Effect of Carbohydrates on the Formation Process and Performance of Micro-Arc Oxidation Coatings on AZ31B Magnesium Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of MAO Coatings
2.3. Characterization of MAO Coatings
2.4. Corrosion Resistance Test of MAO Coatings
3. Results and Discussions
3.1. MAO Process
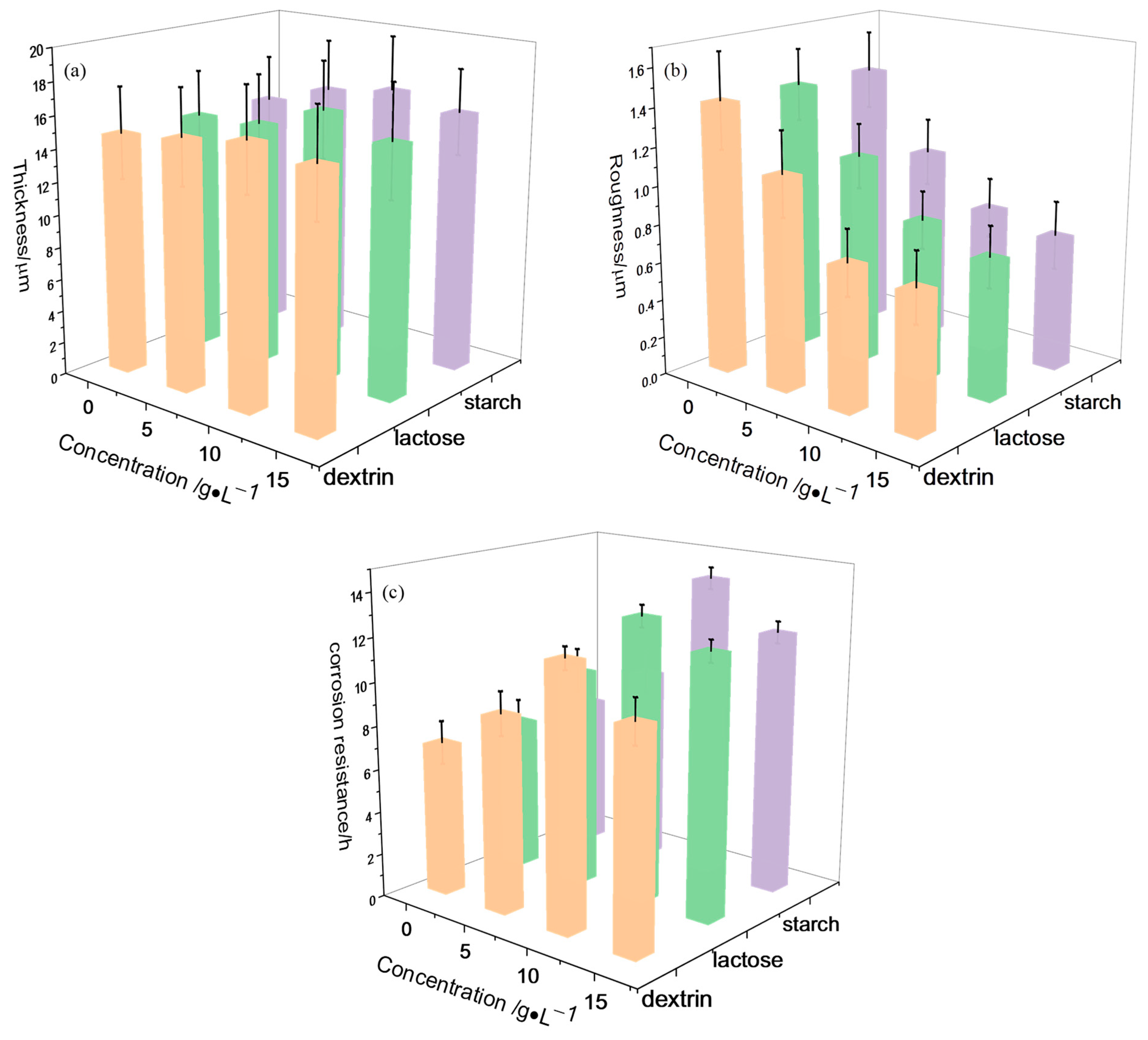
3.2. Properties of MAO Coatings
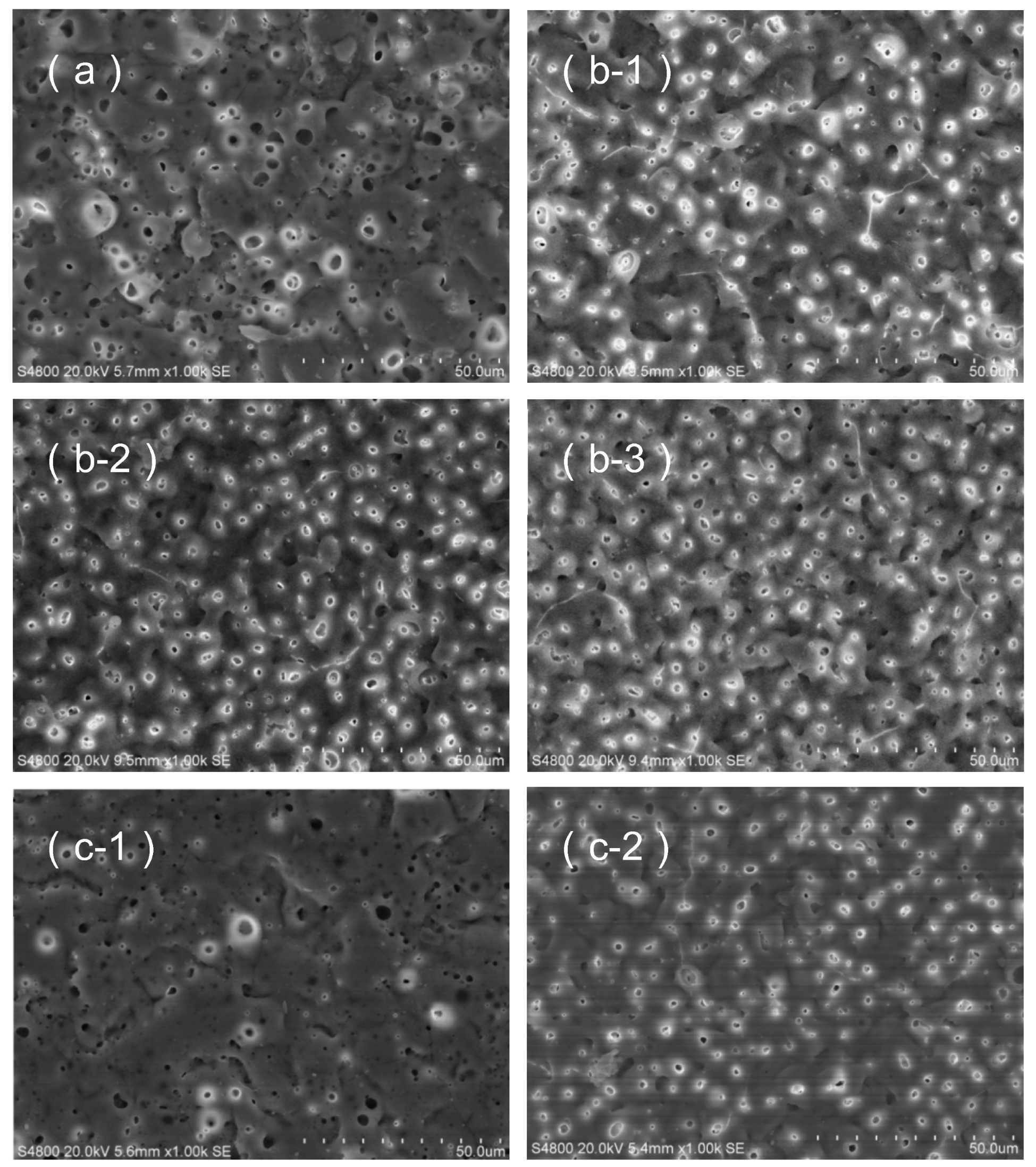
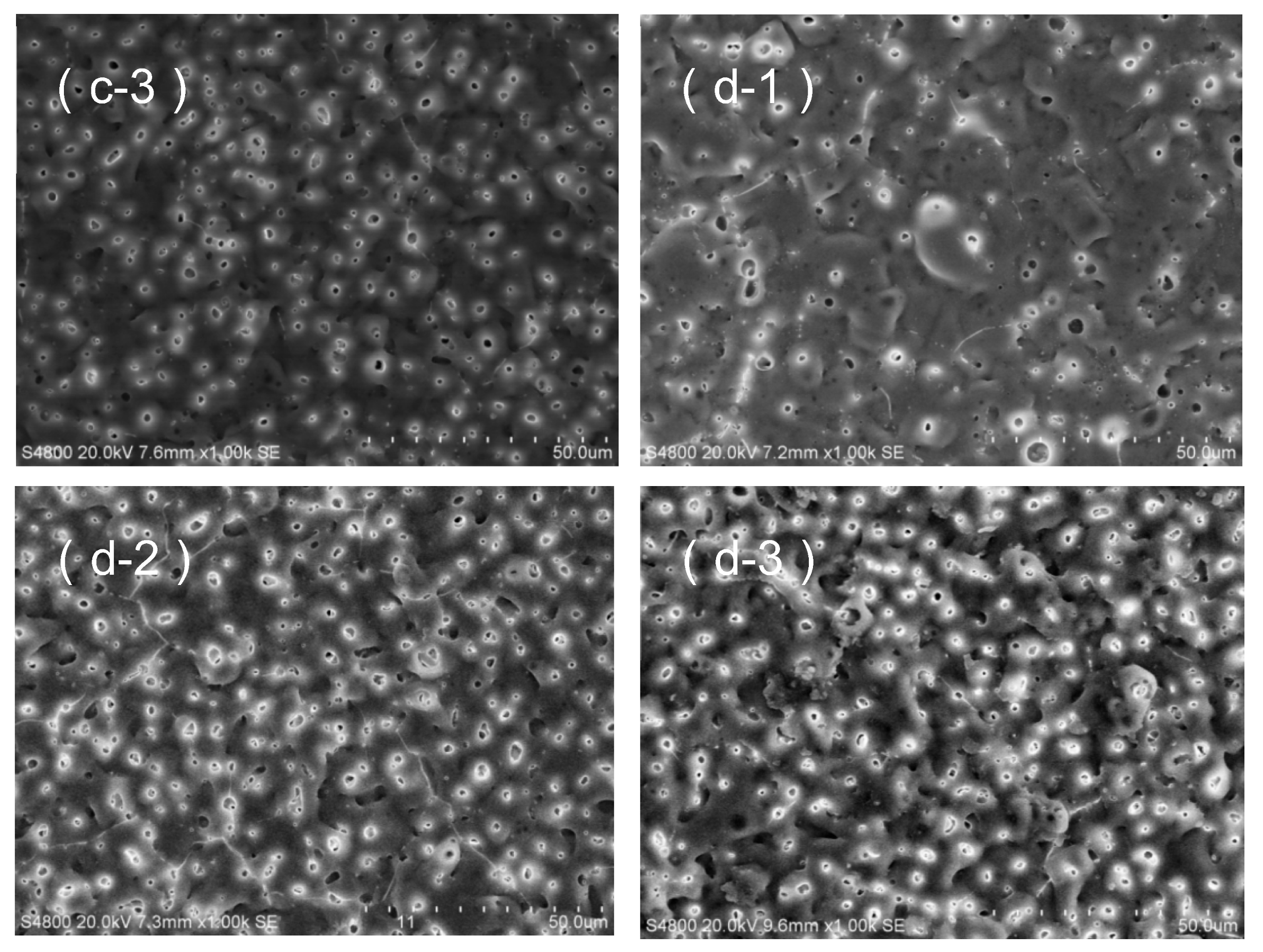
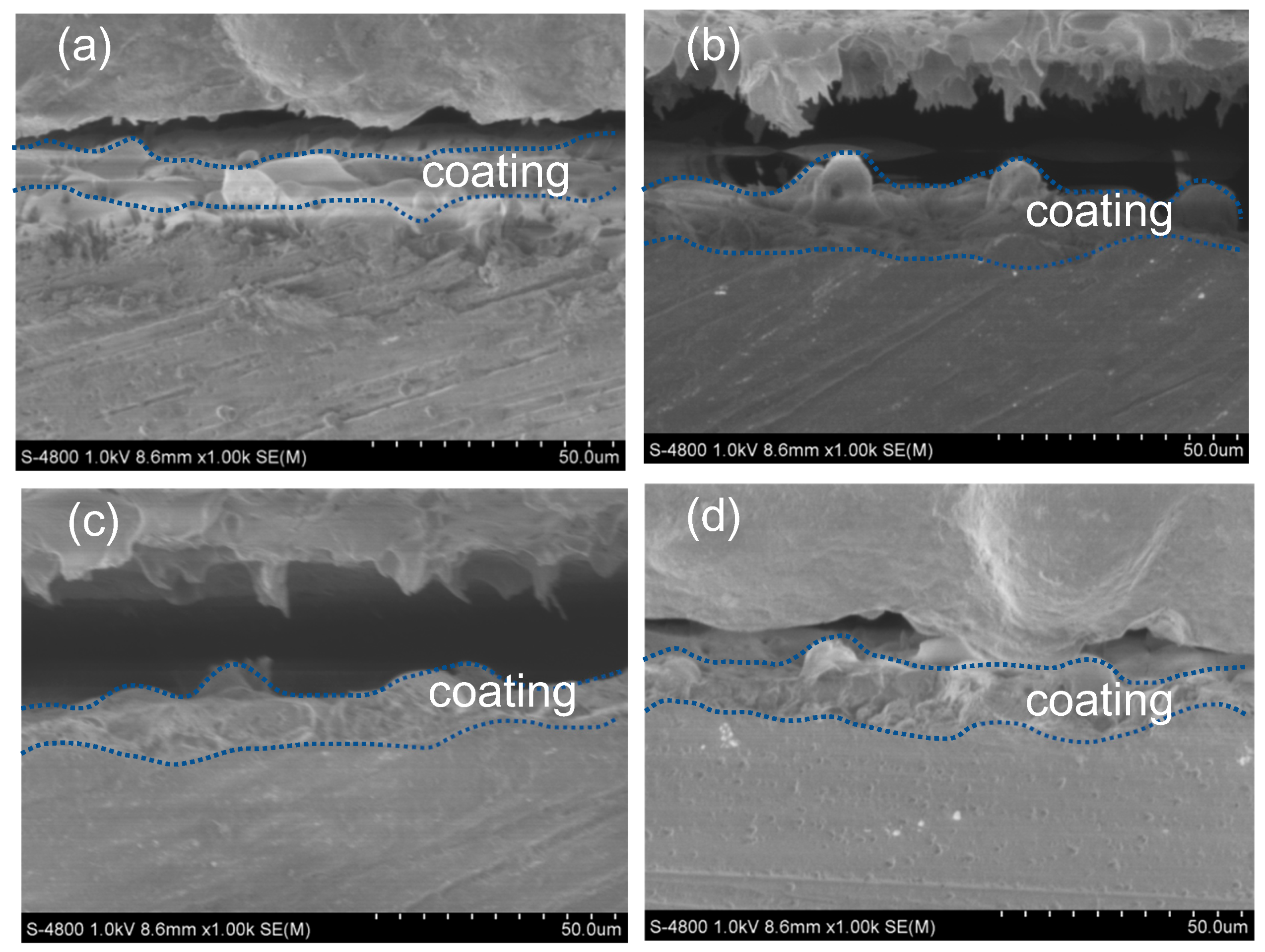
3.3. Morphological Characteristics
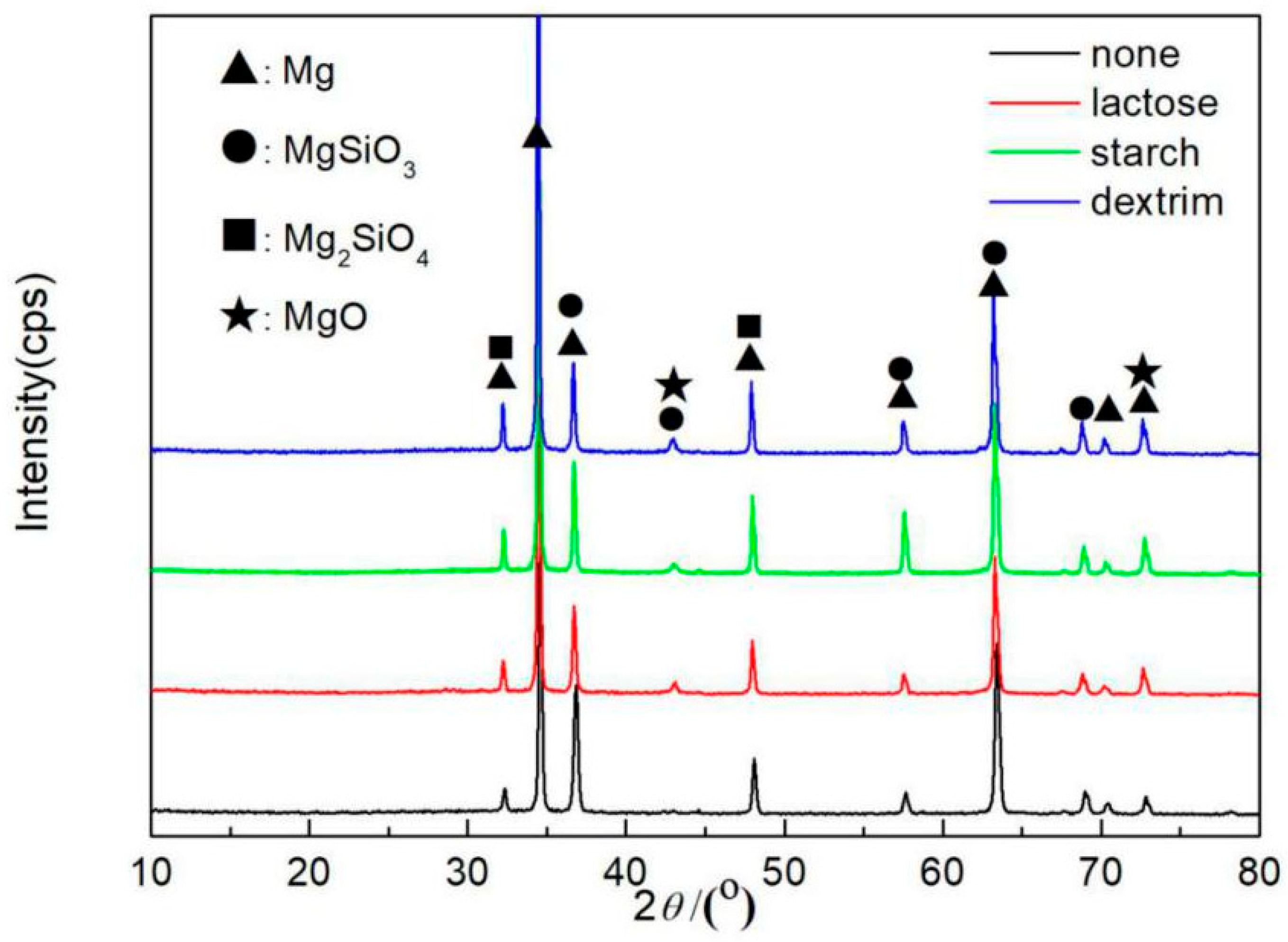
3.4. Phase Analysis
3.5. Electrochemical Properties of MAO Coatings
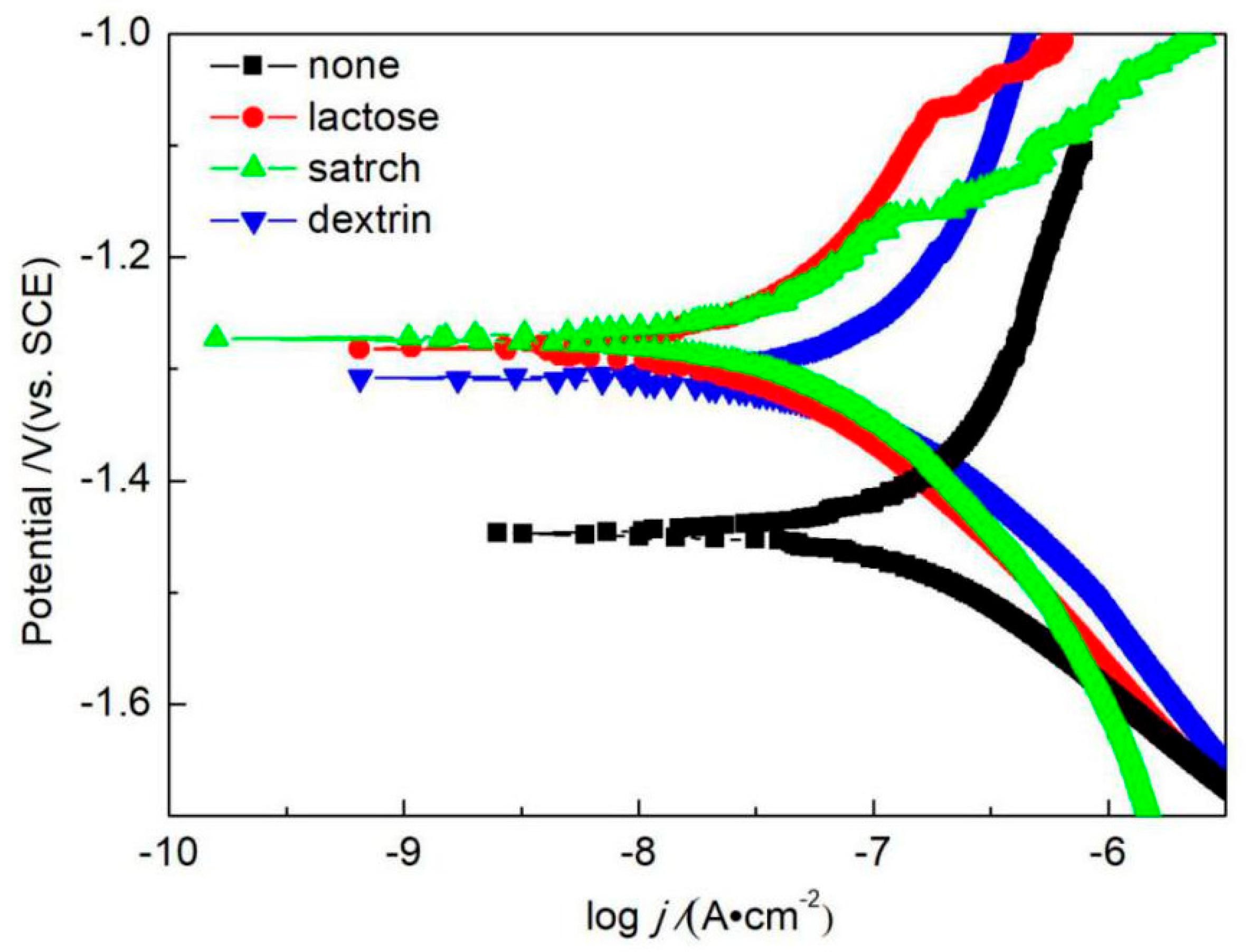
3.5.1. Polarization Curves
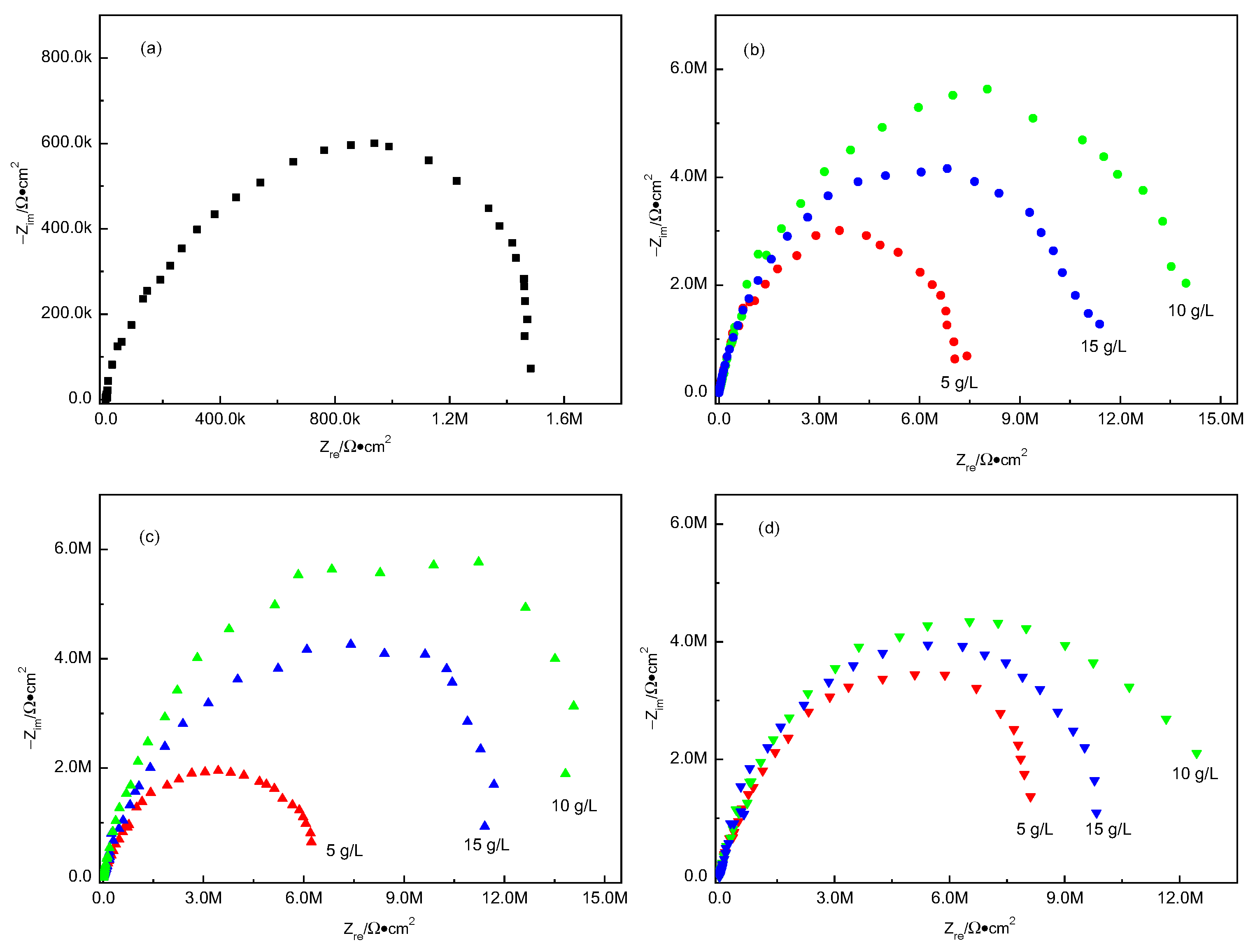
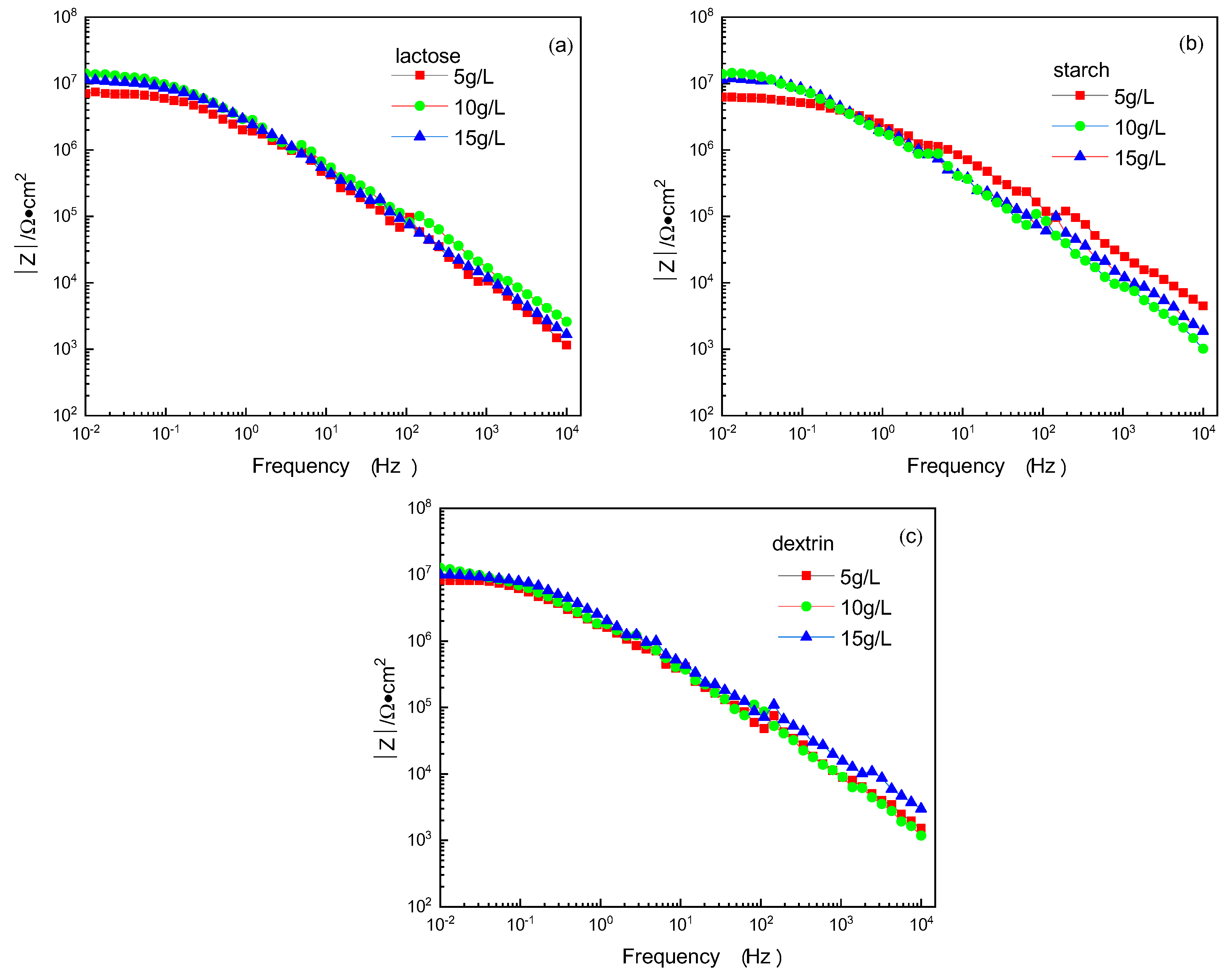
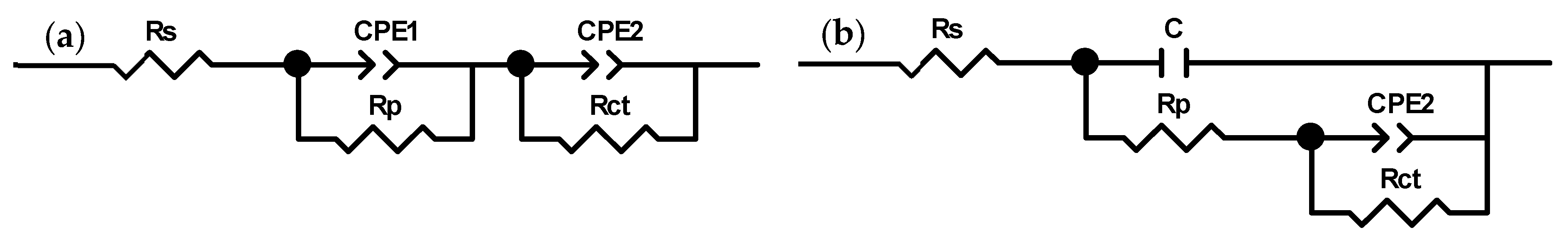
3.5.2. Electrochemical Impedance Spectroscopy
4. Conclusions
- (1)
- Spark discharge was suppressed with the additions of lactose, starch, and dextrin into the alkaline electrolyte in the MAO process. Also, the thickness and corrosion resistance of the MAO coatings with a smooth surface structure were improved.
- (2)
- The additives of lactose, starch, and dextrin affected the MAO process but did not participate in the film-forming reaction. The XRD results show that the MAO coatings were mainly composed of MgO, MgSiO3, and Mg2SiO4.
- (3)
- Through the polarization curves and EIS test, it was found that the addition of lactose, starch, and dextrin to the alkaline electrolyte significantly improved the corrosion resistance of the MAO coatings. When the concentration of lactose, starch, and dextrin additives was 10 g/L, the MAO coatings had the best corrosion resistance. In this framework, the corrosion