Thermal Inkjet Printing: Prospects and Applications in the Development of Medicine
Abstract
1. Introduction
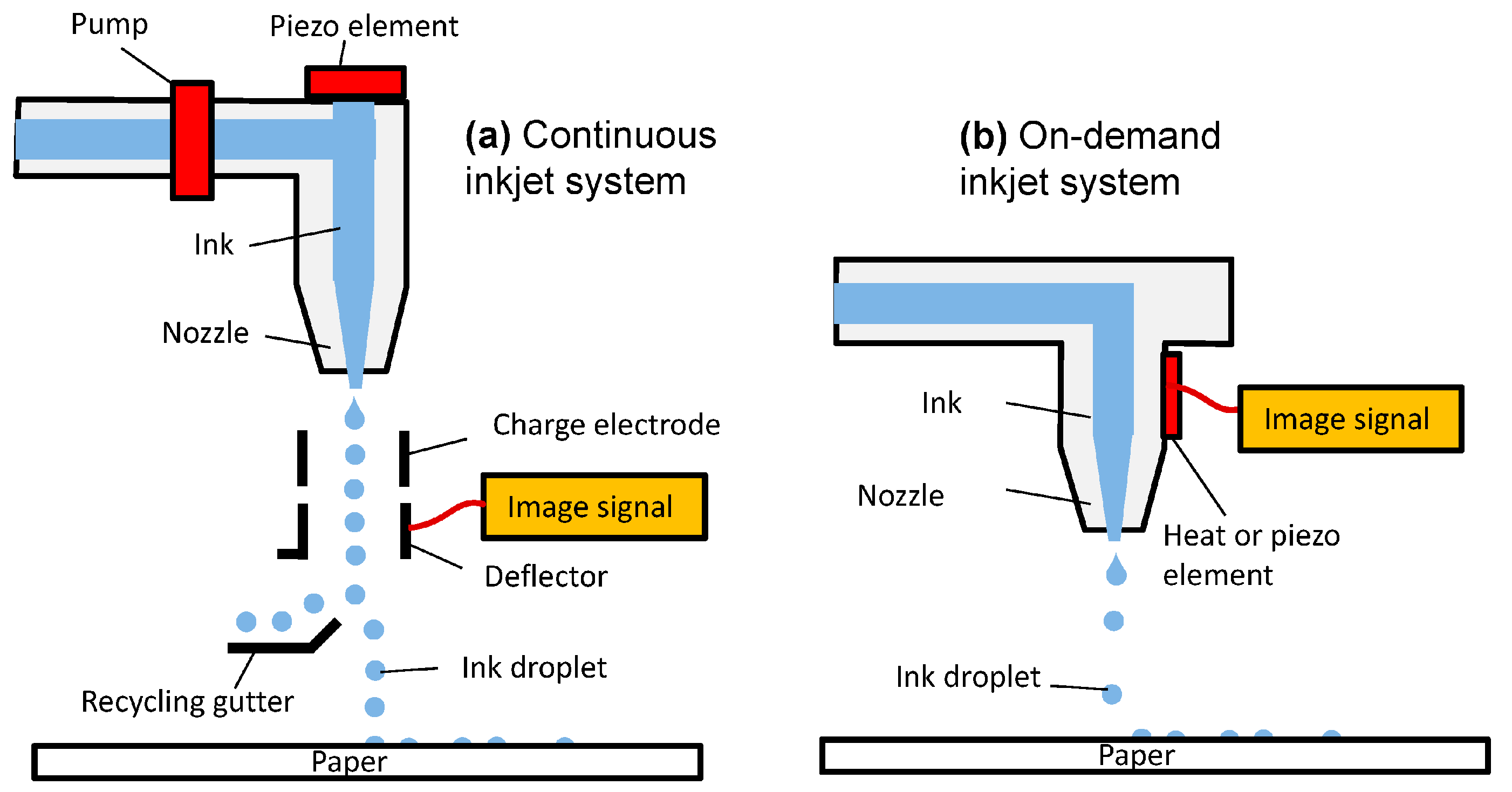
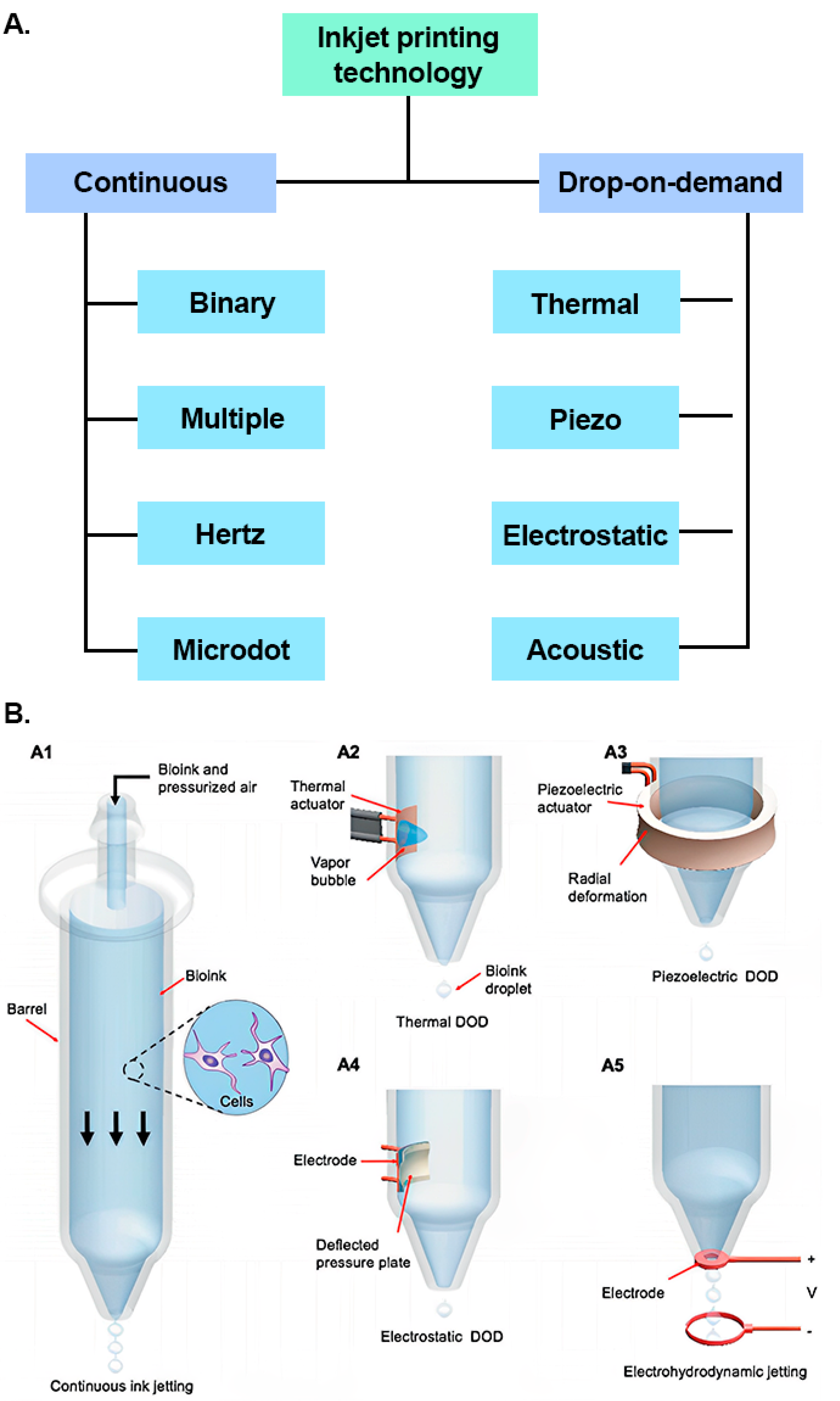
1.1. Inkjet Printing Technology
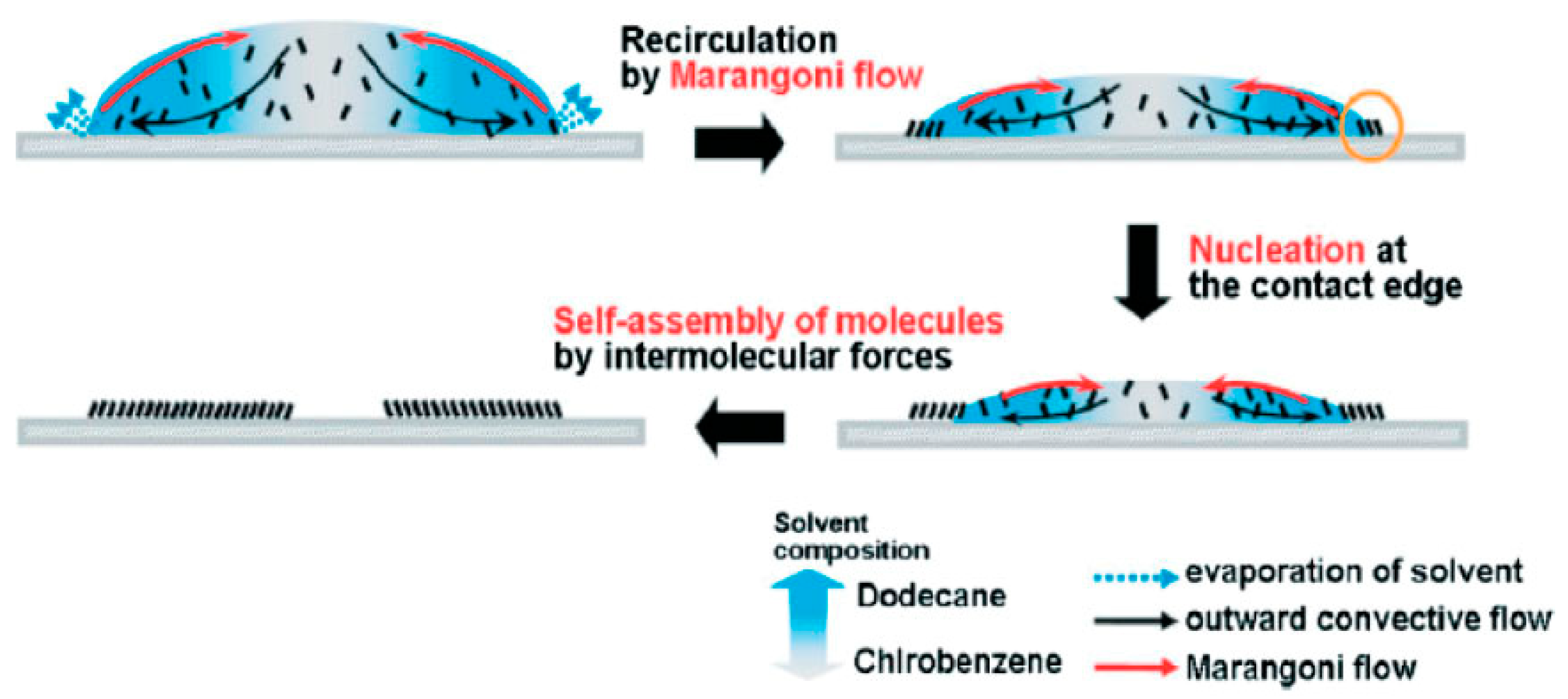
1.2. Inks for Inkjet Printing
1.3. Overview of Different Types of Inkjet Printing Technology
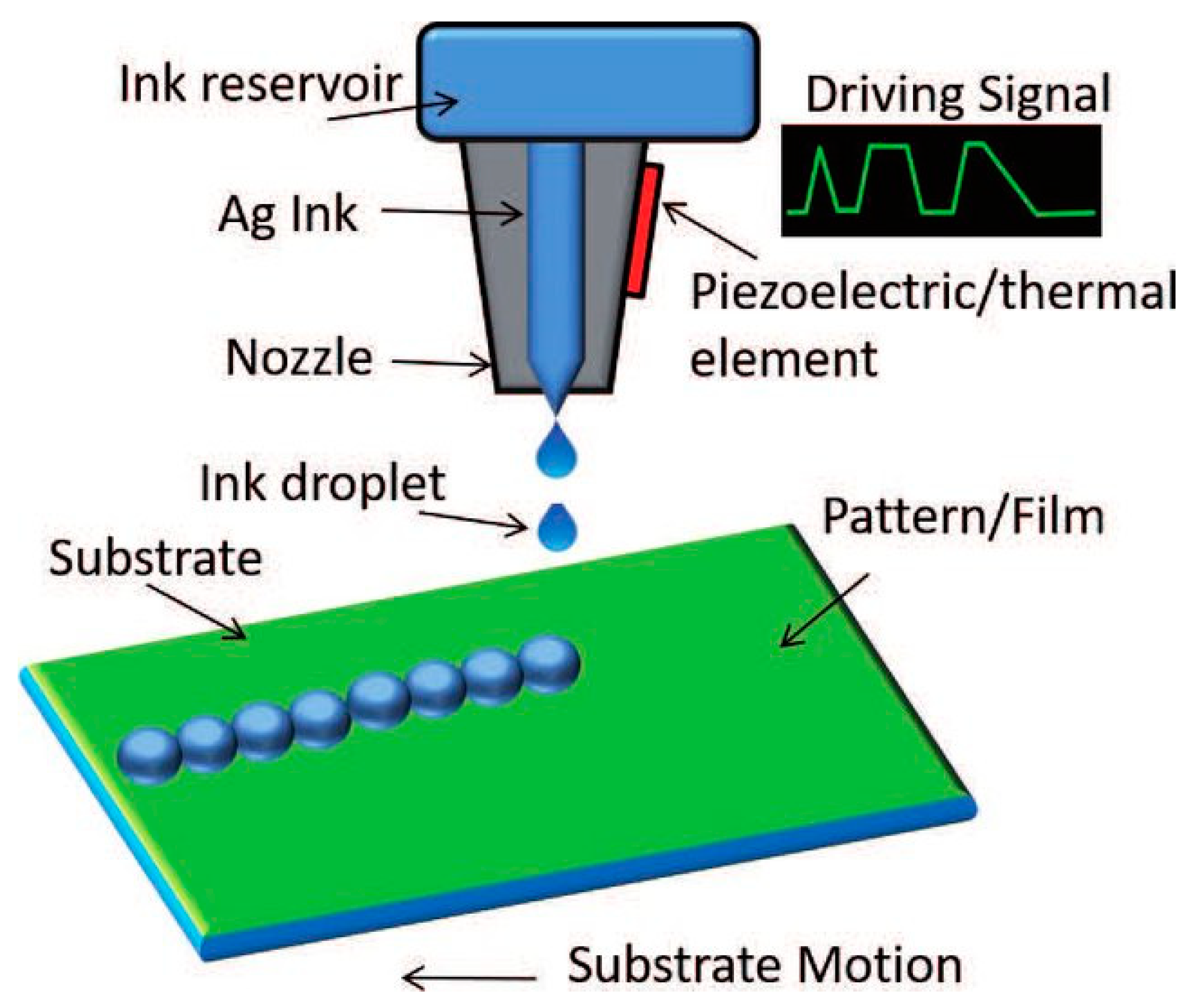
