The Gradient of Spontaneous Oscillations Across Cortical Hierarchies Measured by Wearable Magnetoencephalography
Abstract
1. Introduction
2. Materials and Methods
2.1. Principle of Spatial Gradient Estimation of Spontaneous Oscillations
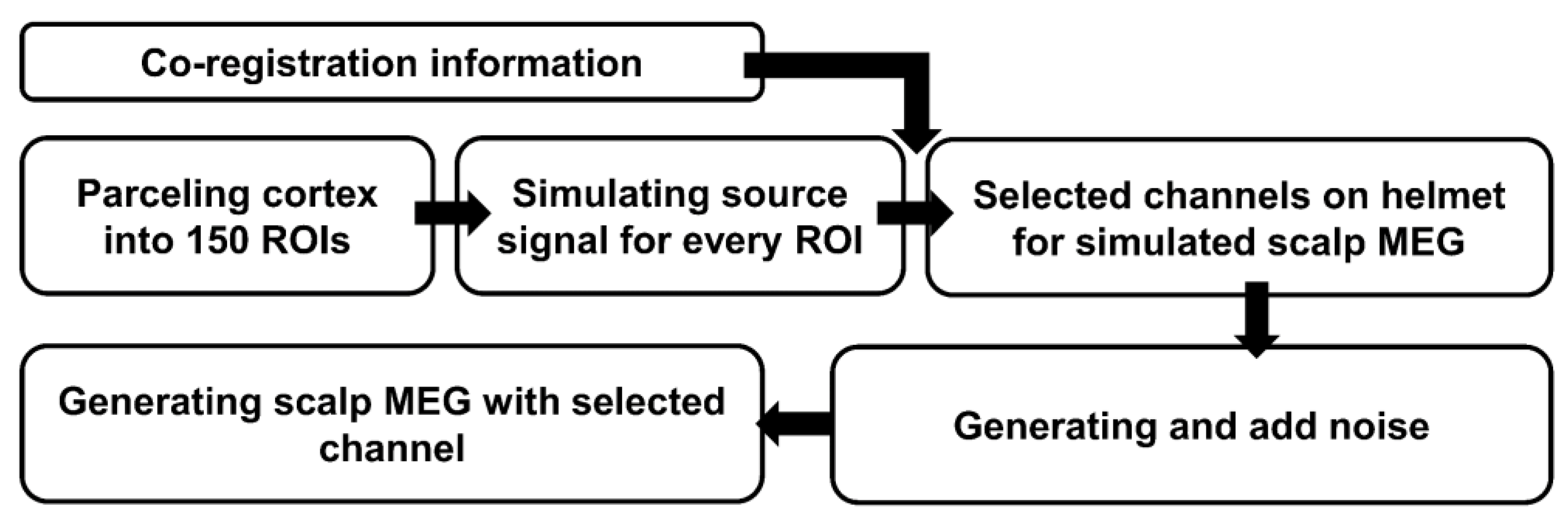
2.2. Simulation and MEG Experiments
2.2.1. Anatomical Coordinates of Regions of Interest (ROIs)
2.2.2. Co-Registration Data and Forward Model
2.2.3. Background Noise Data Acquisition
2.2.4. Simulated MEG Signals
2.2.5. SQUID-MEG Experiment
2.2.6. Participants of OPM-MEG Experiment
2.2.7. EO-EC OPM-MEG Experiment
2.2.8. MEG Data Preprocessing
2.3. Cortical Timeseries Reconstruction
- (1)
- Minimum Norm Estimate (MNE) [51]: MNE estimates the current source distribution over the cortical surface based on the minimum norm criterion. It uses L2 regularization to suppress noise. It assumes that the energy of the source distribution is minimized while satisfying the measured magnetic or electric field data. MNE solves a linear inverse problem to estimate source strengths.
