Production Technologies, Regulatory Parameters, and Quality Control of Vaccine Vectors for Veterinary Use
Abstract
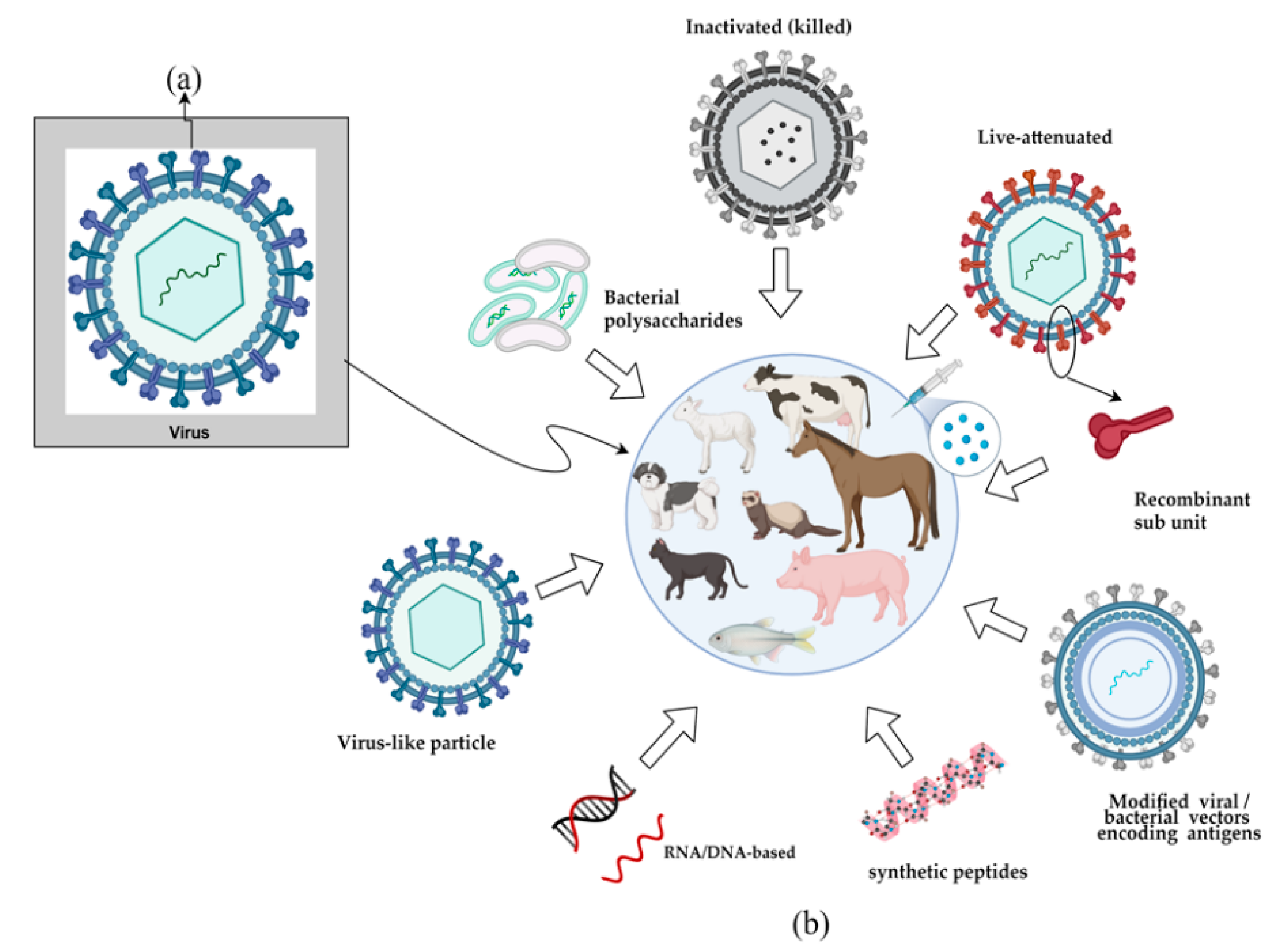
1. Introduction
| Platform | Characteristics | Restrictions | Refs |
|---|---|---|---|
| Whole virus | |||
| Attenuated | Entire virus passed on in successive cultivations to lose infective capacity. | Production requires cell culture of the virus and exhaustive safety tests as they are more immunogenic. | [21] |
| Inactivated | The intact virus is inactivated by chemical or physical methods. | Vaccines based on the inactivated virus require an initial high amount of virus. | |
| Subunit | |||
| Proteins | Proteins or their fragments are injected directly into the animal. | Generally, require adjuvants or multiple doses to achieve the desired immune response. | [21] |
| Virus-like particle | Self-assembled viral structural proteins that resemble the virus, however, lack genetic material. | The biggest challenge of this platform is to ensure that the epitopes are in an adequate conformation after translation and that the expressed proteins are not allergenic. | [22] |
| Nucleic acid | |||
| DNA | Insertion of the DNA that encodes the viral antigen into a plasmid. | They are platforms under experimentation for animal purposes. | [23] |
| RNA | Messenger RNA encapsulated in a lipid membrane. | ||
| Vector encoding antigen | |||
| Replicating and non-replicating | Non-infective pathogens are genetically modified with the insertion of one or more genes that express antigenic particles, which may or may not multiply in the animal organism. | Requires level 2 biosafety labs for production; it has reduced efficacy due to pre-existing immunity to selected vectors. | [24] |
| Vaccine Strategy | Disease | Animal | Consequences | Refs |
|---|---|---|---|---|
| Bacterial ghost construction | Avian Colibacillosis | Avian | Mortality of poultry bacterial infections—it causes a variety of disease manifestations in poultry including yolk sac infection, omphalitis, respiratory tract infection, swollen head syndrome, septicemia, polyserositis, coligranuloma, enteritis, cellulitis and salpingitis | [30,31] |
| Avirulent suspension of Salmonella typhimurium AWC 591 | Salmonellosis | Commercial poultry | Economic losses and risks to public health such as diarrhea, fever, and stomach cramps | [32] |
| Modified live vaccine (MLV) infectious bovine rhinotracheitis | Rhinotracheitis | Cattle | Respiratory disease complex | [33] |
| The gene for protein 2 (VP2) of infectious bursal disease virus was cloned into a Pichia pastoris expression system | Infectious bursal disease (also known as Gumboro disease) | Avian | Immunosuppressive viral disease due to widespread destruction of lymphocytes | [34] |
| Replacement of the capsid-encoding gene (P1) from the vaccine strain O1 Manisa | Foot-and-mouth disease virus | Cattle, pigs, sheep, and many wildlife species | Economically devastating disease; reduced animal productivity and the restrictions on international trade in animal products | [35,36] |
| Recombinant vaccines based on Brucella Outer Membrane Protein (OMP) antigens | Brucellosis | Calves, sheep, cattle, goats, pigs, and dogs, among others | High economic losses due to restrictions on international trade in animal products; the signs and symptoms include fever, joint pain (arthritis, spondylitis, sacroiliitis), endocarditis and fatigue. | [37] |
| Recombinant vaccines based on their major toxins and their genetic origins (iota (ia), alpha (cpa), beta (cpb), and epsilon (etx), and toxoid vaccines, bacterin-toxoid vaccine | Clostridial diseases | Cattle, sheep, and goats | botulism, tetanus, enterotoxaemia, gas gangrene, necrotic enteritis, pseudomembranous colitis, blackleg, and black disease causing severe economic losses in livestock and poultry industries | [38] |
| Disease | Example (Supplier)/Vaccine Strategy | Recommended Vaccination Schedule |
|---|---|---|
| Feline Panleukopenia/ Infectious Enteritis (Parvovirus) | Fevaxyn® Pentofel (Zoetis Belgium SA)/Fevaxyn Pentofel contains the following inactivated viruses: feline panleukopenia virus, feline rhinotracheitis virus, feline calicivirus, feline leukemia virus, and the inactivated bacterium feline Chlamydophila felis. | Cats of 9 weeks or older. Two doses at an interval of 3 to 4 weeks. |
