Abstract
We have developed a dissolved oxygen (DO) method with differential equation (DE) correction. We measured the oxygen content in La-based and Y-based superconductors, and succeeded in measuring the oxygen content simply in one-third of the time required by the iodometric titration method. However, there was a problem with Bi-based superconductors where the measured oxygen content was smaller compared to the iodometric titration method. We hypothesized that not only O2 but also Cl2 gas is generated when dissolving Bi-based superconductors and developed a dissolved oxygen/chlorine (DO/Cl) method with DE correction. This method uses only a dissolved oxygen sensor and a dissolved chlorine sensor to measure the dissolved oxygen and dissolved chlorine content in Bi2Sr2−xLaxCuOy, allowing for the calculation of copper valence and oxygen content. The results from the DO/Cl method with DE correction show that the measured copper valence and oxygen content differ very little from those obtained using the iodometric titration method, with discrepancies within 0.016 and 0.008, respectively. Additionally, this method reduces the measurement time by one-third compared to the iodometric titration method. The results demonstrate that the DO/Cl method with DE correction can effectively measure the copper valence and oxygen content in cuprate superconductors, and using hydrochloric acid as the experimental solution is superior to sulfuric acid and nitric acid.
1. Introduction
It is widely recognized that the Cu valence can significantly influence the transition temperature (Tc) of copper oxide superconductors [1,2]. The Cu valence correlates with the carrier concentration, and the valence can be deduced from the oxygen content by adhering to the charge neutrality principle. Considering Bi2Sr2−xLaxCuOy [3], with assumed valences of Bi3+, Sr2+, La3+, and O2−, the formula relating the oxygen content (y), La content (x), and copper valence (v) simplifies to y = (10 + x + v)/2.
Over recent decades, a variety of methods for measuring oxygen content have been introduced (Table 1). Physical methods to measure oxygen content include thermogravimetric analysis (TGA) [4,5], X-ray photoelectron spectroscopy (XPS), Auger electron spectroscopy (AES), and oxygen analyzers. Among these, TGA is a traditional method for measuring the loss of oxide material mass during controlled heating. Thermogravimetric analyzers (TGAs) range in price from approximately JPY 4,843,800 to JPY 24,219,000. Nevertheless, this method falls short of ascertaining the absolute oxygen content. Moreover, XPS and AES can measure the oxygen content in samples that contain at least 1 µg, but these methods are sensitive to the surface [6]. X-ray photoelectron spectrometers (XPS) range from approximately JPY 48,438,000 to JPY 129,168,000, with high-end models exceeding JPY 161,460,000. Auger electron spectrometers (AES) range from approximately JPY 32,292,000 to JPY 113,022,000. Impurities like carbonate or hydroxide tend to form near the surface. To mitigate the impact of these impurities, the surface needs to be scraped under high vacuum conditions. Oxygen analyzers are priced between approximately JPY 1,614,600 and JPY 8,073,000.
Chemical analysis offers a more precise measurement of oxygen content compared to physical methods, achieving an accuracy of approximately 0.1%. The iodometric titration method, for instance, is frequently employed to ascertain the oxygen content in single-phase HgBa2CuO4+δ [7]. Furthermore, the enhanced coulometry technique has demonstrated reproducibility within 0.01% for YBa2Cu3Oy reference samples weighing 310 mg [2,8].

Table 1.
Oxygen content measurement methods.
Table 1.
Oxygen content measurement methods.
| Type | Method | Weight | Error | Time | Merit | Demerit |
|---|---|---|---|---|---|---|
| Physical analysis | Thermogravimetric analysis (TGA) [9] | 0.1 mg | 0.1% | 1 h | High accuracy | Cannot ascertain the precise oxygen content. |
| X-ray Photoelectron Spectroscopy (XPS) [10] | 1 μg | >10% | 1 h | The depth profile can be determined. | Sensitive to the surface Lacks precision | |
| Auger Electron Spectroscopy (AES) [10] | 1 μg | ~10% | 1 h | The depth profile can be determined. | Sensitive to the surface Lacks precision | |
| Oxygen Analyzer [11] | 1 g | ~10% | 10 min | Simple measuring | Requires a large sample Limited accuracy | |
| Chemical analysis | Coulometric [12] | 50 mg | 0.1% | 1 h | Minor error Automatic measurement | Long measurement time |
| Iodometric [13] | 50 mg | 0.1% | 1 h | Simple measuring | Long measurement time |
We previously reported a novel technique called the dissolved oxygen (DO) method, along with a correction procedure, to determine the oxygen content in La2−xSrxCuO4 using a DO sensor. This method was also successfully applied to measure the oxygen content in YBa2Cu3Oy, and succeeded in measuring the oxygen content simply in one-third of the time required by the iodometric titration method. When compared to the iodometric titration method, the corrected errors for the Cu valence (v) and oxygen content (y) were confined to 0.026 and 0.039, respectively.
