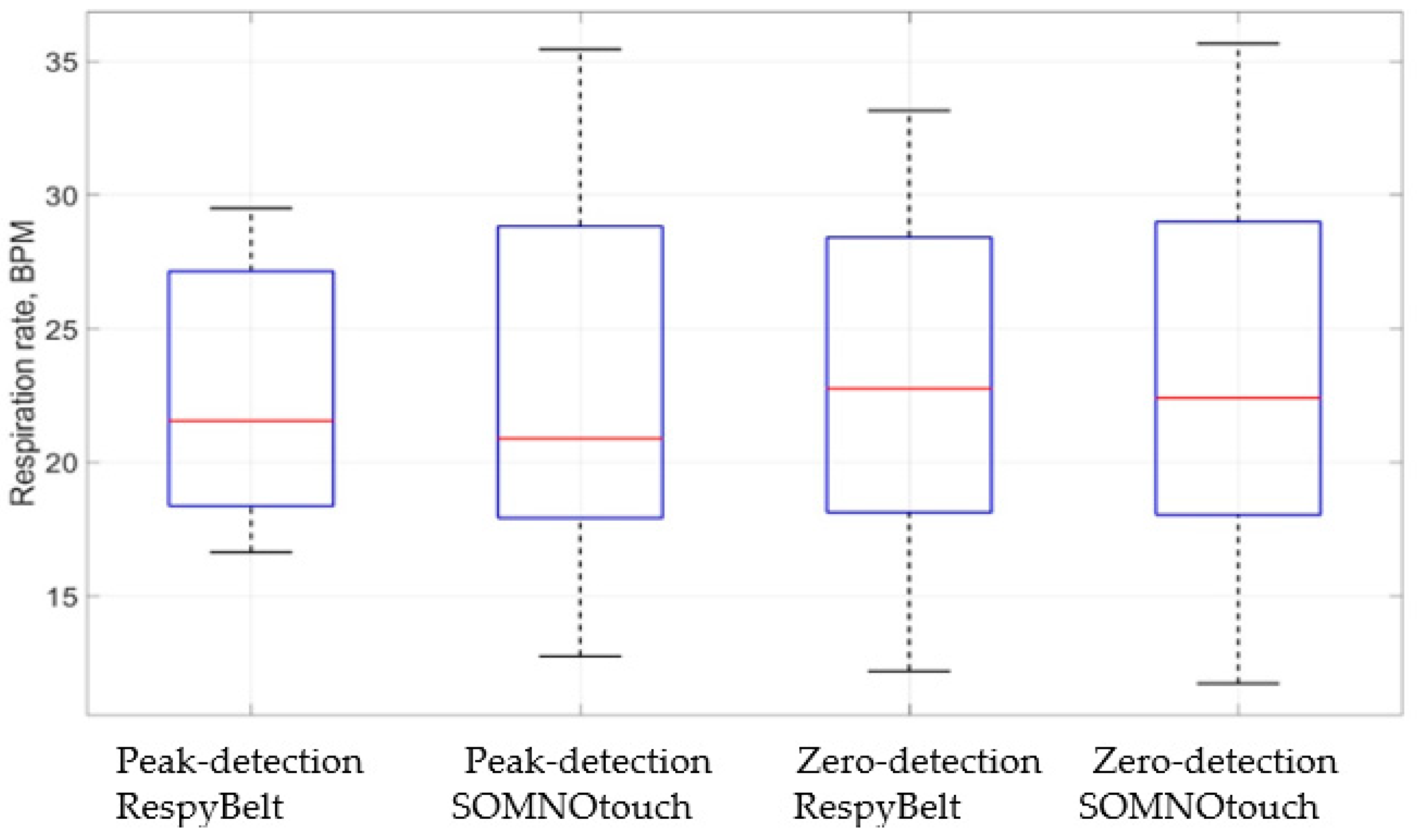
| Device | Respiration Rate Measurement | Mean Respiration Rate (bpm) | Minimum Respiration Rate (bpm) | Maximum Respiration Rate (bpm) | Standard Deviation of Respiration Rate (bpm) |
|---|---|---|---|---|---|
| RespyBelt | Peak detection | 22.7 | 16.6 | 29.5 | 6.0 |
| Zero crossing | 22.9 | 12.2 | 33.1 | 4.3 | |
| SOMNOtouch | Peak detection | 22.7 | 12.8 | 35.4 | 3.4 |
| Zero crossing | 23.4 | 11.7 | 35.7 | 4.1 |
| Test | Shapiro–Wilk Test | Paired t-Test |
|---|---|---|
| RespyBelt vs. SOMNOtouch | Test statistics= 0.948 p = 0.696 | p = 0.727 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saatchi, R.; Elphick, H.; Rowson, J.; Wesseler, M.; Marris, J.; Shortland, S.; Thomas, L. Development of a New Prototype Paediatric Central Sleep Apnoea Monitor. Technologies 2024, 12, 116. https://doi.org/10.3390/technologies12070116
Saatchi R, Elphick H, Rowson J, Wesseler M, Marris J, Shortland S, Thomas L. Development of a New Prototype Paediatric Central Sleep Apnoea Monitor. Technologies. 2024; 12(7):116. https://doi.org/10.3390/technologies12070116
Chicago/Turabian StyleSaatchi, Reza, Heather Elphick, Jennifer Rowson, Mark Wesseler, Jacob Marris, Sarah Shortland, and Lowri Thomas. 2024. "Development of a New Prototype Paediatric Central Sleep Apnoea Monitor" Technologies 12, no. 7: 116. https://doi.org/10.3390/technologies12070116
APA StyleSaatchi, R., Elphick, H., Rowson, J., Wesseler, M., Marris, J., Shortland, S., & Thomas, L. (2024). Development of a New Prototype Paediatric Central Sleep Apnoea Monitor. Technologies, 12(7), 116. https://doi.org/10.3390/technologies12070116