| Feline Calicivirus | Purevax RC (Boehringer Ingelheim, Ingelheim am Rhein, Germany)/Attenuated feline rhinotracheitis herpesvirus (FVH F2 strain) and inactivated feline calicivirus antigens (FCV 431 and G1 strains) | Only cats of 8 weeks or older receive the first injection; the second injection is 3 to 4 weeks later. Revaccination: the first revaccination should be carried out one year after the primary vaccination, and subsequent revaccinations: at intervals of up to three years. |
| Feligen RCP (Virbac)/a modified live vaccine providing immunization of healthy cats against feline rhinotracheitis virus, feline calicivirus and feline panleucopaenia virus. | Cats from minimum 9 weeks of age. Two doses at an interval of 3 to 4 weeks. Annual boosters are recommended after that | |
| Feline Leukaemia Virus | Purevax FeLV (Boehringer Ingelheim, Ingelheim am Rhein, Germany)/virus canaripox recombinante FeLV (vCP97). The vaccine strain is a recombinant canarypox virus that expresses the FeLV-A env and gag genes. Under natural conditions, only subgroup A is infectious and immunization against subgroup A induces total protection against subgroups A, B, and C. After inoculation, the virus expresses the protective proteins but does not replicate in the cat. Thus, the vaccine induces an immune state against the feline leukemia virus. | Cats of 8 weeks of age or older. Primary vaccination: first injection: from the age of 8 weeks. Second injection: 3 to 4 weeks later. Revaccination: annual |
| Feline Rhinotracheitis (Herpesvirus) | Purevax RC (Boehringer Ingelheim, Ingelheim am Rhein, Germany)/Attenuated feline rhinotracheitis herpesvirus (FHV F2 strain) and inactivated feline calicivirus (FCV 431 and G1 strains) antigens | Cats of 8 weeks of age or older. Against feline viral rhinotracheitis, for the reduction in clinical signs and against calicivirus infection for the reduction in clinical signs. Primary vaccination: first injection: from 8 weeks. Second injection: 3 to 4 weeks later. Revaccination: the first revaccination should be carried out one year after the primary vaccination, subsequent revaccinations at intervals of up to three years. |
| Feline Rabies | Purevax Rabies (Boehringer Ingelheim, Ingelheim am Rhein, Germany)/Contains rabies recombinant canarypox virus (vCP65); Rabisin (Boehringer Ingelheim, Ingelheim am Rhein, Germany)/inactivated rabies antigen (viral glycoproteins) | Cats 12 weeks of age and older. The cats should be revaccinated every year |
| Canine Rabies | Rabvac 1 (Boehringer Ingelheim Ingelheim am Rhein, Germany)/a inactivated virus vaccine; Defensor (Zoetis, Belgium SA)/ Rabico virus strain PV-Paris (Pasteur) replicated in a stable cell line, chemically inactivated; Rabisin (Boehringer Ingelheim, Ingelheim am Rhein, Germany)/inactivated rabies antigen (viral glycoproteins). | Rabvac 1:3 months of age or older. Revaccinate one year later and annually thereafter. Defensor: heath dogs and cats: a single dose at 3 months of age or older. Annual revaccination with a single dose is recommended. Rabisin: inactivated rabies antigen (viral glycoproteins) |
| Canine distemper virus, Canine Adenovirus Type 2, infectious hepatitis, Canine Parvovirus (modified live viruses), Coronavirose canina, and Leptospira Canicola-Icterohaemorrhagiae (L. canicola and L. icterohaemorrhagiae) | V8 Nobivac® Canine (MSD, NJ, USA)/vaccine combination—modified live virus vaccine and a live attenuated vaccine | Puppies from 45 days of age, there are 3 or 4 doses in a row with intervals of 21 to 30 days between them |
| Canine distemper, infectious hepatitis, parainfluenza, parvovirus, coronavirus, and leptospirosis (Canicola and Icterohaemorrhagiae serovars), leptospirosis (Grippotyphosa and Pomona) | V10 Vanguard Plus (Zoetis, Belgium SA)/live attenuated vaccine | After V8 applications, the adult dog must be vaccinated with V10 from 6 weeks of age or older. |
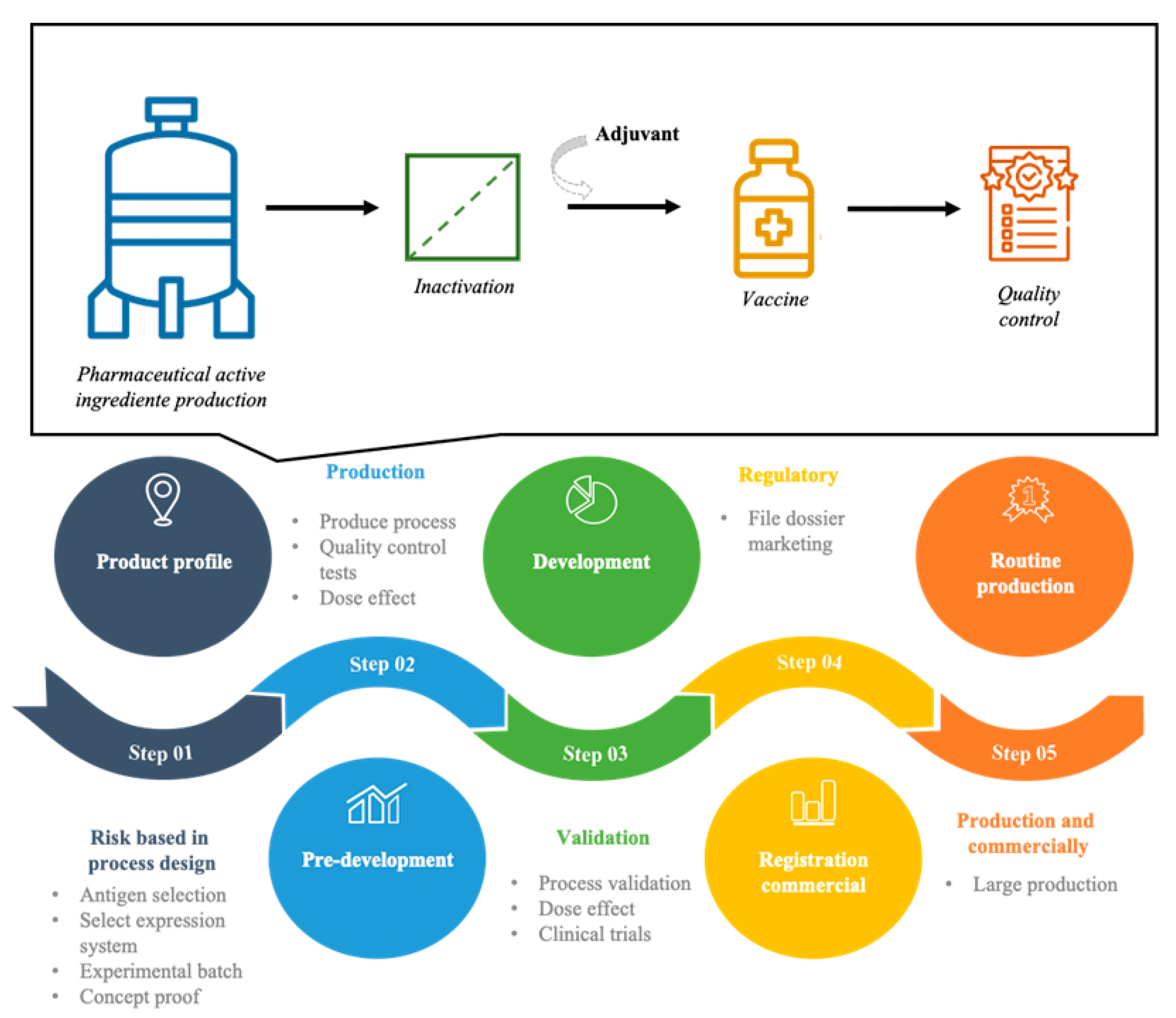
2. Production of Vaccines
2.1. Bacteria Seed
2.2. Virus Seed
2.3. Computational Based Vaccine
2.4. Challenges in Vaccine Production
2.5. Production Methods
3. Inactivation
3.1. Bacterial Vaccines