In this study, we hypothesized that not only O2 but also Cl2 gas is generated when dissolving Bi-based superconductors, and developed a dissolved oxygen/chlorine (DO/Cl) method with differential equation (DE) correction. This method uses not only a dissolved oxygen sensor but also a dissolved chlorine sensor to measure the dissolved oxygen and dissolved chlorine content in Bi2Sr2−xLaxCuOy, allowing for the calculation of copper valence and oxygen content. Notably, the total cost of the sensors and USB transducer used in the DO/Cl method with DE correction in this study is only around JPY 200,000, making it cheaper compared to other physical analysis methods.
2. Experimental
2.1. Preparation
Figure 1 illustrates a flow chart outlining the preparation process and subsequent determination for Bi2Sr2−xLaxCuOy with x values of 0, 0.2, 0.4, 0.6, and 1. Bi2O3, SrCO3, La2O3, and CuO were ground and mixed using a solid-state reaction method, then calcined at 700 °C for 12 h in air. After cooling in the furnace, the mixture was ground again, mixed, and pressed into pellets. These pellets were then sintered at 850 °C for 20 h to synthesize the desired Bi2Sr2−xLaxCuOy compounds.
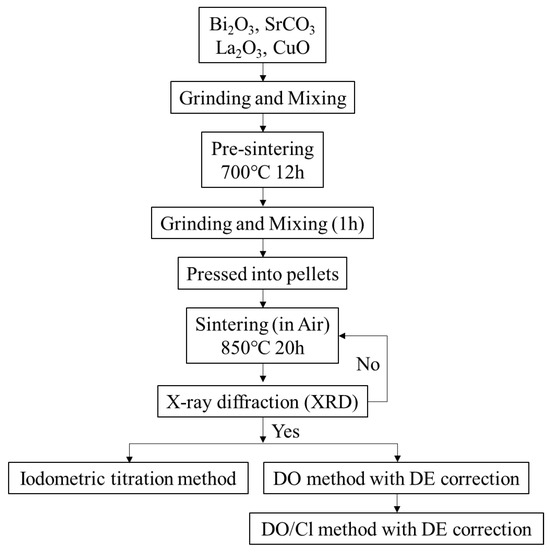
Figure 1.
Experimental flow chart.
The phase purity of the samples was confirmed through X-ray diffraction (XRD) analysis, confirming the presence of a single phase. Samples that did not exhibit single-phase characteristics were subjected to an additional sintering cycle at 850 °C for 20 h.
To quantify the oxygen content, the iodometric titration method was employed as the standard. The obtained values were then compared with those determined through the methods of DO/Cl and DO/Cl with DE correction.
2.2. XRD
To verify the Bi2Sr2−xLaxCuOy phase at room temperature, X-ray diffraction (XRD) analysis was conducted using a RIGAKU Ultima IV diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation. The instrument was set to a voltage of 40 kV and a current of 20 mA. Measurements were taken over a 2θ range of 3 to 60° with a scanning speed of 5°/min. Detailed X-ray diffraction conditions for the X-ray diffraction analysis are provided in Table 2.

Table 2.
XRD parameters.
2.3. Iodometric Titration Method
The iodometric titration method can be categorized into multiple titrations or a single titration [7]. In our experiment, we utilized a single titration method. Figure 2 depicts the experimental setup and procedure.
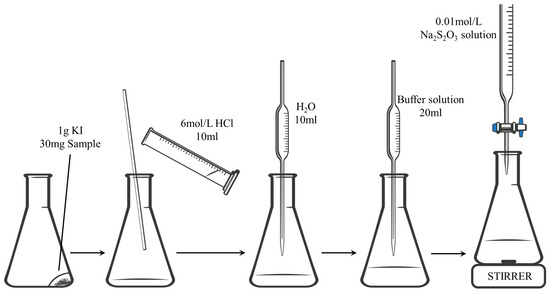
Figure 2.
Experimental setup and procedure diagram for the iodometric titration method.
Bi2Sr2−xLaxCuOy powder (30 mg) was placed at the bottom of a beaker and then covered with 1 g of KI powder. Subsequently, 10 mL of 6 mol/L HCl was added to the beaker containing the mixture. The mixture was stirred with a glass rod for 10 min under an Ar gas flow to dissolve the powder. Next, 10 mL of distilled water was added, followed by 20 mL of a buffer solution (diluted acetic acid solution, used to adjust the pH to approximately 3). Then, 1 mL of starch solution was added as an indicator, turning the solution purple. Finally, a 0.01 mol/L of Na2S2O3 solution was titrated until the color disappeared.