potentials of the MAO coatings were −1.28 V, −1.27 V, and −1.31 V, respectively; the corrosion current densities were 2.21 × 10−8 A/cm2, 1.90 × 10−8 A/cm2, and 3.22 × 10−8 A/cm2; and the Rct values were 1.62 × 107 ohm·cm−2, 1.68 × 107 ohm·cm−2, and 1.47 × 107 ohm·cm−2, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Leiva-García, R.; Connolly, B.; Matthews, A.; Yerokhin, A. Smart functionalization of ceramic-coated AZ31 magnesium alloy. ACS Appl. Mater. Interfaces 2020, 12, 30833–30846. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, J.; Jian, S.; Chen, C.; Aktug, S.; Ger, M. The effect of fluoride on the formation of an electroless Ni-P plating film on MAO-coated AZ31B magnesium alloy. J. Mater. Res. Technol. 2019, 8, 5823–5832. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, L.; Xie, Z.; Tang, L.; Wang, F.; Zhong, C. A self-healing coating based on facile pH-responsive nanocontainers for corrosion protection of magnesium alloy. J. Magnes. Alloys 2022, 10, 836–849. [Google Scholar] [CrossRef]
- Song, G. An irreversible dipping sealing technique for anodized ZE41 Mg alloy. Surf. Coat. Technol. 2009, 203, 3618–3625. [Google Scholar] [CrossRef]
- Evangelides, H. Method of Electrolytically Coating Magnesium and Electrolyte Therefore. U.S. Patent 2723952, 15 November 1955. [Google Scholar]
- Dow Chemical Co. Bath for and Method of Producing a Corrosion Resistant Coating upon Light Metals. GB Patent 762195, 28 November 1956. [Google Scholar]
- Shi, Z.; Song, G.; Atrens, A. Influence of anodising current on the corrosion resistance of anodised AZ91D magnesium alloy. Corros. Sci. 2006, 48, 1939–1959. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L. Effect of AC voltage on anodic film of AZ91D magnesium alloy. Chin. J. Nonferrous Met. 2008, 18, 1472–1478. [Google Scholar]
