High Affinity of Nanoparticles and Matrices Based on Acid-Base Interaction for Nanoparticle-Filled Membrane
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Silica@PSSNa and Silica@PSSA
2.3. Preparation of P1VIm-co-PBA
2.4. Fabrication of Silica@PSSNa/P1VIm-co-PBA and Silica@PSSA/P1VIm-co-PBA
2.5. Characterization
2.6. Retention Stability of Nanoparticles
2.7. Proton Conductivity
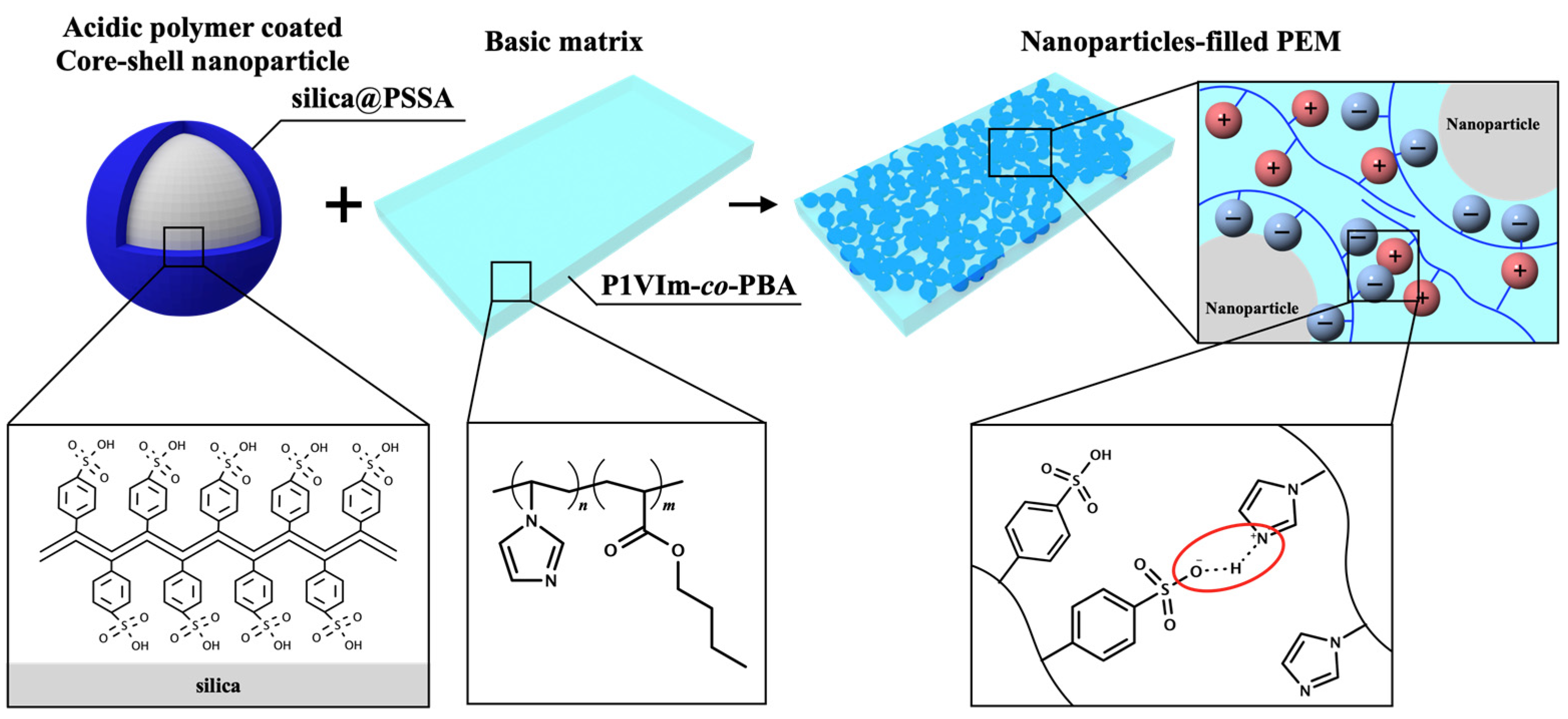
3. Results and Discussion
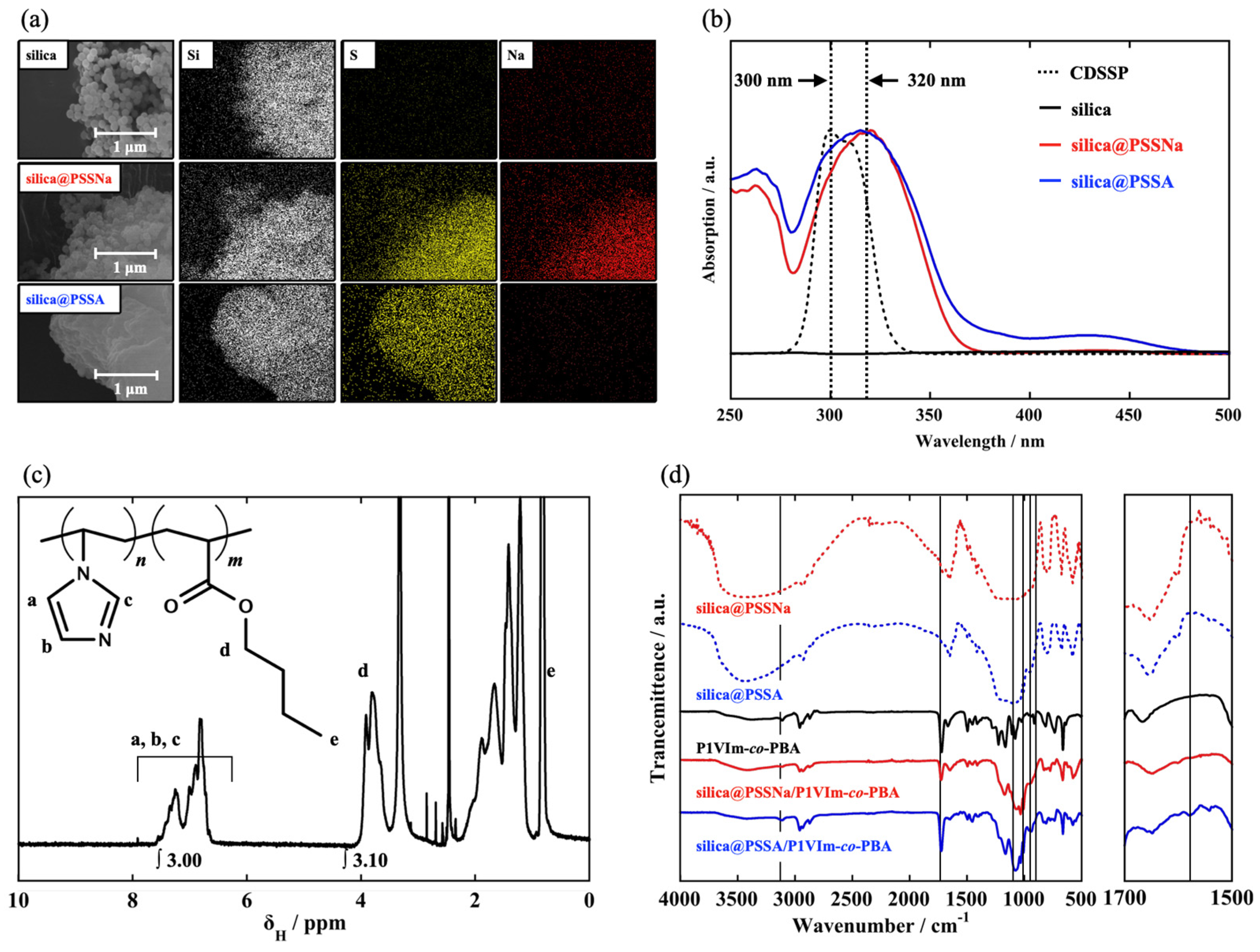
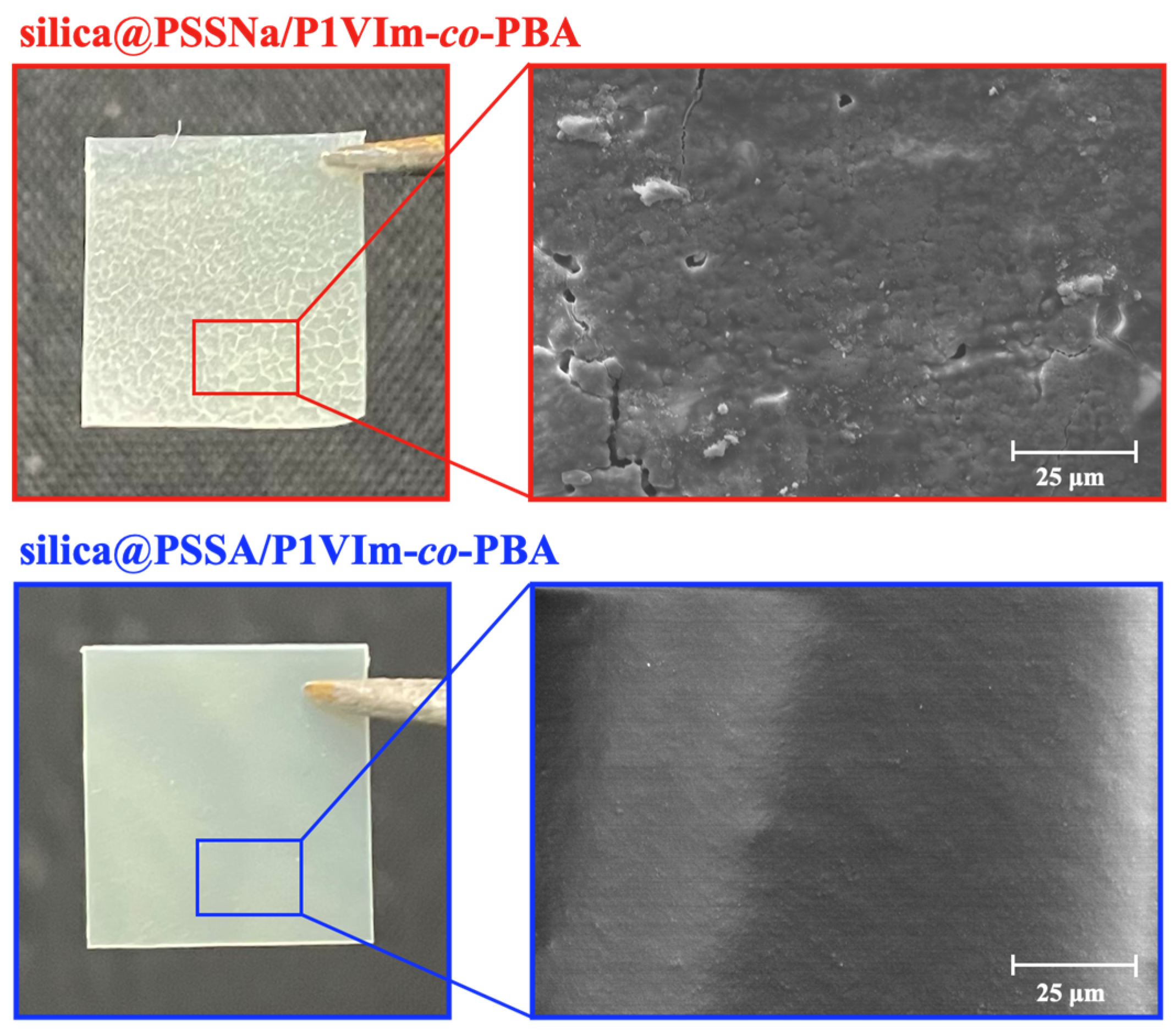
3.1. Characterization of Core-Shell Nanoparticles, Basic Matrix, and Composite Membrane
3.2. Effects of Acid-Base Interaction
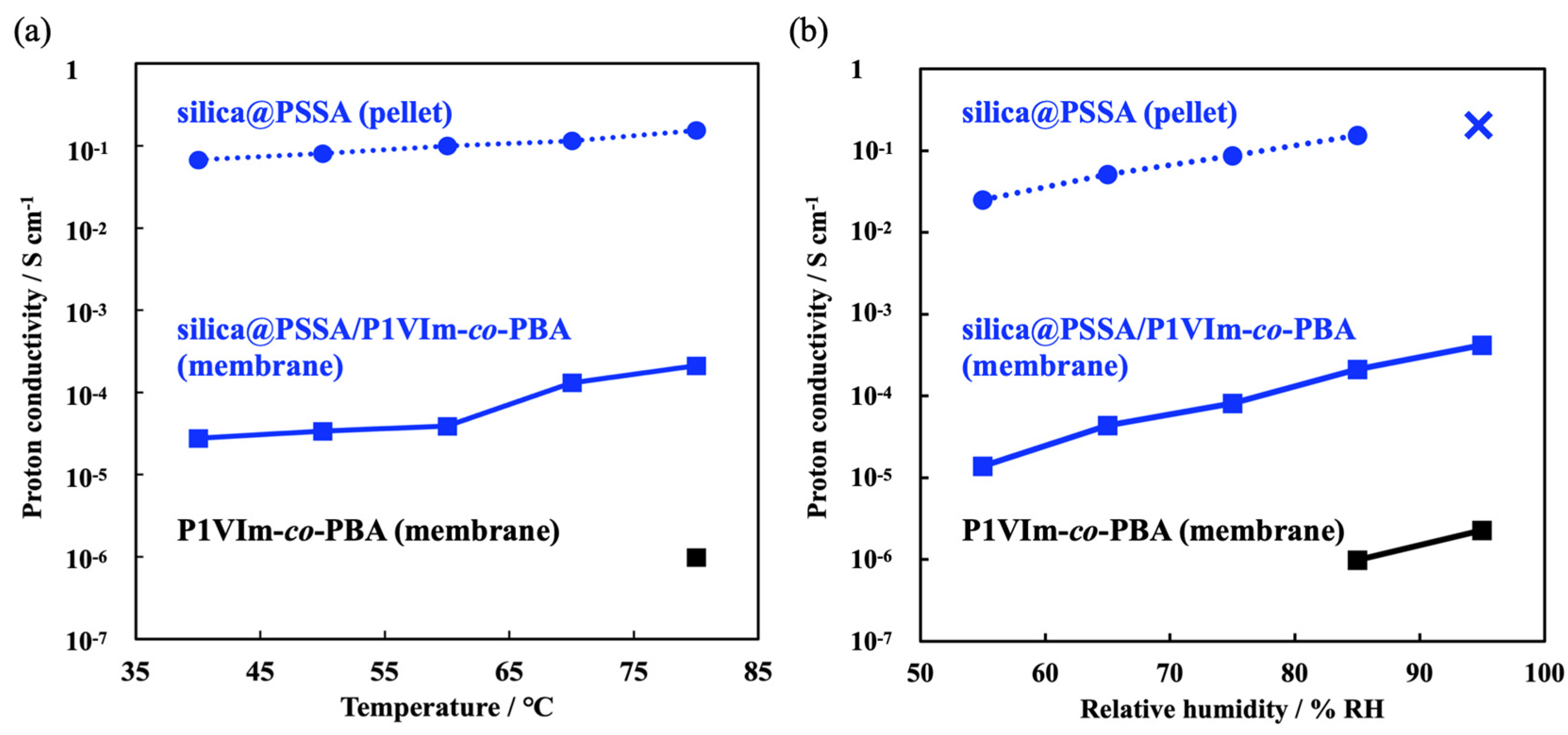
3.3. Proton Conductivities of Silica@PSSA, P1VIm-co-PBA and Silica@PSSA/P1VIm-co-PBA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhara, S.; Shekhar Samanta, N.; Das, P.P.; Uppaluri, R.V.S.; Purkait, M.K. Ravenna Grass-Extracted Alkaline Lignin-Based Polysulfone Mixed Matrix Membrane (MMM) for Aqueous Cr(VI) Removal. ACS Appl. Polym. Mater. 2023, 5, 6399–6411. [Google Scholar] [CrossRef]
- Daglar, H.; Keskin, S. Combining Machine Learning and Molecular Simulations to Unlock Gas Separation Potentials of MOF Membranes and MOF/Polymer MMMs. ACS Appl. Mater. Interfaces 2022, 14, 32134–32148. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, Z.; Naji, L.; Hadadi Asl, V.; Khanbabaei, G.; Dezhagah, F. The influence of fumed silica content and particle size in poly (amide 6-b-ethylene oxide) mixed matrix membranes for gas separation. Sep. Purif. Technol. 2018, 199, 47–56. [Google Scholar] [CrossRef]