2.4. Dissolved Oxygen Method
The dissolved oxygen (DO) method was performed as described in the literature [14]. Initially, Ar gas was passed through 100 mL of 1 mol/L aq. HCl solution at a flow rate of 0.5 L/min while stirring to remove any dissolved oxygen. This degassing process, facilitated by argon bubbling, effectively reduced the background DO levels in the solution. After 10 min of treatment, the DO concentration stabilized within a range of 0 to 1 mg/L. Subsequently, 50 mg of Bi2Sr2−xLaxCuOy was introduced into the solution. The resultant change in DO concentration was monitored and recorded. This entire measurement process was completed within 15 min.
Figure 3 illustrates the schematic diagram of the DO method. The DO sensor, interfaced with a PC through a USB transducer (DKK-TOA Corporation, Yamagata, Japan), is capable of gauging the DO concentration across a span of 0–19.99 mg/L. The transducer’s dimensions are approximately 20 mm × 20 mm × 180 mm. It relays DO data to the connected PC in real-time, at one-second intervals, ensuring that the information is both displayed for immediate observation and archived for future reference. Moreover, the system is designed to automatically adjust the DO readings to account for variations in temperature, chloride ion concentration, and atmospheric pressure.
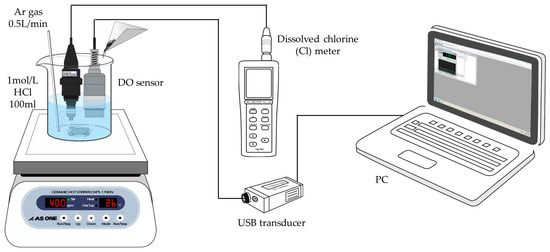
Figure 3.
The schematic view of DO/Cl method with DE correction.
The fundamental principles governing dissolved oxygen measurement are extensively discussed in the literature [14]. When dissolving a metal oxide in an HCl solution, the metal’s valence stabilizes to a fixed value. Specifically, if the initial valence of Cu is monovalent or trivalent, it converts to the divalent state upon dissolution. If the initial valence is higher than two, it also changes to the divalent state during dissolution, as follows:
CuO1+z + 2H+ → Cu2+ + H2O + z/2 O2↑.
For Cu with a valence of less than two, it undergoes the following changes:
CuO1−z + 2H+ + z/2 O2 → Cu2+ + H2O.
Since Equation (1) expresses the correlation between Cu valence and the DO content, the oxygen content of the oxide can be computed in the following manner.
| m(CuO1+z) = w(CuO1+z)/M(CuO1+z) mO2 = Δc V/MO2 1:z/2 = m(CuO1+z):mO2 = w(CuO1+z)/M(CuO1+z):Δc V/MO2 Δc V/MO2 = (z/2) × w(CuO1+z)/M(CuO1+z) |
We can obtain the equation to determine z:
where m(CuO1+z), w(CuO1+z), M(CuO1+z), mO2, Δc, V, and MO2 are molar number of CuO1+z, weight of CuO1+z (g), formula weight of CuO1+z, molar number of O2, DO change (mg/L), volume of the solution (L), and formula weight of O2, respectively. In the case of the Bi2Sr2−xLaxCuOy superconductor, Bi2Sr2−xLaxCuOy, CuO1+z is substituted for CuO1+z.
z = 2 × Δc V/MO2 × M(CuO1+z)/w(CuO1+z),
2.5. DO Method with Differential Equation Correction
When dissolving a sample, it not only dissolves oxygen but also partially degasses from the solution. Therefore, the change in dissolved oxygen (DO) does not fully represent all the dissolved oxygen. Here, a correction method is suggested [14]. If we assume that the rate of dissolution at time t1 and the rate of degassing into the air both depend proportionally on the DO content, their relationship can be formulated as
dΔC/dt = −αΔC
Rewriting with dt and ΔC on opposite sides:
dΔC/ΔC = −α dt
By integrating both sides of the equation:
∫ dΔC/ΔC = −∫ α dt
Integrating the left-hand side, we get:
where C1 is the constant of integration. Next, we express the result in exponential form:
ln |ΔC| = −αt + C1,
|ΔC| = e−αt + C2
Since |ΔC| is greater than zero, we can remove the absolute value symbol and combine C2 into a single constant C1, resulting in:
ΔC = e−αt + C1
If we denote ΔC at t = 0 as ΔC0,
where ΔC and ΔC0 denote the dissolved oxygen (DO) amount at time t and at t = 0, respectively. This illustrates that after dissolution, the DO content exhibits an exponential decrease.