- Wu, X.; Su, P.; Jiang, Z.; Meng, S. Influences of current density on tribological characteristics of ceramic coatings on ZK60 Mg alloy by plasma electrolytic oxidation. ACS Appl. Mater. Interfaces 2010, 2, 808–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Duan, X.; Zhao, Y. Effect of treatment time on a PEO-coated AZ31 magnesium alloy. Mater. Corros. 2021, 72, 1885–1893. [Google Scholar] [CrossRef]
- Choi, Y.; Salman, S.; Kuroda, K.; Okido, M. Improvement in corrosion characteristics of AZ31 Mg alloy by square pulse anodizing between transpassive and active regions. Corros. Sci. 2012, 63, 5–11. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, Y.; Park, D.; Yoo, B.; Shin, D. Corrosion resistance of oxide layers formed on AZ91 Mg alloy in KMnO4 electrolyte by plasma electrolytic oxidation. Electrochim. Acta 2009, 54, 5479–5485. [Google Scholar] [CrossRef]
- Barchiche, C.; Rocca, E.; Juers, C.; Hazan, J.; Steinmetz, J. Corrosion resistance of plasma-anodized AZ91D magnesium alloy by electrochemical methods. Electrochim. Acta 2007, 53, 417–425. [Google Scholar] [CrossRef]
- Barchiche, C.; Veys-Renaux, D.; Rocca, E. A better understanding of PEO on Mg alloys by using a simple galvanostatic electrical regime in a KOH-KF-Na3PO4 electrolyte. Surf. Coat. Technol. 2011, 205, 4243–4248. [Google Scholar] [CrossRef]
- Lamaka, S.; Knorchild, G.; Snihirova, D.; Taryba, M.; Zheludkevich, M.; Ferreira, M. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Say, W.; Chen, C.; Hsieh, S. Electrochemical characterization of non-chromate surface treatments on AZ80 magnesium. Mater. Charact. 2008, 59, 1400–1406. [Google Scholar] [CrossRef]