3.2. Bacterial Toxoids
3.3. Viral Vaccines
4. Choice of Composition and Strain of Vaccines
5. Final Bulk and Final Batch
6. Vaccines Assays and Quality Control
- Test for the detection of Mycoplasma contamination
- ii
- Test of residual moisture (RM)
- iii
- Test of residual formaldehyde
7. Vaccines’ Labeling
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tedeschi, P.; Fine, A.H.; Helgeson, J.I. Assistance Animals: Their Evolving Role in Psychiatric Service Applications. In Handbook on Animal-Assisted Therapy: Theoretical Foundations and Guidelines for Practice; Fine, A.H., Ed.; Academic Press: Cambridge, UK, 2010; pp. 421–438. [Google Scholar] [CrossRef]
- Flegr, J.; Preiss, M. Friends with Malefit. The Effects of Keeping Dogs and Cats, Sustaining Animal-Related Injuries and Toxoplasma Infection on Health and Quality of Life. PLoS ONE 2019, 14, 1–30. [Google Scholar] [CrossRef]
- Maaten, T.S.D.; Turner, D.; Tilburg, J.V. Benefits and Risks for People and Livestock of Keeping Companion Animals: Searching for a Healthy Balance. J. Comp. Pathol. 2016, 155, S8–S17. [Google Scholar] [CrossRef]
- Jorge, S.; Dellagostin, O.A. The Development of Veterinary Vaccines: A Review of Traditional Methods and Modern Biotechnology Approaches. Biotechnol. Res. Innov. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Meeusen, E.N.T.; Walker, J.; Peters, A.; Pastoret, P.; Jungersen, G. Current Status of Veterinary Vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; Mcwhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef]
- Pollard, A.J. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Besnard, L.; Fabre, V.; Fettig, M.; Gousseinov, E.; Kawakami, Y.; Laroudie, N.; Scanlan, C.; Pattnaik, P. Clarification of Vaccines: An Overview of Filter Based Technology Trends and Best Practices. Biotechnol. Adv. 2016, 34, 1–13. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Ferreira, M.R.A.; Moreira, G.M.S.G.; Eduardo, C.; Cunha, P.; Mendonça, M.; Salvarani, F.M.; Moreira, Â.N.; Conceição, F.R. Clostridium Perfringens: Production Strategies and Applications as Veterinary Vaccines. Toxins 2016, 2, 340. [Google Scholar] [CrossRef]
- Fabbri, A.; Travaglione, S.; Falzano, L.; Fiorentini, C. Bacterial Protein Toxins: Current and Potential Clinical Use. Curr. Med. Chem. 2008, 15, 1116–1125. [Google Scholar] [CrossRef]
- Ghimire, T.R. The Mechanisms of Action of Vaccines Containing Aluminum Adjuvants: An in Vitro vs in Vivo Paradigm. Springerplus 2015, 4, 181. [Google Scholar] [CrossRef]
- Van Gelder, P.; Makoschey, B. Production of Viral Vaccines for Veterinary Use. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 103–109. [Google Scholar]
- Hansen, L.J.J.; Daoussi, R.; Vervaet, C.; Remon, J.; Beer, T.R.M.D. Freeze-Drying of Live Virus Vaccines: A Review. Vaccine 2015, 33, 5507–5519. [Google Scholar] [CrossRef]
- Choi, Y.; Chang, J. Viral Vectors for Vaccine Applications. Clin. Exp. Vaccine Res. 2013, 2, 97–105. [Google Scholar] [CrossRef]
- Barrett, P.N.; Mundt, W.; Kistner, O.; Howard, M.K.; Barrett, P.N.; Mundt, W.; Kistner, O.; Vero, M.K.H.; Barrett, P.N.; Mundt, W.; et al. Towards Cell Culture-Based Viral Vaccines Vero Cell Platform in Vaccine Production: Moving towards Cell Culture-Based Viral Vaccines. Expert Rev. Vaccines 2014, 0584, 607–618. [Google Scholar] [CrossRef]
- Xia, J.; Cui, J.; He, X.; Liu, Y.; Yao, K.; Cao, S.; Han, X. Genetic and Antigenic Evolution of H9N2 Subtype Avian Influenza Virus in Domestic Chickens in Southwestern China, 2013–2016. PLoS ONE 2017, 12, 2013–2016. [Google Scholar] [CrossRef]
- Ewer, K.J.; Lambe, T.; Rollier, C.S.; Spencer, A.J.; Hill, A.V.S.; Dorrell, L. Viral Vectors as Vaccine Platforms: From Immunogenicity to Impact. Curr. Opin. Immunol. 2016, 41, 47–54. [Google Scholar] [CrossRef]
- Rabie, N.S.; Girh, Z.M.S.A. Bacterial Vaccines in Poultry. Bull. Natl. Res. Cent. 2020, 44, 15. [Google Scholar] [CrossRef]
- Guzman, F.; Vargas, M.I.; Sossai, S.; Patarroyo, J.H.; Patarroyo, A.M.V.; Gonza, C.Z.L. Use of Biodegradable PLGA Microspheres as a Slow Release Delivery System for the Boophilus Microplus Synthetic Vaccine SBm7462. Vet. Immunol. Immunopathol. 2005, 107, 281–290. [Google Scholar] [CrossRef]
- Callaway, E. The Race for Coronavirus Vaccines: A Graphical Guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef]