ΔC = ΔC0 e−α
Taking the natural logarithm of ΔC
Figure 4a,b depict ΔC and the natural logarithm of ΔC as functions of time during the dissolution of a cuprate superconductor sample, respectively.
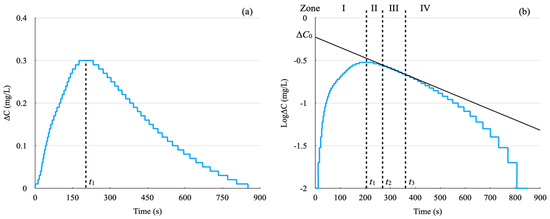
Figure 4.
(a) shows the dissolved oxygen concentration, ΔC, plotted against time, while (b) depicts the natural logarithm of ΔC as a function of time.
As depicted in Figure 4a, for 0 ≤ t < t1, the dissolved oxygen (DO) content increases as the sample continues to dissolve. Beyond t1, the dissolution rate slows down, leading to a decrease in the DO content. For times beyond t1 the data in Figure 4a,b should be fitted to Equations (4) and (5), respectively.
As noted in Equation (5), taking the logarithm of the DO content results in a linear relationship. Therefore, additional analysis will focus on Figure 4b.
In Figure 4b, the graph is segmented into four zones: Zone I (0 ≤ t < t1), Zone II (t1 ≤ t < t2), Zone III (t2 ≤ t < t3), and Zone IV (t3 ≤ t),
In Zone I, the dissolution of the sample leads to an increase in oxygen concentration.
In Zone II, the rate of oxygen release surpasses the rate of oxygen generation, resulting in a decrease in oxygen concentration in the solution. This phase is influenced by both the generation of oxygen through sample dissolution and the release of oxygen from the solution.
Zone III is characterized by the cessation of oxygen generation from sample dissolution, with oxygen release from the solution becoming predominant. Equation (5) applies in Zone III, resulting in a linear graph. Here, t2 denotes the time when sample dissolution concludes, coinciding with the complete dissolution of the sample. For the Bi2Sr2−xLaxCuOy sample, t2 falls between 200 and 350 s. We determined t2 and t3 using the least squares method. When t2 and t3 lie on a straight line, the correlation function of the least squares method reaches its minimum value and remains constant. t2 and t3 were decided within the range where the correlation function is minimized and constant.
In Zone IV, the variation in oxygen concentration becomes smaller than the detection limit, causing the graph to deviate from a linear trend. This zone occurs when the dissolved oxygen level is within the range of log10ΔC ≤ −0.9~1.5. This value aligns closely with the measurement error of the dissolved oxygen sensor, which may vary slightly depending on specific conditions. After the sample dissolution is complete at t2, we can extrapolate the straight line from Zone III back to t = 0 using Equation (5) to determine ΔC0. This approach is known as the DO method with DE correction.
The amount of oxygen in the solution is represented by a sequential reaction model of oxygen generation and release. However, after the dissolution ends (from t2 to t3), since no oxygen is generated, it can be approximated only by the dissolved oxygen release Equation (4). The presence of a range (from t2 to t3) showing an exponential decrease indicates that the above approximation is valid.
2.6. DO/Cl Method with DE Correction
It is acknowledged that during the dissolution of certain oxides in hydrochloric acid, not only is oxygen released, but chlorine may also be liberated. This phenomenon can introduce potential errors in the DO method with DE correction. The reaction can be described by the following equation:
Cu2+z + z Cl− → Cu2+ + z/2 Cl2↑
If Cl2 gas is released during the dissolution of a superconductor, it will be detected by a dissolved chlorine sensor. The value of z obtained is then added to the Cu valence measured using the DO method with DE correction, resulting in the DO/Cl method with DE correction.
Two sensors, a dissolved chlorine sensor (RC-24P) (DKK-TOA Corporation, Yamagata, Japan) and the DO sensor, were placed into the solution. Once the dissolved oxygen and chlorine concentrations stabilized, approximately 50 mg of Bi2Sr2−xLaxCuOy was added to the solution to observe changes in the concentrations of dissolved O2 and Cl2. The Cu valence was then calculated using Equations (1) and (6).
2.7. Effect of Different Acidic Solutions in the DO/Cl Method with DE Correction
We investigated the application of different acidic solutions, including HCl, H2SO4, and HNO3. Historically, HCl solution has been commonly used in the method. Here, we selected H2SO4 solution and HNO3 solutions with the same concentration of 1 mol/L as the acidic media in our experiments to explore their effects on the experimental results.