- (2)
- Linearly Constrained Minimum Variance (LCMV) [44]: The LCMV method aims to find a set of spatial filters that minimize the variance of the source estimates while satisfying the linear constraints imposed by the sensor data. Although the LCMV method itself does not directly apply regularization to the source current distribution, it indirectly achieves regularization effects by optimizing the design of the spatial filters.
- (3)
- Exact Low-Resolution Electromagnetic Tomography (eLORETA) [52]: eLORETA is a source localization technique based on the weighted least squares method that assumes a continuous distribution of source current density over the cortical surface with spatial correlations between adjacent source points. It estimates source current density by minimizing the energy of the source distribution while satisfying the measured data.
- (4)
- Dynamic Statistical Parametric Mapping (dSPM) [53]: dSPM combines statistical parametric mapping with source localization to detect and localize statistically significant source activities from MEG data. It estimates the source distribution using MNE or similar methods and then applies statistical tests to assess whether the activity at each source point is significantly different from noise levels. dSPM works by computing statistical significance maps for each source point, showing the degree to which source activity deviates significantly from baseline levels.
2.4. Parameterizing Transient Oscillation
2.5. The Evaluation Indexes
2.5.1. The Root Mean Square Error (RMSE)
2.5.2. The Correlation of Cross-Cortical Gradient Estimation Between Two MEG Data Results
3. Results
3.1. The Performance of Gradient Estimation on Simulated MEG
3.2. The Result of EC-EO OPM-MEG
4. Discussion
4.1. The Cross-Cortical Gradient of Spontaneous Oscillations
4.2. Limitations and Future Directions
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paquola, C.; Wael, R.V.D.; Wagstyl, K.; Bethlehem, R.A.I.; Hong, S.-J.; Seidlitz, J.; Bullmore, E.T.; Evans, A.C.; Misic, B.; Margulies, D.S.; et al. Microstructural and Functional Gradients Are Increasingly Dissociated in Transmodal Cortices. PLoS Biol. 2019, 17, e3000284. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Himberger, K.D.; Chien, H.-Y.; Honey, C.J. Principles of Temporal Processing Across the Cortical Hierarchy. Neuroscience 2018, 389, 161–174. [Google Scholar] [CrossRef]
- Murray, J.D.; Bernacchia, A.; Freedman, D.J.; Romo, R.; Wallis, J.D.; Cai, X.; Padoa-Schioppa, C.; Pasternak, T.; Seo, H.; Lee, D.; et al. A Hierarchy of Intrinsic Timescales across Primate Cortex. Nat. Neurosci. 2014, 17, 1661–1663. [Google Scholar] [CrossRef]
- Jasmin, K.; Lima, C.F.; Scott, S.K. Understanding Rostral–Caudal Auditory Cortex Contributions to Auditory Perception. Nat. Rev. Neurosci. 2019, 20, 425–434. [Google Scholar] [CrossRef]
- Mahjoory, K.; Schoffelen, J.-M.; Keitel, A.; Gross, J. The Frequency Gradient of Human Resting-State Brain Oscillations Follows Cortical Hierarchies. eLife 2020, 9, e53715. [Google Scholar] [CrossRef] [PubMed]
- Doelling, K.B.; Assaneo, M.F. Neural Oscillations Are a Start toward Understanding Brain Activity Rather than the End. PLoS Biol. 2012, 19, e3001234. [Google Scholar] [CrossRef]