- Yoo, B.; Shin, K.; Hwang, D.; Lee, D.; Shin, D. Effect of surface roughness on leakage current and corrosion resistance of oxide layer on AZ91 Mg alloy prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2010, 256, 6667–6672. [Google Scholar] [CrossRef]
- Bai, A.; Chen, Z. Effect of electrolyte additives on anti-corrosion ability of micro-arc oxide coatings formed on magnesium alloy AZ91D. Surf. Coat. Technol. 2009, 203, 1956–1963. [Google Scholar] [CrossRef]
- Kazanski, B.; Kossenko, A.; Zinigrad, M.; Lugovskoy, A. Fluoride ions as modifiers of the oxide layer produced by plasma electrolytic oxidation on AZ91D magnesium alloy. Appl. Surf. Sci. 2013, 287, 461–466. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.; Li, L.; Chen, Z.; Wang, H.; Zhang, Z. The anodization of ZK60 magnesium alloy in alkaline solution containing silicate and the corrosion properties of the anodized films. Appl. Surf. Sci. 2007, 253, 9387–9394. [Google Scholar] [CrossRef]
- Hsiao, H.; Tsung, H.; Tsai, W. Anodization of AZ91D magnesium alloy in silicate-containing electrolytes. Surf. Coat. Technol. 2005, 199, 127–134. [Google Scholar] [CrossRef]
- Xue, D.; Yun, Y.; Schulz, M.; Shanov, V. Corrosion protection of biodegradable magnesium implants using anodization. Mater. Sci. Eng. C 2011, 31, 215–223. [Google Scholar] [CrossRef]
- Ou, A.; Yu, G.; Hu, B.; He, X.; Zhang, J.; Yi, H.; Chen, Y. Effect of triethanolamine on anodizing process of magnesium alloys. CIESC J. 2009, 60, 2118–2123. [Google Scholar]
- Guo, X.; An, M.; Yang, P.; Li, H.; Su, C. Effects of benzotriazole on anodized film formed on AZ31B magnesium alloy in environmental-friendly electrolyte. J. Alloys Compd. 2009, 482, 487–497. [Google Scholar] [CrossRef]