3. Results and Discussion
3.1. XRD Analysis
Figure 5 presents the XRD patterns of Bi2Sr2−xLaxCuOy (0 ≤ x ≤ 1). The observed peaks within this range confirm the absence of impurity phases. Notably, the XRD patterns agree with those expected for single-phase Bi2Sr2−xLaxCuOy, verifying the single-phase nature of the synthesized samples.
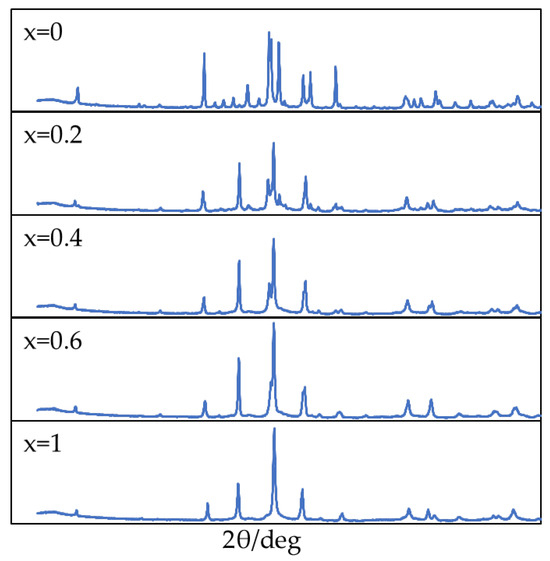
Figure 5.
XRD patterns of Bi2Sr2−xLaxCuOy.
In Figure 6 and Figure 7, we juxtaposed the XRD patterns for x = 0 and x = 1 against the corresponding single-phase JCPDS standards.
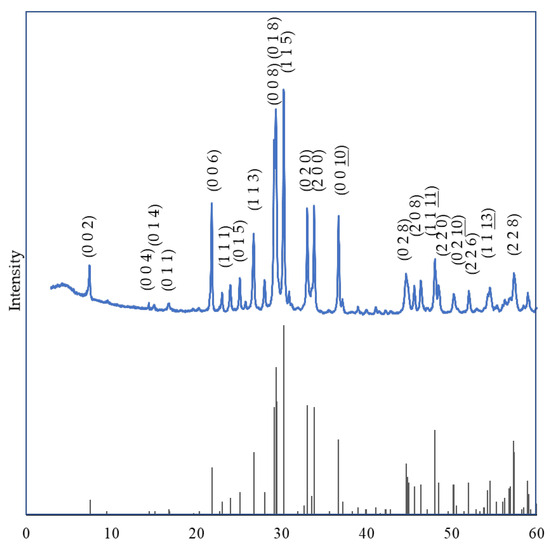
Figure 6.
Comparison of XRD Patterns of Bi2Sr2−xLaxCuOy (x = 0) with JCPDS.
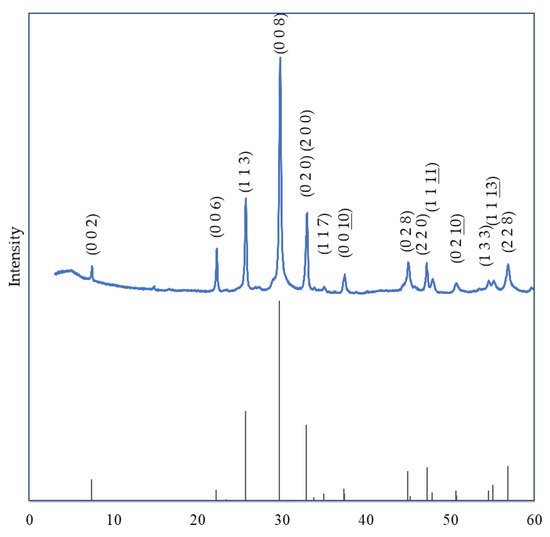
Figure 7.
Comparison of XRD Patterns of Bi2Sr2−xLaxCuOy (x = 1) with JCPDS.
3.2. Iodometric Titration Method Results as a Standard
Table 3 provides the Cu valence (v) and oxygen content (y) for Bi2Sr2−xLaxCuOy across the range 0 ≤ x ≤ 1. The values are deduced using the relation y = (10 + x + v)/2. The iodometric titration indicates a significant increase in oxygen content (y) with increased La doping levels. Enhanced La doping leads to the substitution of La3+ ions for Sr2+ ions. This modification could promote the integration of additional oxygen atoms.

Table 3.
Cu valence (v) and oxygen content (y) of Bi2Sr2−xLaxCuOy.