- Aframehr, W.; Molki, B.; Bagheri, R.; Sarami, N. Capturing CO2 by a Fixed-Site-Carrier Polyvinylamine-/Matrimid-Facilitated Transport Membrane. ACS Appl. Polym. Mater. 2022, 4, 3380–3393. [Google Scholar] [CrossRef]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.; Xiong, R.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zheng, X.; Xie, Z. Zirconium-Based Nanoscale Metal-Organic Framework/Poly(epsilon-caprolactone) Mixed-Matrix Membranes as Effective Antimicrobials. ACS Appl. Mater. Interfaces 2017, 9, 41512–41520. [Google Scholar] [CrossRef]
- Ray, M.; Samantaray, P.K.; Negi, Y.S. In Situ Polymerization-Mediated Cross-Linking of the MOF Using Poly(1-vinylimidazole) in SPEEK Fuel Cells. ACS Appl. Polym. Mater. 2023, 5, 4704–4715. [Google Scholar] [CrossRef]
- Elakkiya, S.; Arthanareeswaran, G.; Ismail, A.F.; Das, D.B.; Suganya, R. Polyaniline coated sulfonated TiO2 nanoparticles for effective application in proton conductive polymer membrane fuel cell. Eur. Polym. J. 2019, 112, 696–703. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Pan, S.; Wen, Q.; Dan, X.; Li, Y.; Ning, F.; He, C.; Li, W.; Shen, M.; He, L.; Tian, B.; et al. Enhanced Triple-Phase Interface in PEMFC by Proton Conductor Absorption on the Pt Catalyst. ACS Appl. Energy Mater. 2023, 6, 763–772. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Y.; Wang, D.; Hasan, M.M.; Suwansoontorn, A.; Li, H.; Du, G.; Liu, Z.; Nagao, Y. Simple and universal synthesis of sulfonated porous organic polymers with high proton conductivity. Mater. Chem. Front. 2020, 4, 2339–2345. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, S.; Xiang, Y.; Jiang, S.P. Pt-based nanoparticles on non-covalent functionalized carbon nanotubes as effective electrocatalysts for proton exchange membrane fuel cells. RSC Adv. 2014, 4, 46265–46284. [Google Scholar] [CrossRef]