- Cao, F.; Cao, J.; Zhang, Z.; Zhang, J.; Cao, C. Plasma electrolytic oxidation of AZ91D magnesium alloy with different additives and its corrosion behavior. Mater. Corros. 2007, 58, 696–703. [Google Scholar] [CrossRef]
- Yabuki, A.; Sakai, M. Anodic films formed on magnesium in organic, silicate-containing electrolytes. Corros. Sci. 2009, 51, 793–798. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Chen, C.; Zhang, J.; Cui, L. Adsorption orientation of sodium of polyaspartic acid effect on anodic films formed on magnesium alloy. Appl. Surf. Sci. 2011, 257, 7579–7585. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Ghalebsaz-Jeddi, N.; Hashemzadeh, F.; Jahani, H. Corrosion inhibition of carbon steel in hydrochloric acid by some polyethylene glycols. Electrochim. Acta 2006, 51, 3848–3854. [Google Scholar] [CrossRef]
- Tu, X.; Chen, L.; Wu, J. Effect of glucose on properties of anodizing film on AZ31B magnesium alloy. Chin. J. Nonferrous Met. 2013, 23, 727–734. [Google Scholar]
- Verdier, S.; Boinet, M.; Maximovitch, S.; Dalard, F. Formation, structure and compostion of anodic films on AM60 magnesium alloy obtained by DC plasma anodizing. Corros. Sci. 2005, 47, 1429–1444. [Google Scholar] [CrossRef]
- Guo, H.; An, M.; Huo, H.; Xu, S.; Wu, L. Microstructure characteristic of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation in alkaline silicate solutions. Appl. Surf. Sci. 2006, 252, 7911–7916. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Yang, F.; Xu, S.; Wu, L. Environmental friendly anodizing of AZ91D magnesium alloy in alkaline borate–benzoate electrolyte. J. Alloys Compd. 2009, 509, 6440–6446. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, J.; Li, S.; Zhang, J. Preparation and characterization of anodic films on AZ31B Mg alloy formed in the silicate electrolyte with ethylene glycol oligomers as additives. Appl. Surf. Sci. 2012, 258, 8985–8990. [Google Scholar] [CrossRef]