3.3. DO Method Analysis
Figure 8 illustrates the temporal variation of dissolved oxygen (DO) concentration, denoted as ΔC (mg/L), in hydrochloric acid. A consistent sample weight of 50 mg of Bi2Sr2−xLaxCuOy is used throughout the experiments. The dissolution process employs 100 mL of 1 mol/L aqueous HCl solution. Upon the introduction of Bi2Sr2−xLaxCuOy into the solution at time zero (t = 0), a notable increase in DO content is observed. The elevation in DO concentration is attributed to the oxygen release delineated in Equation (1). Over time, the dissolved oxygen gradually diffuses into the atmosphere, leading to a decrease in the solution’s oxygen concentration.
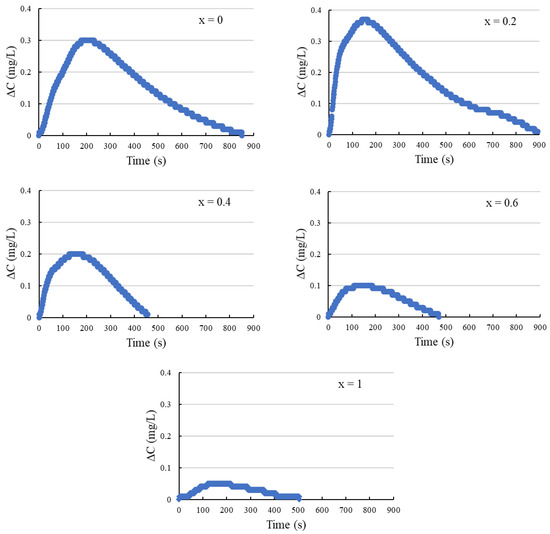
Figure 8.
Change of DO concentration ΔC (mg/L) when Bi2Sr2−xLaxCuOy is dissolved.
We quantify this change by measuring the difference (ΔC) between the peak DO value and the initial DO value at t = 0. Utilizing the DO concentration fluctuation and the solution’s volume, we can deduce the copper valence (v) and oxygen content (y). Table 4 shows the Cu valence and oxygen content of Bi2Sr2−xLaxCuOy measured using the DO method. The Cu valence (v) obtained by the DO method ranges from 2.005 to 2.071, while those obtained by the iodometric titration method range from 2.331 to 2.467, indicating that the values determined by the DO method are lower than those obtained through the iodometric titration method. This discrepancy suggests that the DO method yields lower valence values, likely due to oxygen release during the sample’s dissolution phase.

Table 4.
Cu valence (v) and oxygen content (y) of Bi2Sr2−xLaxCuOy.
3.4. DO Method with DE Correction
The oxygen contents measured by the DO method undergo refinement using a differential equation correction, as shown in Table 4. Figure 9 shows the relationship between the logarithm of the change in DO concentration (log ΔC) and time. After dissolution, ΔC exhibits an exponential decline, while log ΔC decreases linearly. By extrapolating log ΔC to t = 0, we infer the true initial value of ΔC, denoted as ΔC0 in Figure 9. This extrapolation forms the basis of the DO method with DE correction.
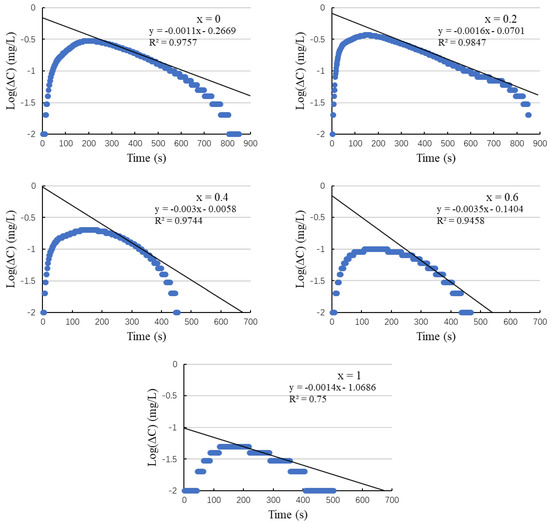
Figure 9.
Logarithmic ΔC as a function of time.
As outlined in Section 2.2, Section 2.3, Section 2.4 and Section 2.5, the linear segment of the graph—indicative of a stable degassing rate—occurs once the Bi2Sr2−xLaxCuOy sample is completely fully dissolved, specifically from t = 200 to 350 s, until log10ΔC reaches approximates −0.5. For instance, the x = 0 sample achieves a logarithmically consistent degassing rate between 176 s (complete dissolution) and 232 s (log10ΔC = −0.5). The coefficient of determination for the least squares analysis of this portion of the graph is greater than 0.9757, indicating an excellent level of linearity. The data in Figure 9 allows for precise identification of the linear portion across various samples.