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Kopell, N.J.; Gritton, H.J.; Whittington, M.A.; Kramer, M.A. Beyond the Connectome: The Dynome. Neuron 2014, 83, 1319–1328. [Google Scholar] [CrossRef]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T.; et al. Parameterizing Neural Power Spectra into Periodic and Aperiodic Components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef]
- Keitel, A.; Gross, J. Individual Human Brain Areas Can Be Identified from Their Characteristic Spectral Activation Fingerprints. PLoS Biol. 2016, 14, e1002498. [Google Scholar] [CrossRef] [PubMed]
- Zich, C.; Quinn, A.J.; Bonaiuto, J.J.; O’Neill, G.; Mardell, L.C.; Ward, N.S.; Bestmann, S. Spatiotemporal Organization of Human Sensorimotor Beta Burst Activity. eLife 2023, 12, e80160. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.; Rea, M.; Boto, E.; Rier, L.; Shah, V.; Hill, R.M.; Osborne, J.; Doyle, C.; Holmes, N.; Coleman, S.C.; et al. Measurement of Frontal Midline Theta Oscillations Using OPM-MEG. NeuroImage 2023, 271, 120024. [Google Scholar] [CrossRef] [PubMed]
- Azanova, M.; Herrojo Ruiz, M.; Belianin, A.; Klucharev, V.; Nikulin, V. Resting-State Theta Oscillations and Reward Sensitivity in Risk Taking. Front. Neurosci. 2021, 15, 608699. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; De Blasio, F.M. EEG Differences between Eyes-Closed and Eyes-Open Resting Remain in Healthy Ageing. Biol. Psychol. 2017, 129, 293–304. [Google Scholar] [CrossRef]
- Leroy, S.; Major, S.; Bublitz, V.; Dreier, J.P.; Koch, S. Unveiling Age-Independent Spectral Markers of Propofol-Induced Loss of Consciousness by Decomposing the Electroencephalographic Spectrum into Its Periodic and Aperiodic Components. Front. Aging Neurosci. 2023, 14, 1076393. [Google Scholar] [CrossRef]
- McSweeney, M.; Morales, S.; Valadez, E.A.; Buzzell, G.A.; Yoder, L.; Fifer, W.P.; Pini, N.; Shuffrey, L.C.; Elliott, A.J.; Isler, J.R.; et al. Age-Related Trends in Aperiodic EEG Activity and Alpha Oscillations during Early- to Middle-Childhood. NeuroImage 2023, 269, 119925. [Google Scholar] [CrossRef]
- Lundqvist, M.; Miller, E.K.; Nordmark, J.; Liljefors, J.; Herman, P. Beta: Bursts of Cognition. Trends Cogn. Sci. 2024, 28, 662–676. [Google Scholar] [CrossRef]
- Liang, X.; Wang, R.; Wu, H.; Ma, Y.; Liu, C.; Gao, Y.; Yu, D.; Ning, X. A Novel Time–Frequency Parameterization Method for Oscillations in Specific Frequency Bands and Its Application on OPM-MEG. Bioengineering 2024, 11, 773. [Google Scholar] [CrossRef]
- Van Ede, F.; Quinn, A.J.; Woolrich, M.W.; Nobre, A.C. Neural Oscillations: Sustained Rhythms or Transient Burst-Events? Trends Neurosci. 2018, 41, 415–417. [Google Scholar] [CrossRef]
- Brady, B.; Bardouille, T. Periodic/Aperiodic Parameterization of Transient Oscillations (PAPTO)–Implications for Healthy Ageing. NeuroImage 2022, 251, 118974. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Law, R.; Tsutsui, S.; Moore, C.I.; Jones, S.R. The Rate of Transient Beta Frequency Events Predicts Behavior across Tasks and Species. eLife 2017, 6, e29086. [Google Scholar] [CrossRef] [PubMed]