- Lai, X.; Kang, Z.; Li, Y. Duplex surface modification combined with micro-arc oxidation and polymer plating on AZ31 magnesium alloy and their functional properties. Chin. J. Nonferrous Met. 2011, 21, 1299–1307. [Google Scholar]
- Kamil, M.; Kaseem, M.; Lee, Y.; Ko, Y. Microstructure characteristics of oxide layer formed by plasma electrolytic oxidation: Nanocrystalline and amorphous structure. J. Alloys Compd. 2017, 707, 167–171. [Google Scholar] [CrossRef]
- Montemor, M.; Ferreira, M. Electrochemical study of modified bis-[triethoxysilylpropyl] tetrasulfide silane films applied on the AZ31 Mg alloy. Electrochim. Acta 2007, 52, 7486–7495. [Google Scholar] [CrossRef]
- Kim, J.; Wong, K.; Wong, P.; Kulinich, S.; Metson, J.; Mitchell, K. Characterization of AZ91 magnesium alloy and organosilane adsorption on its surface. Appl. Surf. Sci. 2007, 253, 4197–4207. [Google Scholar] [CrossRef]
- Zeng, R.; Li, X.; Liu, Z.; Zhang, F.; Li, S.; Cui, H. Corrosion resistance of Zn–Al layered double hydroxide/poly(lactic acid) composite coating on magnesium alloy AZ31. Front. Mater. Sci. 2015, 9, 355–365. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Della Rovere, C.A.; Alano, J.H.; Silva, R.; Nascente, P.A.P.; Otubo, J.; Kuri, S.E. Characterization of passive films on shape memory stainless steels. Corros. Sci. 2012, 57, 154–161. [Google Scholar] [CrossRef]
- Qian, J.; Li, D.; Wang, C.; Guo, B. EIS Study on Corrosion Process of Anodized Film on AZ91D Magnesium Alloy. Rare Metal Mater. Eng. 2006, 35, 1280–1284. [Google Scholar]
- Birss, V.; Xia, S.; Yue, R.; Rateick, R. Characterization of Oxide Films Formed on Mg-Based WE43 Alloy Using AC/DC Anodization in Silicate Solutions. J. Electrochem. Soc. 2004, 151, B1–B10. [Google Scholar] [CrossRef]