- Inoue, M.; Sakashita, R.; Kagaya, S.; Gemmei-Ide, M.; Yao, Y.; Suwansoontorn, A.; Nagano, S.; Yamamoto, S.; Mitsuishi, M.; Nagao, Y.; et al. Mechanism of High Proton Mobility in the Two-Dimensional Nanospace at the Interlayer of a Multilayer Polymer Nanosheet Film. J. Phys. Chem. C 2023, 127, 24046–24055. [Google Scholar] [CrossRef]
- Nagao, Y. Progress on highly proton-conductive polymer thin films with organized structure and molecularly oriented structure. Sci. Technol. Adv. Mater. 2020, 21, 79–91. [Google Scholar] [CrossRef]
- Nohara, T.; Arita, T.; Tabata, K.; Saito, T.; Shimada, R.; Nakazaki, H.; Suzuki, Y.; Sato, R.; Masuhara, A. Novel Filler-Filled-Type Polymer Electrolyte Membrane for PEFC Employing Poly(vinylphosphonic acid)-b-polystyrene-Coated Cellulose Nanocrystals as a Filler. ACS Appl. Mater. Interfaces 2022, 14, 8353–8360. [Google Scholar] [CrossRef]
- Saito, T.; Nohara, T.; Tabata, K.; Nakazaki, H.; Makino, T.; Matsuo, Y.; Sato, K.; Abadie, C.; Masuhara, A. Relationship between the Proton Conductive Performance and Water Uptake Ratio on a Filler-Filled Polymer Electrolyte Membrane. Energy Fuels 2022, 36, 13924–13929. [Google Scholar] [CrossRef]
- Arita, T. Efficient Production of Block-copolymer-coated Ceramic Nanoparticles by Sequential Reversible Addition–Fragmentation Chain-transfer Polymerizations with Particles (SqRAFTwP). Chem. Lett. 2013, 42, 801–803. [Google Scholar] [CrossRef]
- Shito, K.; Matsui, J.; Takahashi, Y.; Masuhara, A.; Arita, T. Proton Conductivity of Poly(acrylic acid)-b-Polystyrene-coated Silica Nanoparticles Synthesized by Reversible Addition–Fragmentation Chain Transfer Polymerization with Particles. Chem. Lett. 2018, 47, 9–12. [Google Scholar] [CrossRef]