- Maslow, J.N. Vaccine Development for Emerging Virulent Infectious Diseases. Vaccine 2017, 35, 5437–5443. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Khuroo, M.; Khuroo, M.S.; Sofi, A.A.; Khuroo, N.S. COVID-19 Vaccines: A Race Against Time in the Middle of Death and Devastation! J. Clin. Exp. Hepatol. 2020, 10, 610–621. [Google Scholar] [CrossRef]
- Koirala, A.; Jin Joo, Y.; Khatami, A.; Chiu, C.; Britton, P.N. Vaccines for COVID-19: The Current State of Play. Paediatr. Respir. Rev. 2020, 35, 43–49. [Google Scholar] [CrossRef]
- Heppell, J.; Davis, H.L. Application of DNA Vaccine Technology to Aquaculture. Adv. Drug Deliv. Rev. 2000, 43, 29–43. [Google Scholar] [CrossRef]
- Fomsgaard, A.; Liu, M.A. The Key Role of Nucleic Acid Vaccines for One Health. Viruses 2021, 13, 258. [Google Scholar] [CrossRef]
- Dhama, K.; Mahendran, M.; Gupta, P.K.; Rai, A. DNA Vaccines and Their Applications in Veterinary Practice: Current Perspectives. Vet. Res. Commun. 2008, 32, 341–356. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19). Available online: https://covid19.who.int/ (accessed on 30 July 2022).
- Monath, T.P. Vaccines against Diseases Transmitted from Animals to Humans: A One Health Paradigm. Vaccine 2013, 31, 5321–5338. [Google Scholar] [CrossRef]
- Ebrahimi-nik, H.; Bassami, M.R.; Mohri, M.; Rad, M.; Khan, M.I. Bacterial Ghost of Avian Pathogenic E. Coli (APEC) Serotype O78: K80 as a Homologous Vaccine against Avian Colibacillosis. PLoS ONE 2018, 13, e0194888. [Google Scholar] [CrossRef]
- Kabir, S.M.L. Avian Colibacillosis and Salmonellosis: A Closer Look at Epidemiology, Pathogenesis, Diagnosis, Control and Public Health Concerns. Int. J. Environ. Res. Public Health 2010, 7, 89. [Google Scholar] [CrossRef]
- Muniz, E.C.; Verdi, R.; Leão, J.A.; Back, A.; Pinheiro, V.; Correa, E.; Verdi, R.; Leão, J.A.; Back, A. Evaluation of the Effectiveness and Safety of a Genetically Modified Live Vaccine in Broilers Challenged with Salmonella Heidelberg. Avian Pathol. 2017, 46, 676–682. [Google Scholar] [CrossRef]
- Lark, C.; William, W.; Kayla, M.G.; Jillian, J.A. Influence of a Bovine Respiratory Disease Vaccine with a Temperature—Sensitive Modified Live or Killed Infectious Bovine Rhinotracheitis Component on Oestrous Cycle Parameters and Anti—Müllerian Hormone Concentration in Nulliparous Heifers. Reprod. Domest. Anim. 2019, 54, 1470–1476. [Google Scholar] [CrossRef]
- Pitcovski, J.; Gutter, B.; Gallili, G.; Goldway, M.; Perelman, B.; Gross, G.; Krispel, S.; Barbakov, M.; Michael, A. Development and Large-Scale Use of Recombinant VP2 Vaccine for the Prevention of Infectious Bursal Disease of Chickens. Vaccine 2003, 21, 4736–4743. [Google Scholar] [CrossRef]
- You, S.; Jo, H.; Choi, J.; Ko, M.; Shin, S.H.; Lee, M.J.; Kim, S.; Kim, B.; Park, J. Evaluation of Novel Inactivated Vaccine for Type C Foot-and-Mouth Disease in Cattle and Pigs. Vet. Microbiol. 2019, 234, 44–50. [Google Scholar] [CrossRef]
- Jamal, S.M.; Belsham, G.J. Foot-and-Mouth Disease: Past, Present and Future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef]
- Rezaei, M.; Rabbani-khorasgani, M.; Zarkesh-Esfahani, S.H.; Emamzadeh, R.; Abtahi, H. Prediction of the Omp16 Epitopes for the Development of an Epitope-Based Vaccine Against Brucellosis. Infect. Disord. Drug Targets 2019, 19, 36–45. [Google Scholar] [CrossRef]
- Abdolmohammadi Khiav, L.; Zahmatkesh, A. Vaccination against Pathogenic Clostridia in Animals: A Review. Trop. Anim. Health Prod. 2021, 53, 284. [Google Scholar] [CrossRef]
- Mans, K.L.; Hernández-triana, L.M.; Banyard, A.C.; Fooks, A.R.; Johnson, N. Japanese Encephalitis Virus Infection, Diagnosis and Control in Domestic Animals. Vet. Microbiol. 2017, 201, 85–92. [Google Scholar] [CrossRef]
- Maki, J.; Guiot, A.L.; Aubert, M.; Brochier, B.; Cliquet, F.; Hanlon, C.A.; King, R.; Oertli, E.H.; Rupprecht, C.E.; Schumacher, C.; et al. Oral Vaccination of Wildlife Using a Vaccinia—Rabies-Glycoprotein Recombinant Virus Vaccine (RABORAL V-RG®): A Global Review. Vet. Res. 2017, 48, 57. [Google Scholar] [CrossRef]
- Corbel, M.J. Brucellosis in Humans and Animals Brucellosis in Humans and Animals; World Health Organization: Geneva, Switzerland, 2006.