- Xia, S.; Yue, R.; Rateick, R.; Birss, V. Electrochemical Studies of AC/DC Anodized Mg Alloy in NaCl Solution. J. Electrochem. Soc. 2004, 151, B179–B187. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Yang, F.; Zhang, Z. Anodizing of AZ91D magnesium alloy in borate-terephthalic acid electrolyte. Acta Phys-Chim. Sin. 2011, 27, 2385–2392. [Google Scholar]
- Ningshen, S.; Kamachi, M.; Amarendra, G.; Gopalan, P.; Dayal, R.; Khatak, H. Hydrogen effects on the passive film formation and pitting susceptibility of nitrogen containing type 316L stainless steels. Corros. Sci. 2006, 48, 1106–1121. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, F.; Li, W. Electrochemical behavior of anodized Mg alloy AZ91D in chloride containing aqueous solution. Corros. Sci. 2005, 47, 2816–2831. [Google Scholar] [CrossRef]

| Addition/10 g/L | Element (wt%) | ||||
|---|---|---|---|---|---|
| O | Na | Mg | Si | Al | |
| None | 55.7 | 6.2 | 28.3 | 9.1 | 0.7 |
| Lactose | 51.6 | 3.1 | 32.6 | 9.2 | 1.0 |
| Starch | 53.8 | 3.1 | 32.7 | 9.6 | 0.9 |
| Dextrin | 54.3 | 3.5 | 32.2 | 9.1 | 0.9 |
| Addition/10 g·L−1 | Ecorr/V | jcorr/A·cm−2 |
|---|---|---|
| None | −1.447 ± 0.002 | 1.34 × 10−7 ± 0.24 × 10−7 |
| Lactose | −1.284 ± 0.004 | 2.78 × 10−8 ± 0.18 × 10−8 |
| Starch | −1.273 ± 0.003 | 2.43 × 10−8 ± 0.16 × 10−8 |
| Dextrin | −1.307 ± 0.005 | 6.08 × 10−8 ± 0.28 × 10−8 |
| Addition | c/g·L−1 | Rs/ohm·cm−2 | C/μF·cm−2 | Rp/ohm·cm−2 | CPE1/ (μF·cm−2)1/n | n1 | CPE2/ (μF·cm−2)1/n | n2 | Rct/107 ohm·cm−2 |
|---|---|---|---|---|---|---|---|---|---|
| None | 0 | 91.57 | -- | 2485 | 0.55 × 10−2 | 0.91 | 0.33 × 10−2 | 0.93 | 0.13 |
| Lactose | 5 | 117.9 | 1.32 × 10−2 | 8391 | -- | -- | 8.92 × 10−2 | 0.67 | 0.83 |