- Song, T.; Deng, J.; Deng, L.; Bai, L.; Zhang, X.; Zhang, S.; Szabo, P.; Daugaard, A.E. Poly(vinylimidazole-co-butyl acrylate) membranes for CO2 separation. Polymer 2019, 160, 223–230. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Wu, H.; Huang, Y.; Hou, Y.; Wu, Q.; Wu, J. Effect of Counterions on the Physicomechanical Properties of Copper-Nitrogen-Coordinated Metallosupramolecular Elastomers. ACS Appl. Mater. Interfaces 2022, 14, 57281–57289. [Google Scholar] [CrossRef]
- Koseki, K.; Arita, T.; Tabata, K.; Nohara, T.; Sato, R.; Nagano, S.; Masuhara, A. Effect of Surface Silanol Density on the Proton Conductivity of Polymer-Surface-Functionalized Silica Nanoparticles. ACS Sustain. Chem. Eng. 2021, 9, 10093–10099. [Google Scholar] [CrossRef]
- Tabata, K.; Nohara, T.; Nakazaki, H.; Makino, T.; Saito, T.; Arita, T.; Masuhara, A. Proton conductivity dependence on the surface polymer thickness of core-shell type nanoparticles in a proton exchange membrane. Nanoscale Adv. 2022, 4, 4714–4723. [Google Scholar] [CrossRef]
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.D.; Oliveira, V.B.; et al. Poly(4-styrene sulfonic acid)/bacterial cellulose membranes: Electrochemical performance in a single-chamber microbial fuel cell. Bioresour. Technol. Rep. 2020, 9, 100376. [Google Scholar] [CrossRef]
- Kamjornsupamitr, T.; Sangthumchai, T.; Saejueng, P.; Sumranjit, J.; Hunt, A.J.; Budsombat, S. Composite proton conducting membranes from chitosan, poly(vinyl alcohol) and sulfonic acid-functionalized silica nanoparticles. Int. J. Hydrogen Energy 2021, 46, 2479–2490. [Google Scholar] [CrossRef]
- Park, J.T.; Koh, J.H.; Roh, D.K.; Shul, Y.G.; Kim, J.H. Proton-conducting nanocomposite membranes based on P(VDF-co-CTFE)-g-PSSA graft copolymer and TiO2–PSSA nanoparticles. Int. J. Hydrogen Energy 2011, 36, 1820–1827. [Google Scholar] [CrossRef]