- Rashid, A.; Rasheed, K.; Akhtar, M. Factors Influencing Vaccine Efficacy—A General Review. J. Anim. Plant Sci. 2009, 19, 22–25. [Google Scholar]
- Buddle, B.M.; Vordermeier, H.M.; Chambers, M.A. Efficacy and Safety of BCG Vaccine for Control of Tuberculosis in Domestic Livestock and Wildlife. Front. Vet. Sci. 2018, 5, 259. [Google Scholar] [CrossRef]
- Pascual, D.W.; Yang, X.; Wang, H.; Goodwin, Z.; Hoffman, C.; Clapp, B. Alternative Strategies for Vaccination to Brucellosis. Microbes Infect. 2019, 20, 599–605. [Google Scholar] [CrossRef]
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef]
- Demain, A.L. Microbial Biotechnology. Trends Biotechnol. 2000, 18, 26–31. [Google Scholar] [CrossRef]
- Zaragoza, N.E.; Orellana, C.A.; Moonen, G.A.; Moutafis, G.; Marcellin, E. Vaccine Production to Protect Animals Against Pathogenic Clostridia. Toxins 2019, 11, 525. [Google Scholar] [CrossRef]
- Campos, I.B.; Cardoso, C.P.; Fernando, J.; Muriel, F.; Leite, L.C.C.; Moffitt, K.L.; Jie, Y.; Richard, L.; Gonçalves, V.M. Process Intensification for Production of Streptococcus Pneumoniae Whole—Cell Vaccine. Biotechnol. Bioeng. 2020, 117, 1661–1672. [Google Scholar] [CrossRef]
- Fang, C.; Guilbault, C.; Li, X.; Elahi, S.M.; Ansorge, S.; Kamen, A.; Gilbert, R. Development of Suspension Adapted Vero Cell Culture Process Technology for Production of Viral Vaccines. Vaccine 2019, 37, 6996–7002. [Google Scholar] [CrossRef]
- Ottiger, H.P. Monitoring Veterinary Vaccines for Contaminating Viruses. Dev. Biol. Stand. 2006, 126, 309–319. [Google Scholar]
- Yang, D.; Nakagawa, K.; Ito, N.; Kim, H.; Hyun, B.; Nah, J.; Sugiyama, M.; Song, J. A Single Immunization with Recombinant Rabies Virus (ERAG3G) Confers Complete Protection against Rabies in Mice. Clin. Exp. Vaccine 2014, 3, 176–184. [Google Scholar] [CrossRef]
- Reciilard, P. Cell-Culture Vaccines for Veterinary Use. In Laboratory Techniques in Rabies; Meslin, F.X., Ed.; World Health Organization: Geneva, Switzerland, 1958; pp. 314–324. [Google Scholar]
- Prkno, A.; Ho, D.; Kaiser, M.; Goerigk, D.; Pfe, M.; Winter, K.; Vahlenkamp, T.W.; Beer, M.; Starke, A. Field Trial Vaccination against Cowpox in Two. Viruses 2020, 12, 234. [Google Scholar] [CrossRef]
- Dalsass, M.; Brozzi, A.; Medini, D.; Rappuoli, R. Comparison of Open-Source Reverse Vaccinology Programs for Bacterial Vaccine Antigen Discovery. Front. Immunol. 2019, 10, 113. [Google Scholar] [CrossRef]
- Soleymani, S.; Tavassoli, A.; Reza, M. An Overview of Progress from Empirical to Rational Design in Modern Vaccine Development, with an Emphasis on Computational Tools and Immunoinformatics Approaches. Comput. Biol. Med. 2022, 140, 105057. [Google Scholar] [CrossRef]
- Souza, J.G.D.; Fernandes, M.A.C. A Novel Deep Neural Network Technique for Drug—Target Interaction. Pharmaceutics 2021, 14, 625. [Google Scholar] [CrossRef]
- Awasthi, A.; Sharma, G.; Agrawal, P. Chapter 20-Computational Approaches for Vaccine Designing. In Bioinformatics—Methods and Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 317–335. [Google Scholar] [CrossRef]
- Orellaa, F.A.D.; Gustavo, L.; Achecoa, C.P.; Liveirab, S.C.O.; Iyoshia, A.M.; Zevedoa, V.A. Corynebacterium Pseudotuberculosis: Microbiology, Biochemical Properties, Pathogenesis and Molecular Studies of Virulence. Vet. Res. 2006, 37, 201–218. [Google Scholar] [CrossRef]
- Soares, S.C.; Trost, E.; Ramos, R.T.J.; Carneiro, A.R.; Santos, A.R.; Pinto, A.C.; Barbosa, E.; Aburjaile, F.; Ali, A.; Diniz, C.A.A.; et al. Genome Sequence of Corynebacterium Pseudotuberculosis Biovar Equi Strain 258 and Prediction of Antigenic Targets to Improve Biotechnological Vaccine Production. J. Biotechnol. 2013, 167, 135–141. [Google Scholar] [CrossRef]
- Leonardo, C.; Alves, J.; Nogueira, W.; César, L.; Cybelle, A.; Ramos, R.; Azevedo, V.; Silva, A.; Folador, A. Prediction of New Vaccine Targets in the Core Genome of Corynebacterium Pseudotuberculosis through Omics Approaches and Reverse Vaccinology. Gene 2019, 702, 36–45. [Google Scholar] [CrossRef]
- Bueno, L.L.; Lobo, F.P.; Guimara, C.; Soares, I.S.; Fontes, C.J.; Vinı, M.; Bartholomeu, D.C.; Fujiwara, R.T. Identification of a Highly Antigenic Linear B Cell Epitope within Plasmodium Vivax Apical Membrane Antigen 1. PLoS ONE 2011, 6, e21289. [Google Scholar] [CrossRef]
- Michel-tod, L.; Bigey, P.; Reche, P.A.; Pinazo, M.; Gasc, J.; Alonso-padilla, J. Design of an Epitope-Based Vaccine Ensemble for Animal Trypanosomiasis by Computational Methods. Vaccines 2020, 8, 130. [Google Scholar] [CrossRef]
- Shan, R.; Lina, G.; Yao, D.; Chaoli, W.; Li, F.; Yongen, X. Design and Evaluation of a Multi-Epitope Assembly Peptide Vaccine against Acinetobacter Baumannii Infection in Mice. Swiss Med. Wkly. 2019, 149, 2324. [Google Scholar] [CrossRef]