3.5. Comparison of DO Method with DE Correction and Iodometric Titration Method for Oxygen Content in Bi2Sr2−xLaxCuOy
We conducted a comparative analysis of the oxygen content in Bi2Sr2−xLaxCuOy samples using two distinct methods: the DO method with DE correction and the traditional iodometric titration method.
In Table 4 and Figure 10a, we present a comparison of the oxygen content measurements obtained from both methods. The results indicate a consistent difference in the oxygen content values. The oxygen contents measured by the DO method with DE correction are approximately 0.138 to 0.160 lower than those obtained via the iodometric titration method.
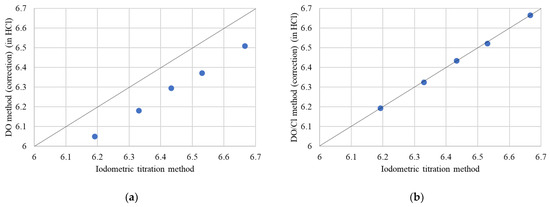
Figure 10.
Oxygen content measured by (a) DO method with DE correction and (b) DO/Cl method with DE correction vs. the Iodometric titration method.
3.6. Comparison of DO/Cl Method with DE Correction and Iodometric Titration Method for Oxygen Content in Bi2Sr2−xLaxCuOy
The dissolution of a sample in an HCl solution often results in the formation of Cl2 gas. Consequently, we observed an increase in dissolved chlorine, which was measured alongside the increase in dissolved oxygen (DO/Cl method with DE correction). The results are listed in Table 4. By incorporating the Cl method, the copper (Cu) valences aligned more closely with those obtained through iodometric titration.
Figure 10b illustrates a comparative analysis of the oxygen content determined by the DO/Cl method with DE correction against the iodometric titration method. The observed differences in oxygen content range from approximately 0.002 to 0.008, underscoring the precision of the DO/Cl method with DE correction.
In summary, the utilization of the DO/Cl method with DE correction enhances the accuracy of the measurements. A comparative evaluation of the results from the DO/Cl method with DE correction against those from the uncorrected DO method with DE correction reveals a marked improvement, attesting to the efficacy of the technique.
In terms of measurement efficiency, the experimental duration required for the DO/Cl method with DE correction is significantly shorter—approximately one-third of the time needed for the iodometric titration method. The DO/Cl method with DE correction not only demonstrates superior precision but also offers a reduced measurement timeframe, establishing itself as an efficient and reliable technique for determining the oxygen content in Bi2Sr2−xLaxCuOy.
3.7. Effect of Acidic Solutions on DO/Cl Method with DE Correction
In Table 5, Cu valence (v) and oxygen content (y) of Bi2Sr2−xLaxCuOy by the DO/Cl method with DE correction in various acids are listed. In Figure 11, we present a comparative analysis of the oxygen content as measured by the DO/Cl method with DE correction in acids of HCl, H2SO4, and HNO3 against the iodometric titration method. The oxygen contents obtained via the DO/Cl method with DE correction in H2SO4 and HNO3 were consistently lower than those measured by the iodometric titration method, with differences ranging from approximately 0.139 to 0.179 and 0.15 to 0.21, respectively. The discrepancies in oxygen content are attributed to the non-production of Cl2 gas through Equation (6). It is to be noted that no Cl2 gas is produced by dissolving in H2SO4 and HNO3 acids.

Table 5.
Cu valence (v) and oxygen content (y) of Bi2Sr2−xLaxCuOy by DO/Cl method with DE correction in various acids.
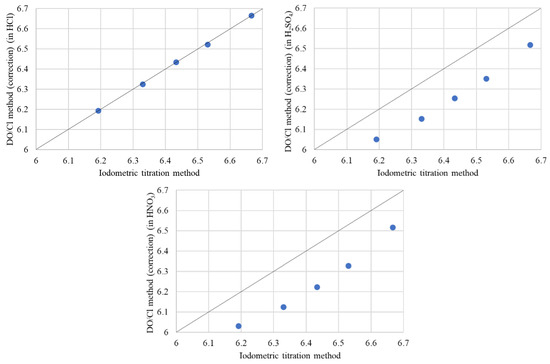
Figure 11.
Oxygen content measured by the DO/Cl method with DE correction (in HCl, H2SO4, HNO3) vs. the Iodometric titration method.
Upon consideration of the experimental findings, it is evident that employing an HCl solution as the acidic medium in the DO/Cl method with DE correction enhances the accuracy of the experimental results.
4. Conclusions
We hypothesized that not only O2 but also Cl2 gas is generated when dissolving Bi-based superconductors and developed a dissolved oxygen/chlorine (DO/Cl) method with DE. This method uses not only a dissolved oxygen sensor but also a dissolved chlorine sensor to measure the dissolved oxygen and dissolved chlorine content in Bi2Sr2−xLaxCuOy, allowing for the calculation of copper valence and oxygen content. The results from the DO/Cl method with DE correction show that the measured copper valence and oxygen content differ very little from those obtained using the iodometric titration method, with differences within 0.016 and 0.008, respectively.