- Pekel, N.; Şahiner, N.; Güven, O. Development of new chelating hydrogels based on N-vinyl imidazole and acrylonitrile. Radiat. Phys. Chem. 2000, 59, 485–491. [Google Scholar] [CrossRef]
- Deng, W.; Lobovsky, A.; Iacono, S.T.; Wu, T.; Tomar, N.; Budy, S.M.; Long, T.; Hoffman, W.P.; Smith, D.W., Jr. Poly (acrylonitrile-co-1-vinylimidazole): A new melt processable carbon fiber precursor. Polymer 2011, 52, 622–628. [Google Scholar] [CrossRef]
- Yang, F.J.; Liu, Q.F.; Wu, X.B.; He, Y.Y.; Shu, X.G.; Huang, J. High ionic conduction, toughness and self-healing poly(ionic liquid)-based electrolytes enabled by synergy between flexible units and counteranions. RSC Adv. 2021, 11, 35687–35694. [Google Scholar] [CrossRef]
- Sevil, F.; Bozkurt, A. Proton conducting polymer electrolytes on the basis of poly(vinylphosphonic acid) and imidazole. J. Phys. Chem. Solids 2004, 65, 1659–1662. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Z.; Wei, Z.; Zhang, W.; Wu, J.; Li, Y.; Li, J.; Qu, K.; Cai, W. Non-destructive fabrication of Nafion/silica composite membrane via swelling-filling modification strategy for high temperature and low humidity PEM fuel cell. Renew. Energy 2020, 153, 935–939. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Cho, S.; Kim, D.; Kim, J.; Song, G.; Singh, R.; Kim, C. Boosting the proton conduction using protonated imidazole for advanced ion conducting membrane. J. Membr. Sci. 2021, 620, 118904. [Google Scholar] [CrossRef]
- Honda, K.; Yoshida, K.; Sato, K.; Ida, H.; Takahashi, Y. In situ visualization of LbL-assembled film nanoscale morphology using scanning ion conductance microscopy. Electrochim. Acta 2023, 469, 143152. [Google Scholar] [CrossRef]