- Majidiani, H.; Dalimi, A.; Ghaffarifar, F.; Pirestani, M.; Ghaffari, A.D. Microbial Pathogenesis Computational Probing of Toxoplasma Gondii Major Surface Antigen 1 (SAG1) for Enhanced Vaccine Design against Toxoplasmosis. Microb. Pathog. 2020, 147, 104386. [Google Scholar] [CrossRef]
- Roopngam, P.E. Liposome and Polymer-Based Nanomaterials for Vaccine Applications. Nanomedicine 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Sadozai, H.; Saeidi, D. Recent Developments in Liposome-Based Veterinary Therapeutics. Hindawi 2013, 2013, 16752. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as Vaccine Delivery Systems: A Review of the Recent Advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Celis-giraldo, C.T.; Julio, L.; Muro, A.; Patarroyo, M.A. Nanovaccines against Animal Pathogens: The Latest Findings. Vaccines 2021, 9, 988. [Google Scholar] [CrossRef]
- Williams, P.D.; Paixão, G. On-Farm Storage of Livestock Vaccines May Be a Risk to Vaccine Efficacy: A Study of the Performance of on-Farm Refrigerators to Maintain the Correct Storage Temperature. BMC Vet. Res. 2018, 14, 136. [Google Scholar] [CrossRef]
- Fanelli, A.; Mantegazza, L.; Hendrickx, S.; Capua, I. Thermostable Vaccines in Veterinary Medicine: State of the Art and Opportunities to Be Seized. Vaccines 2022, 10, 245. [Google Scholar] [CrossRef]
- Abdi, R.D.; Amsalu, K.; Merera, O.; Asfaw, Y.; Gelaye, E.; Yami, M.; Sori, T. Serological Response and Protection Level Evaluation in Chickens Exposed to Grains Coated with I2 Newcastle Disease Virus for Effective Oral Vaccination of Village Chickens. BMC Vet. Res. 2016, 12, 150. [Google Scholar] [CrossRef]
- Lankester, F.J.; Wouters, P.A.W.M.; Czupryna, A.; Palmer, G.H.; Mzimbiri, I.; Cleaveland, S.; Francis, M.J.; Sutton, D.J.; Sonnemans, D.G.P. Thermotolerance of an Inactivated Rabies Vaccine for Dogs. Vaccine 2016, 34, 5504–5511. [Google Scholar] [CrossRef]
- Schat, K.; Baranowski, E. Animal Vaccination and the Evolution of Viral Pathogens. Rev. Sci. Tech. 2007, 26, 327–338. [Google Scholar] [CrossRef]
- Ferrari, G.; Paton, D.; Duffy, S.; Bartels, C.; Knight-jones, T. Foot and Mouth Disease Vaccination and Post-Vaccination Monitoring (Guidelines); Metwally, S., Münstermann, S., Eds.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2016. [Google Scholar]
- Cunningham, A.L.; Garçon, N.; Leo, O.; Friedland, L.R.; Strugnell, R.; Laupèze, B.; Doherty, M.; Stern, P. Vaccine Development: From Concept to Early Clinical Testing. Vaccine 2016, 34, 6655–6664. [Google Scholar] [CrossRef]
- Shah, R.R.; Hassett, K.J.; Brito, L.A. Overview of Vaccine Adjuvants: Introduction, History, and Current Status. In Vaccine Adjuvants: Methods and Protocols, Methods in Molecular Biology; Fox, C.B., Ed.; Springer Science + Business Media: New York, NY, USA, 2017; Volume 1494, pp. 1–13. [Google Scholar] [CrossRef]
- Gomez, P.L.; Robinson, J.M. Section 1: General Aspects of Vaccination. In Plotkin’s Vaccines; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 51–60. [Google Scholar] [CrossRef]
- Kamminga, T.; Slagman, S.; Martins, V.A.P.; Bijlsma, J.J.E.; Schaap, P.J. Risk-Based Bioengineering Strategies for Reliable Bacterial Vaccine Production. Trends Biotechnol. 2019, 37, 805–816. [Google Scholar] [CrossRef]
- Egorova, M.S.; Kurashova, S.S.; Dzagurova, T.K.; Balovneva, M.V.; Ishmukhametov, A.A.; Tkachenko, E.A. Effect of Virus-Inactivating Agents on the Immunogenicity of Hantavirus Vaccines against Hemorrhagic Fever with Renal Syndrome. Appl. Biochem. Microbiol. 2020, 56, 940–947. [Google Scholar] [CrossRef]
- Tang, L.; Kang, H.; Duan, K.; Guo, M.; Lian, G. Effects of Three Types of Inactivation Agents on the Antibody Response and Immune Protection of Inactivated IHNV Vaccine in Rainbow Trout. Viral Immunol. 2016, 29, 430–435. [Google Scholar] [CrossRef]
- Jagt, H.J.M.; Bekkers, M.L.E.; Bommel, S.A.J.T.V.; Marel, P.V.D.; Schrier, C.C. The Influence of the Inactivating Agent on the Antigen Content of Inactivated Newcastle Disease Vaccines Assessed by the in Vitro Potency Test. Biologicals 2010, 38, 128–134. [Google Scholar] [CrossRef]
- Kuma, R.; Chandra, R.; Shukla, S.K.; Agrawal, D.K.; Kumar, M. Hydropericardium Syndrome (HPS) in India: A Preliminary Study on the Causative Agent and Control of the Disease by Inactivated Autogenous Vaccine. Trop. Anim. Health Prod. 1997, 29, 158–164. [Google Scholar] [CrossRef]
- Herrera-rodriguez, J.; Signorazzi, A.; Holtrop, M.; Vries-idema, J.D.; Huckriede, A. Inactivated or Damaged? Comparing the Effect of Inactivation Methods on Influenza Virions to Optimize Vaccine Production. Vaccine 2020, 37, 1630–1637. [Google Scholar] [CrossRef]
- Fan, C.; Ye, X.; Ku, Z.; Kong, L.; Liu, Q. Beta-Propiolactone Inactivation of Coxsackievirus A16 Induces Structural Alteration and Surface Modification of Viral Capsids. J. Virol. 2017, 91, e00038-17. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jang, Y.H.; Byun, Y.H.; Cheong, Y.; Kim, P.; Lee, Y.J.; Lee, Y.J.; Sung, J.M.; Son, A.; Lee, H.M.; et al. Green Tea Catechin-Inactivated Viral Vaccine Platform. Front. Microbiol. 2017, 8, 2469. [Google Scholar] [CrossRef]