| 10 | 153.1 | 0.82 × 10−2 | 4432 | -- | -- | 7.96 × 10−2 | 0.71 | 1.62 | |
| 15 | 168.5 | 4.76 × 10−2 | 10,680 | -- | -- | 7.12 × 10−2 | 0.72 | 1.21 | |
| Starch | 5 | 128.4 | 5.44 × 10−2 | 4827 | -- | -- | 9.54 × 10−2 | 0.60 | 0.71 |
| 10 | 142.7 | 1.02 × 10−2 | 4262 | -- | -- | 0.12 × 10−2 | 0.68 | 1.68 | |
| 15 | 122.2 | 3.94 × 10−2 | 5586 | -- | -- | 0.10 × 10−2 | 0.72 | 1.33 | |
| Dextrin | 5 | 98.1 | 5.63 × 10−2 | 7752 | -- | -- | 9.37 × 10−2 | 0.70 | 1.09 |
| 10 | 139.3 | 1.13 × 10−2 | 6831 | -- | -- | 1.23 × 10−2 | 0.66 | 1.47 | |
| 15 | 103.2 | 5.19 × 10−2 | 5101 | -- | -- | 9.34 × 10−2 | 0.70 | 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Hu, M.; Tu, X.; Miao, C.; Zhang, Y.; Li, J. Effect of Carbohydrates on the Formation Process and Performance of Micro-Arc Oxidation Coatings on AZ31B Magnesium Alloy. Technologies 2023, 11, 139. https://doi.org/10.3390/technologies11050139
Du Y, Hu M, Tu X, Miao C, Zhang Y, Li J. Effect of Carbohydrates on the Formation Process and Performance of Micro-Arc Oxidation Coatings on AZ31B Magnesium Alloy. Technologies. 2023; 11(5):139. https://doi.org/10.3390/technologies11050139
Chicago/Turabian StyleDu, Yingxiu, Mingyue Hu, Xiaohua Tu, Chengping Miao, Yang Zhang, and Jiayou Li. 2023. "Effect of Carbohydrates on the Formation Process and Performance of Micro-Arc Oxidation Coatings on AZ31B Magnesium Alloy" Technologies 11, no. 5: 139. https://doi.org/10.3390/technologies11050139
APA StyleDu, Y., Hu, M., Tu, X., Miao, C., Zhang, Y., & Li, J. (2023). Effect of Carbohydrates on the Formation Process and Performance of Micro-Arc Oxidation Coatings on AZ31B Magnesium Alloy. Technologies, 11(5), 139. https://doi.org/10.3390/technologies11050139