- Dickhaus, B.N.; Priefer, R. Determination of polyelectrolyte pKa values using surface-to-air tension measurements. Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 15–19. [Google Scholar] [CrossRef]
- Güngör, A.; Özdemir, T.; Genç, R. Investigation of use in 5-FU release: Synthesis of temperature and pH responsive P(NVCL-co-VIm)/PVP hydrogels. Polym. Bull. 2023, 81, 2091–2109. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, H.; Chen, J.; Du, Z.; Mi, J. Interfacial control of polyHIPE with nano-TiO2 particles and polyethylenimine toward actual application in CO2 capture. J. Environ. Chem. Eng. 2017, 5, 2807–2814. [Google Scholar] [CrossRef]
- Lin, B.; Yuan, W.; Xu, F.; Chen, Q.; Zhu, H.; Li, X.; Yuan, N.; Chu, F.; Ding, J. Protic ionic liquid/functionalized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cell applications. Appl. Surf. Sci. 2018, 455, 295–301. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, H.; Li, J.; Tian, X.; Wang, X.; Mao, T.; Xu, J.; Wang, Z. Cross-Linkable Polymeric Ionic Liquid Improve Phosphoric Acid Retention and Long-Term Conductivity Stability in Polybenzimidazole Based PEMs. ACS Sustain. Chem. Eng. 2018, 6, 16352–16362. [Google Scholar] [CrossRef]
- Ohno, H. Design of Ion Conductive Polymers Based on Ionic Liquids. Macromol. Symp. 2007, 249–250, 551–556. [Google Scholar] [CrossRef]
- Wang, X.; Beers, K.M.; Kerr, J.B.; Balsara, N.P. Conductivity and water uptake in block copolymers containing protonated polystyrene sulfonate and their imidazolium salts. Soft Matter 2011, 7, 4446–4452. [Google Scholar] [CrossRef]

| Density | Stress | Strain | Weight of Membrane | |
|---|---|---|---|---|
| [g/cm3] | [MPa] | [%] | [%] | |
| silica@PSSA/P1VIm-co-PBA | 1.06 | 17.0 | 73.5 | 97.1 |
| silica@PSSNa/P1VIm-co-PBA | 0.871 | 7.26 | 58.6 | 71.1 |