- Abd-elghaffar, A.A.; Ali, A.E.; Boseila, A.A.; Amin, M.A. Inactivation of Rabies Virus by Hydrogen Peroxide. Vaccine 2016, 34, 798–802. [Google Scholar] [CrossRef]
- Narayan, S.; Shamsundar, R.; Seetharaman, S. In Vitro Inactivation of the Rabies Virus by Ascorbic Acid. Int. J. Infect. Dis. 2004, 8, 21–25. [Google Scholar] [CrossRef]
- Gupta, R.K.; Sharma, R.; Ahuja, S.; Saxena, S.N. The Effects of Different Inactivating Agents on the Potency, Toxicity and Stability of Pertussis Vaccine. J. Biol. Stand. 1987, 15, 87–98. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and Effectiveness of Influenza Vaccines: A Systematic Review and Meta-Analysis. Lancet 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. In Vaccine Analysis: Strategies, Principles, and Control; Nunnally, B.K., Turula, V.E., D.Sitrin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–80. [Google Scholar] [CrossRef]
- Mondal, S.K.; Neelima, M.; Seetha Rama Reddy, K.; Ananda Rao, K.; Srinivasan, V.A. Validation of the Inactivant Binary Ethylenimine for Inactivating Rabies Virus for Veterinary Rabies Vaccine Production. Biologicals 2005, 33, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, C.W. Kinetics of the Inactivation of Viruses. Bact. Rev. 1964, 28, 150–163. [Google Scholar] [CrossRef]
- Moghadam, A.T.; Afsharpad, K. Application of Fermentor Technology in Production of Diphtheria Toxin. Jundishapur J. Microbiol. 2017, 1, 24–27. [Google Scholar]
- Yuen, C.-T.; Asokanathan, C.; Cook, S.; Lin, N.; Xing, D. Effect of Different Detoxification Procedures on the Residual Pertussis Toxin Activities in Vaccines. Vaccine 2016, 34, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Perugi, F.; Léon, A.; Guéhenneux, F.; Champion-arnaud, P.; Lahmar, M.; Schwamborn, K. Cell Substrates for the Production of Viral Vaccines. Vaccine 2015, 33, 6–13. [Google Scholar] [CrossRef]
- Knezevic, I.; Stacey, G.; Petricciani, J. WHO Study Group on Cell Substrates for Production of Biologicals Geneva, Switzerland, 11–12 June 2007. Biologicals 2008, 36, 203–211. [Google Scholar] [CrossRef]
- Coecke, S.; Balls, M.; Bowe, G.; Davis, J.; Hartung, T.; Hay, R.; Price, A.; Stokes, W.; Schechtman, L.; Stacey, G. Guidance on Good Cell Culture Practice. a Report of the Second ECVAM Task Force on Good Cell Culture Practice. Altern. Lab. Anim. 2005, 33, 261–287. [Google Scholar] [CrossRef]
- Lucey, B.P.; Nelson-rees, W.A.; Hutchins, G.M. Henrietta Lacks, HeLa Cells, and Cell Culture Contamination. Arch. Pathol. Lab. Med. 1951, 133, 1463–1467. [Google Scholar] [CrossRef]
- Marcus-sekura, C.; Richardson, J.C.; Harston, R.K.; Sane, N.; Sheets, R.L. Biologicals Evaluation of the Human Host Range of Bovine and Porcine Viruses That May Contaminate Bovine Serum and Porcine Trypsin Used in the Manufacture of Biological Products. Biologicals 2011, 39, 359–369. [Google Scholar] [CrossRef]
- Lee, H.; Tang, H. Next-Generation Sequencing Technologies and Fragment Assembly Algorithms. In Evolutionary Genomics: Statistical and Computational Methods; Anisimova, M., Ed.; Springer Science + Business Media, LLC: New York, NY, USA, 2012; Volume 855, pp. 155–174. [Google Scholar] [CrossRef]
- Metwally, S.; Viljoen, G.; El Idrissi, A. (Eds.) Veterinary Vaccines: Principles and Applications, 1st ed.; The Food and Agriculture Organization of the United Nations: Rome, Italy; John Wiley & Sons Limited: Oxford, UK, 2021. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Technical Guide for the Elaboration and Use of Monographs for Vaccines and Immunological Veterinary Medicinal Products, 1st ed.; The European Pharmacopoeia: Strasbourg, France, 2016. [Google Scholar]
- Dodet, B. An Important Date in Rabies History. Vaccine 2020, 25, 8647–8650. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.K.; Ni, A.; Hu, B.; Shi, L.I. Antimicrobial Preservative Use in Parenteral Products: Past and Present. J. Pharm. Sci. 2007, 96, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopeia. European Directtorate for the Quality and Healthcare; Updated pa; The European Pharmacopoeia: Strasbourg, France, 2020. [Google Scholar]
- United States Pharmacopeial Convention. The United States Pharmacopeia 2018: USP 41; The National Formulary: NF 36; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2018. [Google Scholar]
- Brazil. Brazilian Pharmacopeia, 6a edição.; Agência Nacional de Vigilância Sanitária: Brasilia, Brazil, 2019. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 10th ed.; Deutscher Apotheker Verlag: Strasbourg, France, 2019. [Google Scholar]
- WHO—World Health Organization. The International Pharmacopoeia, 8th ed.; World Health Organization: Wellington, New Zeland, 2018.