- Pauls, K.A.M.; Nurmi, P.; Ala-Salomäki, H.; Renvall, H.; Kujala, J.; Liljeström, M. Human Sensorimotor Resting State Beta Events and Aperiodic Activity Show Good Test–Retest Reliability. Clin. Neurophysiol. 2024, 163, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Pang, H.; Quan, W.; Fan, W.; Yuan, L.; Zhang, K.; Fang, C. Pulsed Optical Pumping in Electron Spin Vapor. Measurement 2024, 231, 114619. [Google Scholar] [CrossRef]
- Brookes, M.J.; Leggett, J.; Rea, M.; Hill, R.M.; Holmes, N.; Boto, E.; Bowtell, R. Magnetoencephalography with Optically Pumped Magnetometers (OPM-MEG): The next Generation of Functional Neuroimaging. Trends Neurosci. 2022, 45, 621–634. [Google Scholar] [CrossRef]
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving Magnetoencephalography towards Real-World Applications with a Wearable System. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef]
- Rier, L.; Rhodes, N.; Pakenham, D.; Boto, E.; Holmes, N.; Hill, R.M.; Rivero, G.R.; Shah, V.; Doyle, C.; Osborne, J.; et al. The Neurodevelopmental Trajectory of Beta Band Oscillations: An OPM-MEG Study. eLife 2024, 13. [Google Scholar] [CrossRef]
- Cao, F.; Gao, Z.; Qi, S.; Chen, K.; Xiang, M.; An, N.; Ning, X. Realistic Three-Layer Head Phantom for Optically Pumped Magnetometer-Based Magnetoencephalography. Comput. Biol. Med. 2023, 164, 107318. [Google Scholar] [CrossRef]
- Petro, N.M.; Ott, L.; Penhale, S.; Rempe, M.; Embury, C.; Picci, G.; Wang, Y.-P.; Stephen, J.M.; Calhoun, V.D.; Wilson, T.W. Eyes-Closed versus Eyes-Open Differences in Spontaneous Neural Dynamics during Development. NeuroImage 2022, 258, 119337. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Zhang, M.; Ma, C.; Zhang, Y.; Wang, H.; Guo, Q.; Hu, T.; Liu, Z.; Chang, Y.; et al. Detection and Analysis of MEG Signals in Occipital Region with Double-Channel OPM Sensors. J. Neurosci. Meth. 2020, 346, 108948. [Google Scholar] [CrossRef]
- Ciulla, C.; Takeda, T.; Endo, H. MEG Characterization of Spontaneous Alpha Rhythm in the Human Brain. Brain Topogr. 1999, 11, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG Differences between Eyes-Closed and Eyes-Open Resting Conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, W.; Ding, Y.; Feng, Y.; Peng, X.; Shen, L.; Sun, C.; Wu, T.; Wu, Y.; Yang, Y.; et al. Recording Brain Activities in Unshielded Earth’s Field with Optically Pumped Atomic Magnetometers. Sci. Adv. 2020, 6, eaba8792. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Li, R.-Y.; Wei, W.; Feng, Z.-J.; Sun, Y.-K.; Sun, H.-Y.; Tang, Y.-Y.; Zang, Y.-F.; Yao, K. The Acts of Opening and Closing the Eyes Are of Importance for Congenital Blindness: Evidence from Resting-State fMRI. NeuroImage 2021, 233, 117966. [Google Scholar] [CrossRef]
- Li, S.; Cai, T.T.; Li, H. Inference for High-Dimensional Linear Mixed-Effects Models: A Quasi-Likelihood Approach. J. Am. Stat. Assoc. 2022, 117, 1835. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, L.; Hui, F.K.C.; Welsh, A.H. Restricted Maximum Likelihood Estimation in Generalized Linear Mixed Models. arXiv 2024, arXiv:2402.12719. [Google Scholar]
- McCulloch, C.E.; Searle, S.R.; Neuhaus, J.M. Generalized, Linear, and Mixed Models, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-1-118-20996-7. [Google Scholar]