Regarding measurement time, the experimental time of the DO/Cl method with DE correction is approximately one-third that of the iodometric titration method.
Compared to simply using the DO method with DE correction, the DO/Cl method with DE correction greatly improves accuracy.
In the selection of acidic solutions (HCl, H2SO4, and HNO3), using HCl solution for measurement yields the most accurate results. It is concluded that the DO/Cl method (in aq. HCl) with DE correction effectively determines the Cu valence and oxygen content of the superconductor Bi2Sr2−xLaxCuOy.
Author Contributions
Conceptualization, S.K.; Methodology, Y.W., C.Y. and S.K.; Formal analysis, Y.W. and C.Y.; Writing—original draft, Y.W.; Writing—review & editing, Y.W. and S.K.; Supervision, S.K.; Funding acquisition, Y.W. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data substantiating the conclusion of this study are included. Other primary data are available upon reasonable request from the author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Takagi, H.; Ido, T.; Ishibashi, S.; Uota, M.; Uchida, S.; Tokura, Y. Superconductor-to-non superconductor transition in (La1−xSrx)2 CuO4 as investigated by transport and magnetic measurements. Phys. Rev. B 1989, 40, 2254. [Google Scholar] [CrossRef] [PubMed]
- Cava, R.; Hewat, A.; Hewat, E.; Batlogg, B.; Marezio, M.; Rabe, K.; Krajewski, J.; Peck, W.; Rupp, L. Structural anomalies, oxygen ordering and superconductivity in oxygen deficient Ba2YCu3Ox. Phys. C Supercond. 1990, 165, 419–433. [Google Scholar] [CrossRef]
- Akimitsu, J.; Yamazaki, A.; Sawa, H.; Fujiki, H. Superconductivity in the Bi-Sr-Cu-O System. Jpn. J. Appl. Phys. 1987, 26, L2080. [Google Scholar] [CrossRef]
- Karppinen, M.; Fukuoka, A.; Niinistö, L.; Yamauchi, H. Determination of oxygen content and metal valences in oxide superconductors by chemical methods. Supercond. Sci. Technol. 1996, 9, 121. [Google Scholar] [CrossRef]
- Karppinen, M.; Matvejeff, M.; Salomäki, K.; Yamauchi, H. Oxygen content analysis of functional perovskite-derived cobalt oxides. J. Mater. Chem. 2002, 12, 1761–1764. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Yu, Y.; Pei, Z.; Bai, X.; Sun, C.; Huang, R.; Wen, L. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Fukuoka, A.; Tokiwa-Yamamoto, A.; Itoh, M.; Usami, R.; Adachi, S.; Yamauchi, H.; Tanabe, K. Dependence of superconducting properties on the Cu-valence determined by iodometry in HgBa2CuO4+ δ. Phys. C Supercond. 1996, 265, 13–18. [Google Scholar] [CrossRef]
- Yasukawa, Y.; Yamauchi, H.; Karppinen, M. Accurate oxygen-content determination method for decreased sample amounts of superconductive and other functional oxides. Appl. Phys. Lett. 2002, 81, 502–504. [Google Scholar] [CrossRef]
- TGA THERMOSTEP ML Operation Manual; Eltra GmbH: Haan, Germany, 2023; Volume 15, Chapter 3.
- Onishi, K.; Horiike, Y.; Yoshihara, K. Surface Analysis of Solids I; Kodansha: Tokyo, Japan, 1995; Volume 55. (In Japanese) [Google Scholar]
- Salaville, A.; Marciano, J. Oxygen, Nitrogen and Hydrogen Analyzer EMGA-930/EMGA-830: In Pursuit of High Performance, Speed and Operability; Horiba Scientific: Kyoto, Japan, 2009. [Google Scholar]
- Kurusu, K.; Takami, H.; Shintomi, K. Coulometric determination of the average valencies of copper and bismuth in the superconducting bismuth-strontium-calcium-copper-oxygen system. Analyst 1989, 10, 1341–1343. [Google Scholar] [CrossRef]
- Maeno, Y.; Teraoka, H.; Matsukuma, K. Kotai-Butsuri. Solid State Phys. 1991, 26, 235. (In Japanese) [Google Scholar]
- Wei, Y.; Kambe, S. A more accurate method to measure the oxygen content in YBa2Cu3Oy superconductors. J. Ceram. Soc. Jpn. 2023, 131, 789–796. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).