- OIE—World Organisation for Animal Health. Minimum Requirements for the Production and Quality Control of Vaccines; OIE: Paris, France, 2018. [Google Scholar]
- VICH. Organisational Charter of Vich; VICH: Bruxelles, Belgium, 2019. [Google Scholar]
- EMEA. VICH GL34—Biologicals: Testing for the Detection of Mycoplasma Contamination; EMEA: London, UK, 2014. [Google Scholar]
- EMEA. VICH Topic GL26—Biologicals: Testing of Residual Moisture; EMEA: London, UK, 2003. [Google Scholar]
- EMEA. VICH Topic GL25—Biologicals: Testing of Residual Formaldehyde; EMEA: London, UK, 2003. [Google Scholar]
- EMEA. VICH GL17—Stability Testing of Veterinary Medicine Products; EMEA: London, UK, 2001. [Google Scholar]
- EMEA. VICH Topic GL40—Guideline on Test Procedures and Acceptance Criteria for New Biotechnological/Biological Veterinary Medicinal Products; EMEA: London, UK, 2006. [Google Scholar]
- EMA. VICH GL50: Harmonisation of Criteria to Waive Target Animal Batch Safety Testing for Inactivated Vaccines for Veterinary Use; EMA: London, UK, 2018. [Google Scholar]
- EMA. VICH GL55—Harmonisation of Criteria to Waive Target Animal Batch Safety Testing for Live Vaccines for Veterinary Use; EMA: London, UK, 2018. [Google Scholar]
- EMA. VICH GL59—Harmonisation of Criteria to Waive Laboratory Animal Batch Safety Testing for Vaccines for Veterinary Use Testing for Vaccines for Veterinary Use; EMA: Amsterdam, The Netherlands, 2021. [Google Scholar]
- EMEA. VICH Topic GL41: Guideline on Target Animal Safety: Examination of Live Veterinary Vaccines in Target Animals for Absence of Reversion to Virulence; EMEA: London, UK, 2008. [Google Scholar]
- EMEA. VICH Topic GL44—GGuideline on Target Animal Safety for Veterinary Live and Inactied Vaccines; EMEA: London, UK, 2009. [Google Scholar]
- Mbelo, S.; Gay, V.; Blanchard, S.; Abachin, E.; Falque, S.; Lechenet, J.; Poulet, H.; Saint-Vis, B.D. Biologicals Development of a Highly Sensitive PCR / DNA Chip Method to Detect Mycoplasmas in a Veterinary Modi Fi Ed Live Vaccine. Biologicals 2018, 54, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fiorentin, L.; Mores, M.; Trevisol, I.; Antunes, S.; Costa, J.; Soncini, R.; Vieira, N. Test Profiles of Broiler Breeder Flocks Housed in Farms with Endemic Mycoplasma Synoviae Infection. Braz. J. Poult. Sci. 2003, 3, 37–43. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare. 2.6.7. Mycoplasmas. In European Pharmacopoeia; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2020. [Google Scholar]
- Mycoplasma Testing for Cell Substrates Used for the Production of Biotechnological/Biological Products. In Japanese Pharmacopoeia; Stationery Office: London, UK, 2016; pp. 2460–2464.
- Harrak, M.E.; Belkourati, I.; Boumart, Z.; Fakri, F.; Hamdi, J. The Manufacture of Veterinary Vaccines: Quality Control of the Manufacturing Process. In Veterinary Vaccines: Principles and Applications; Metwally, S., Viljoen, G., Idrissi, A.E., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 147–159. [Google Scholar]
- Francis, M.J. A Veterinary Vaccine Development Process Map to Assist in the Development of New Vaccines. Vaccine 2020, 38, 4512–4515. [Google Scholar] [CrossRef]
- Metz, A.B.; Dobbelsteen, G.V.D.; Els, C.V.; Gun, J.V.D.; Levels, L.; Pol, L.V.D. Quality-Control Issues and Approaches in Vaccine Development. Expert Rev. Vaccines 2009, 8, 227–238. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: FDA Review of Vaccine Labeling Requirements for Warnings, Use Instructions, and Precautionary Information; FDA: Rockville, MD, USA, 2004.
| Issue | Test | Guideline | Refs | |
|---|---|---|---|---|
| Quality | Impurities | Test for the detection of Mycoplasma contamination | VICH GL34 | [112] |
| Test of residual moisture | VICH GL26 | [113] | ||
| Test of residual formaldehyde | VICH GL25 | [114] | ||
| Stability | Stability testing of new biotechnological/biological veterinary medicinal products | VICH GL17 | [115] | |
| Specification | Test procedures and acceptance criteria for new biotechnological/biological veterinary medicinal products | VICH GL40 | [116] | |
| Safety | Target animal batch safety | Harmonization of criteria to waive target animal batch safety testing for inactivated vaccines for veterinary use | VICH GL50 (R) | [117] |
| Harmonization of criteria to waive target animal batch safety testing for live vaccines for veterinary use | VICH GL55 | [118] | ||
| Harmonization of criteria to waive laboratory animal batch safety testing for vaccines for veterinary use | VICH GL 59 | [119] | ||
| Target animal safety | Examination of live veterinary vaccines in target animals for absence of reversion to virulence | VICH GL41 | [120] | |
| Target animal safety for veterinary live and inactivated vaccines | VICH GL44 | [121] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, R.d.M.; Silva, A.M.; Silva, C.F.d.; Cardoso, J.C.; Severino, P.; Meirelles, L.M.A.; Silva-Junior, A.A.d.; Viseras, C.; Fonseca, J.; Souto, E.B. Production Technologies, Regulatory Parameters, and Quality Control of Vaccine Vectors for Veterinary Use. Technologies 2022, 10, 109. https://doi.org/10.3390/technologies10050109
Barbosa RdM, Silva AM, Silva CFd, Cardoso JC, Severino P, Meirelles LMA, Silva-Junior AAd, Viseras C, Fonseca J, Souto EB. Production Technologies, Regulatory Parameters, and Quality Control of Vaccine Vectors for Veterinary Use. Technologies. 2022; 10(5):109. https://doi.org/10.3390/technologies10050109
Chicago/Turabian StyleBarbosa, Raquel de M., Amélia M. Silva, Classius F. da Silva, Juliana C. Cardoso, Patricia Severino, Lyghia M. A. Meirelles, Arnobio A. da Silva-Junior, César Viseras, Joel Fonseca, and Eliana B. Souto. 2022. "Production Technologies, Regulatory Parameters, and Quality Control of Vaccine Vectors for Veterinary Use" Technologies 10, no. 5: 109. https://doi.org/10.3390/technologies10050109
APA StyleBarbosa, R. d. M., Silva, A. M., Silva, C. F. d., Cardoso, J. C., Severino, P., Meirelles, L. M. A., Silva-Junior, A. A. d., Viseras, C., Fonseca, J., & Souto, E. B. (2022). Production Technologies, Regulatory Parameters, and Quality Control of Vaccine Vectors for Veterinary Use. Technologies, 10(5), 109. https://doi.org/10.3390/technologies10050109