- Laporte, F.; Charcosset, A.; Mary-Huard, T. Efficient ReML Inference in Variance Component Mixed Models Using a Min-Max Algorithm. PLoS Comput. Biol. 2022, 18, e1009659. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic Parcellation of Human Cortical Gyri and Sulci Using Standard Anatomical Nomenclature. NeuroImage 2010, 53, 1–15. [Google Scholar] [CrossRef]
- Cao, F.; An, N.; Xu, W.; Wang, W.; Li, W.; Wang, C.; Yang, Y.; Xiang, M.; Gao, Y.; Ning, X. OMMR: Co-Registration Toolbox of OPM-MEG and MRI. Front. Neurosci. 2022, 16, 984036. [Google Scholar] [CrossRef]
- Cao, F.; An, N.; Xu, W.; Wang, W.; Li, W.; Wang, C.; Xiang, M.; Gao, Y.; Ning, X. Optical Co-Registration Method of Triaxial OPM-MEG and MRI. IEEE Trans. Med. Imaging 2023, 42, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Nenonen, J.; Stenroos, M.; Gramfort, A.; Dalal, S.S.; Westner, B.U.; Litvak, V.; Mosher, J.C.; Schoffelen, J.-M.; Witton, C.; et al. Comparison of Beamformer Implementations for MEG Source Localization. NeuroImage 2020, 216, 116797. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE Software for Processing MEG and EEG Data. NeuroImage 2014, 86, 446–460. [Google Scholar] [CrossRef]
- Tierney, T.M.; Alexander, N.; Mellor, S.; Holmes, N.; Seymour, R.; O’Neill, G.C.; Maguire, E.A.; Barnes, G.R. Modelling Optically Pumped Magnetometer Interference in MEG as a Spatially Homogeneous Magnetic Field. NeuroImage 2021, 244, 118484. [Google Scholar] [CrossRef]
- Schoffelen, J.-M.; Oostenveld, R.; Lam, N.H.L.; Uddén, J.; Hultén, A.; Hagoort, P. A 204-Subject Multimodal Neuroimaging Dataset to Study Language Processing. Sci. Data 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Hohaia, W.; Saurels, B.W.; Johnston, A.; Yarrow, K.; Arnold, D.H. Occipital Alpha-Band Brain Waves When the Eyes Are Closed Are Shaped by Ongoing Visual Processes. Sci. Rep. 2022, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Yang, J.; Zhang, X.; Shi, M.; Yuan, S.; Wang, L. A Magnetic Compensation System Composed of Biplanar Coils Avoiding Coupling Effect of Magnetic Shielding. IEEE Trans. Ind. Electron. 2023, 70, 2057–2065. [Google Scholar] [CrossRef]
- Hyvarinen, A. Fast and Robust Fixed-Point Algorithms for Independent Component Analysis. IEEE Trans. Neural Netw. 1999, 10, 626–634. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting Magnetic Fields of the Brain: Minimum Norm Estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing Interactions in the Brain with Exact Low-Resolution Electromagnetic Tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic Statistical Parametric Mapping: Combining fMRI and MEG for High-Resolution Imaging of Cortical Activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef]
- Pesnot Lerousseau, J.; Parise, C.V.; Ernst, M.O.; van Wassenhove, V. Multisensory Correlation Computations in the Human Brain Identified by a Time-Resolved Encoding Model. Nat. Commun. 2022, 13, 2489. [Google Scholar] [CrossRef] [PubMed]
- Power, L.; Bardouille, T. Age-related Trends in the Cortical Sources of Transient Beta Bursts during a Sensorimotor Task and Rest. NeuroImage 2021, 245, 118670. [Google Scholar] [CrossRef] [PubMed]