| 85% RH [S/cm] | |||||
|---|---|---|---|---|---|
| 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | |
| silica@PSSA | 6.74 × 10−2 | 8.05 × 10−2 | 9.99 × 10−2 | 1.15 × 10−1 | 1.54 × 10−1 |
| silica@PSSA/P1VIm-co-PBA | 2.77 × 10−5 | 3.38 × 10−5 | 3.88 × 10−5 | 1.31 × 10−4 | 2.11 × 10−4 |
| P1VIm-co-PBA | - | - | - | - | 9.82 × 10−7 |
| 80 °C [S/cm] | |||||
| 55% RH | 65% RH | 75% RH | 85% RH | 95% RH | |
| silica@PSSA | 2.50 × 10−2 | 5.10 × 10−2 | 8.65 × 10−2 | 1.54 × 10−1 | - |
| silica@PSSA/P1VIm-co-PBA | 1.38 × 10−5 | 4.37 × 10−5 | 8.16 × 10−5 | 2.11 × 10−4 | 4.20 × 10−4 |
| P1VIm-co-PBA | - | - | - | 9.82 × 10−7 | 2.26 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makino, T.; Tabata, K.; Saito, T.; Matsuo, Y.; Masuhara, A. High Affinity of Nanoparticles and Matrices Based on Acid-Base Interaction for Nanoparticle-Filled Membrane. Technologies 2024, 12, 24. https://doi.org/10.3390/technologies12020024
Makino T, Tabata K, Saito T, Matsuo Y, Masuhara A. High Affinity of Nanoparticles and Matrices Based on Acid-Base Interaction for Nanoparticle-Filled Membrane. Technologies. 2024; 12(2):24. https://doi.org/10.3390/technologies12020024
Chicago/Turabian StyleMakino, Tsutomu, Keisuke Tabata, Takaaki Saito, Yosimasa Matsuo, and Akito Masuhara. 2024. "High Affinity of Nanoparticles and Matrices Based on Acid-Base Interaction for Nanoparticle-Filled Membrane" Technologies 12, no. 2: 24. https://doi.org/10.3390/technologies12020024
APA StyleMakino, T., Tabata, K., Saito, T., Matsuo, Y., & Masuhara, A. (2024). High Affinity of Nanoparticles and Matrices Based on Acid-Base Interaction for Nanoparticle-Filled Membrane. Technologies, 12(2), 24. https://doi.org/10.3390/technologies12020024