- Beltrachini, L.; von Ellenrieder, N.; Eichardt, R.; Haueisen, J. Optimal Design of On-Scalp Electromagnetic Sensor Arrays for Brain Source Localisation. Hum. Brain Mapp. 2021, 42, 4869–4879. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Shim, J.H.; Kwon, H.; Lee, Y.-H.; Yu, K.-K.; Kwon, M.; Chun, W.Y.; Hirosawa, T.; Hasegawa, C.; Iwasaki, S.; et al. Detection of the 40 Hz Auditory Steady-State Response with Optically Pumped Magnetometers. Sci. Rep. 2022, 12, 17993. [Google Scholar] [CrossRef]
- Wagstyl, K.; Larocque, S.; Cucurull, G.; Lepage, C.; Cohen, J.P.; Bludau, S.; Palomero-Gallagher, N.; Lewis, L.B.; Funck, T.; Spitzer, H.; et al. BigBrain 3D Atlas of Cortical Layers: Cortical and Laminar Thickness Gradients Diverge in Sensory and Motor Cortices. PLoS Biol. 2020, 18, e3000678. [Google Scholar] [CrossRef] [PubMed]
- Huntenburg, J.M.; Bazin, P.-L.; Margulies, D.S. Large-Scale Gradients in Human Cortical Organization. Trends Cogn. Sci. 2018, 22, 21–31. [Google Scholar] [CrossRef]
- Boto, E.; Hill, R.M.; Rea, M.; Holmes, N.; Seedat, Z.A.; Leggett, J.; Shah, V.; Osborne, J.; Bowtell, R.; Brookes, M.J. Measuring Functional Connectivity with Wearable MEG. NeuroImage 2021, 230, 117815. [Google Scholar] [CrossRef]
- Zhang, H.; Watrous, A.J.; Patel, A.; Jacobs, J. Theta and Alpha Oscillations Are Traveling Waves in the Human Neocortex. Neuron 2018, 98, 1269–1281.e4. [Google Scholar] [CrossRef]
- Wei, J.; Chen, T.; Li, C.; Liu, G.; Qiu, J.; Wei, D. Eyes-Open and Eyes-Closed Resting States with Opposite Brain Activity in Sensorimotor and Occipital Regions: Multidimensional Evidences From Machine Learning Perspective. Front. Hum. Neurosci. 2018, 12, 422. [Google Scholar] [CrossRef]
- Jayachandran, M.; Viena, T.D.; Garcia, A.; Veliz, A.V.; Leyva, S.; Roldan, V.; Vertes, R.P.; Allen, T.A. Nucleus Reuniens Transiently Synchronizes Memory Networks at Beta Frequencies. Nat. Commun. 2023, 14, 4326. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.; Boto, E.; Holmes, N.; Hill, R.; Osborne, J.; Rhodes, N.; Leggett, J.; Rier, L.; Bowtell, R.; Shah, V.; et al. A 90-channel Triaxial Magnetoencephalography System Using Optically Pumped Magnetometers. Ann. N. Y. Acad. Sci. 2022, 1517, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Mellor, S.; Tierney, T.M.; OaNeill, G.C.; Alexander, N.; Seymour, R.A.; Holmes, N.; Lopez, J.D.; Hill, R.M.; Boto, E.; Rea, M.; et al. Magnetic Field Mapping and Correction for Moving OP-MEG. IEEE Trans. Biomed. Eng. 2022, 69, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Mellor, S.; Tierney, T.M.; Seymour, R.A.; Timms, R.C.; O’Neill, G.C.; Alexander, N.; Spedden, M.E.; Payne, H.; Barnes, G.R. Real-Time, Model-Based Magnetic Field Correction for Moving, Wearable MEG. NeuroImage 2023, 278, 120252. [Google Scholar] [CrossRef]
- Holmes, N.; Rea, M.; Hill, R.M.; Leggett, J.; Edwards, L.J.; Hobson, P.J.; Boto, E.; Tierney, T.M.; Rier, L.; Rivero, G.R.; et al. Enabling Ambulatory Movement in Wearable Magnetoencephalography with Matrix Coil Active Magnetic Shielding. NeuroImage 2023, 274, 120157. [Google Scholar] [CrossRef]
- Brookes, M.J.; Boto, E.; Rea, M.; Shah, V.; Osborne, J.; Holmes, N.; Hill, R.M.; Leggett, J.; Rhodes, N.; Bowtell, R. Theoretical Advantages of a Triaxial Optically Pumped Magnetometer Magnetoencephalography System. NeuroImage 2021, 236, 118025. [Google Scholar] [CrossRef]
- Boto, E.; Shah, V.; Hill, R.M.; Rhodes, N.; Osborne, J.; Doyle, C.; Holmes, N.; Rea, M.; Leggett, J.; Bowtell, R.; et al. Triaxial Detection of the Neuromagnetic Field Using Optically-Pumped Magnetometry: Feasibility and Application in Children. NeuroImage 2022, 252, 119027. [Google Scholar] [CrossRef]





| Functions | Explanations |
|---|---|
| mne.simulation.simulate_raw | To generate MEG data with source timeseries of all ROI. |
| mne.make_ad_hoc_cov | To quickly generate an ad hoc covariance matrix. |
| mne.simulation.add_noise | To add noise to simulated noise-free MEG data based on a noise covariance matrix. |
| compute_raw_covariance | To calculate a noise covariance matrix using empty room noise data. |
| Reconstruction Method | 64 Channels | 32 Channels | 16 Channels | Mean |
|---|---|---|---|---|
| MNE * | 0.54 | 0.44 | 0.29 | 0.42 |
| LCMV * | 0.26 | 0.33 | 0.37 | 0.32 |
| eLORETA * | 0.49 | 0.49 | 0.29 | 0.42 |
| dSPM * | 0.52 | 0.44 | 0.30 | 0.42 |
| Mean (across methods) | 0.45 | 0.43 | 0.31 | - |
| Reconstruction Method | 64 Channels | 32 Channels | 16 Channels | Mean |
|---|---|---|---|---|
| MNE * | 0.465 | 0.287 | 0.391 | 0.381 |
| LCMV * | 0.479 | 0.226 | 0.397 | 0.367 |
| eLORETA * | 0.470 | 0.307 | 0.382 | 0.386 |
| dSPM * | 0.465 | 0.289 | 0.392 | 0.382 |
| Mean (across methods) | 0.470 | 0.277 | 0.391 | - |
| Reconstruction Method | RMSEPF (Hz) | RMSEPT (s) |
|---|---|---|
| MNE * | 0.43 | 0.479 |
| LCMV * | 0.37 | 0.461 |
| eLORETA * | 0.42 | 0.480 |
| dSPM * | 0.42 | 0.479 |
| Slope | ||||||||
|---|---|---|---|---|---|---|---|---|
| EO | PF | 12.71 | −0.028 | −0.016 | 0.019 | −0.0006 | 0.0002 | 0.0004 |
| PT | 2.376 | −0.0014 | 0.0097 | 0.0052 | −6.00 × 10−5 | 8.77 × 10−5 | −5.08 × 10−5 | |
| EC | PF | 10.46 | −0.002 | −0.001 | 0.002 | −6.36 × 10−5 | −9.18 × 10−7 | −3.36 × 10−5 |
| PT | 2.079 | 0.0008 | −0.0020 | 0.0022 | −1.96 × 10−6 | −5.29 × 10−5 | 1.44 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Ma, Y.; Wu, H.; Wang, R.; Wang, R.; Liu, C.; Gao, Y.; Ning, X. The Gradient of Spontaneous Oscillations Across Cortical Hierarchies Measured by Wearable Magnetoencephalography. Technologies 2024, 12, 254. https://doi.org/10.3390/technologies12120254
Liang X, Ma Y, Wu H, Wang R, Wang R, Liu C, Gao Y, Ning X. The Gradient of Spontaneous Oscillations Across Cortical Hierarchies Measured by Wearable Magnetoencephalography. Technologies. 2024; 12(12):254. https://doi.org/10.3390/technologies12120254
Chicago/Turabian StyleLiang, Xiaoyu, Yuyu Ma, Huanqi Wu, Ruilin Wang, Ruonan Wang, Changzeng Liu, Yang Gao, and Xiaolin Ning. 2024. "The Gradient of Spontaneous Oscillations Across Cortical Hierarchies Measured by Wearable Magnetoencephalography" Technologies 12, no. 12: 254. https://doi.org/10.3390/technologies12120254
APA StyleLiang, X., Ma, Y., Wu, H., Wang, R., Wang, R., Liu, C., Gao, Y., & Ning, X. (2024). The Gradient of Spontaneous Oscillations Across Cortical Hierarchies Measured by Wearable Magnetoencephalography. Technologies, 12(12), 254. https://doi.org/10.3390/technologies12120254