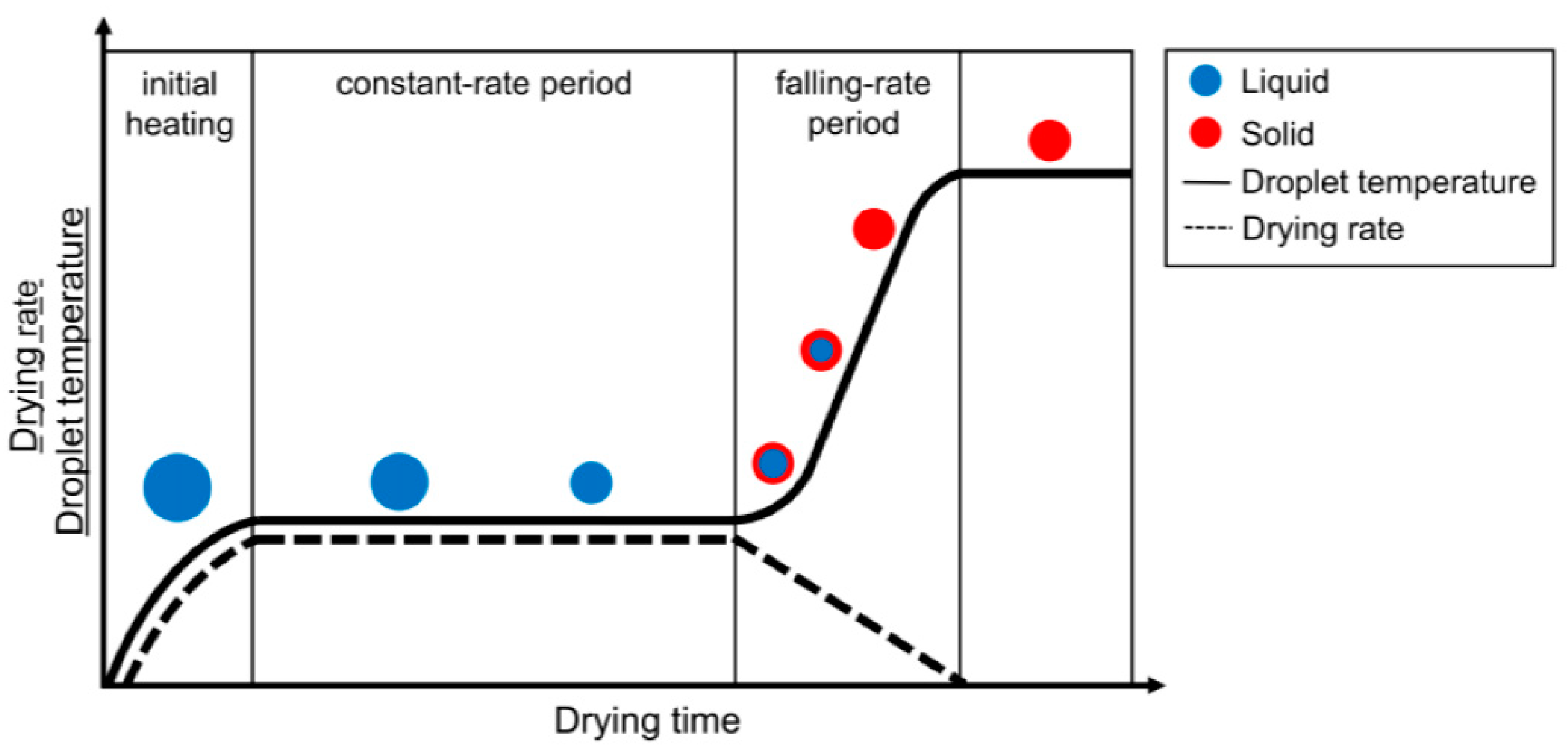
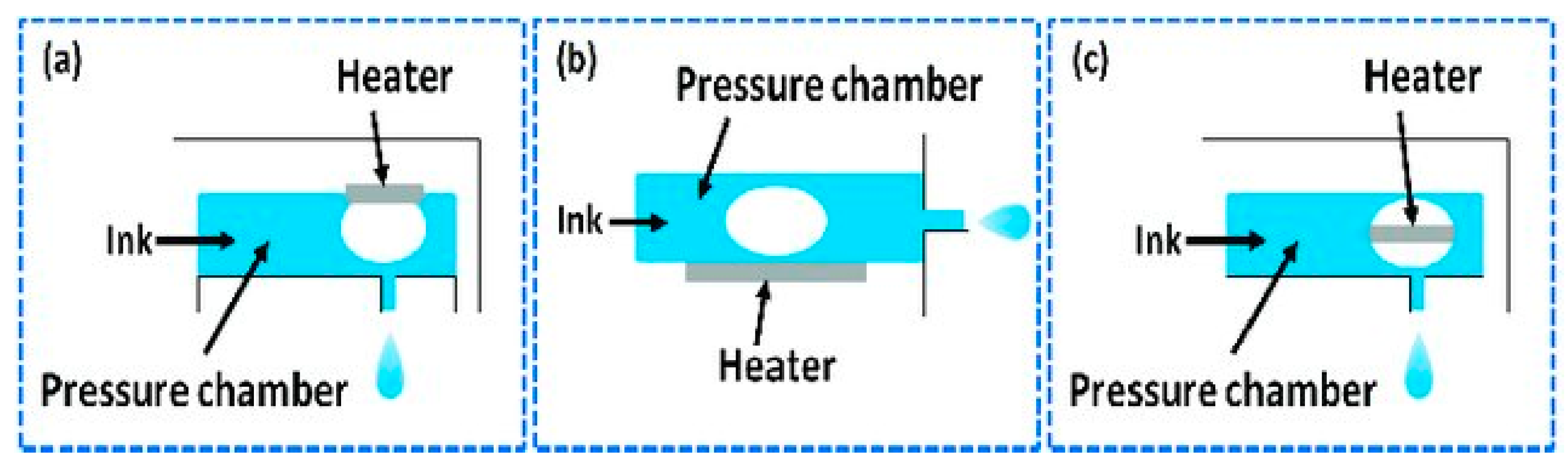
2. Thermal Inkjet Printing
Types of Thermal Inkjet Printer
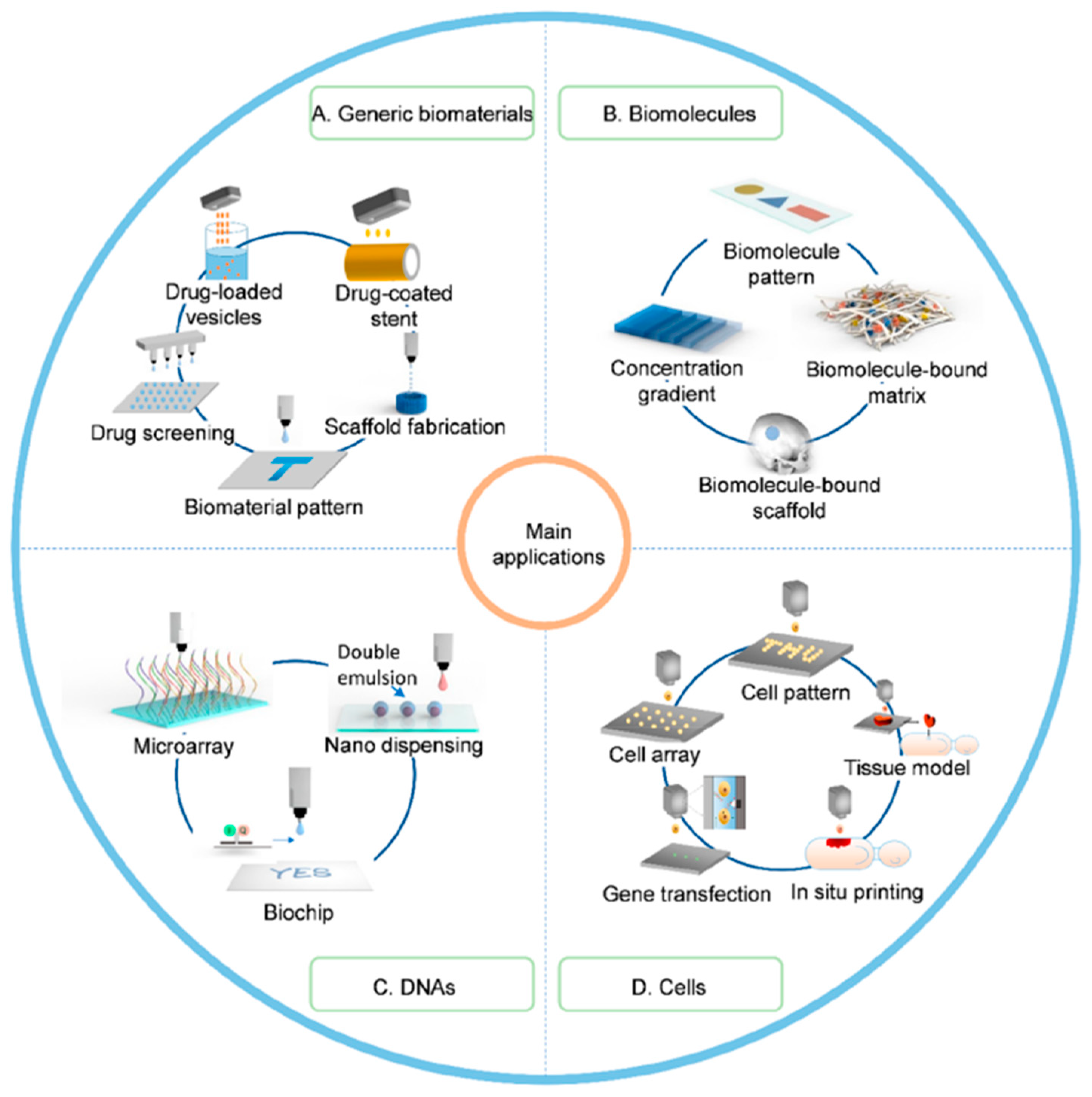
3. Application of Thermal Inkjet Printing
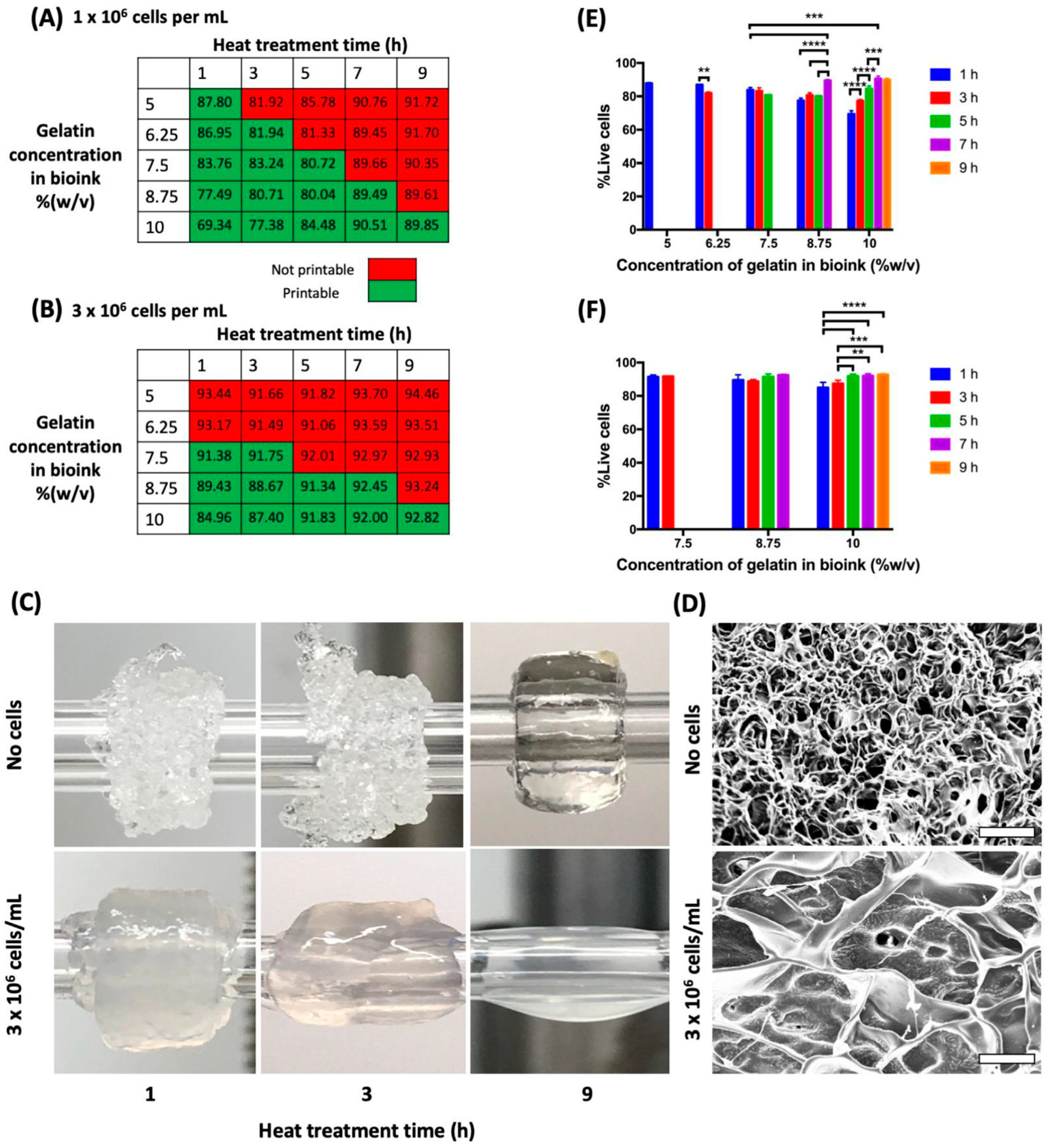
3.1. Bioprinting
3.2. Oral Dosage Form
3.3. Antimicrobial Resistance Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised Dosing: Printing a Dose of One’s Own Medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D. Hot-Melt Extrusion: Pharmaceutical Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Douroumis, D.; Fahr, A. Drug Delivery Strategies for Poorly Water-Soluble Drugs; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet Printing as a Novel Medicine Formulation Technique. J. Control. Release 2011, 156, 179–185. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; McCarthy, J.J. Personalized Medicine: Revolutionizing Drug Discovery and Patient Care. Trends Biotechnol. 2001, 19, 491–496. [Google Scholar] [CrossRef]
- Brittain, H.K.; Scott, R.; Thomas, E. The Rise of the Genome and Personalised Medicine. Clin. Med. 2017, 17, 545. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Sutton, J. Personalized Medicine Could Transform Healthcare. Biomed. Rep. 2017, 7, 3. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges and Progress. Fertil. Steril. 2018, 109, 952. [Google Scholar] [CrossRef] [PubMed]
- Azizi Machekposhti, S.; Mohaved, S.; Narayan, R.J. Inkjet Dispensing Technologies: Recent Advances for Novel Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 101–113. [Google Scholar] [CrossRef]
- Fang, M.; Li, T.; Zhang, S.; Rao, K.V.; Belova, L. Design and Tailoring of Inks for Inkjet Patterning of Metal Oxides. R. Soc. Open Sci. 2020, 7, 200242. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, W.; Wang, W.; Leng, Y.; Zhao, Y. Inkjet Printing Patterns of Highly Conductive Pristine Graphene on Flexible Substrates. Ind. Eng. Chem. Res. 2014, 53, 16777–16784. [Google Scholar] [CrossRef]
- Shah, M.A.; Lee, D.G.; Lee, B.Y.; Hur, S. Classifications and Applications of Inkjet Printing Technology: A Review. IEEE Access 2021, 9, 140079–140102. [Google Scholar] [CrossRef]
- Evans, S.E.; Harrington, T.; Rodriguez Rivero, M.C.; Rognin, E.; Tuladhar, T.; Daly, R. 2D and 3D Inkjet Printing of Biopharmaceuticals—A Review of Trends and Future Perspectives in Research and Manufacturing. Int. J. Pharm. 2021, 599, 120443. [Google Scholar] [CrossRef]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet Printing for Pharmaceutics—A Review of Research and Manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, Q.; Yang, H.; Cai, J.; Huang, L.; Duan, Y.; Xu, Z.; Cen, P. Recent Advances in Inkjet Dispensing Technologies: Applications in Drug Discovery. Expert Opin. Drug Discov. 2012, 7, 761–770. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet Printing of Transdermal Microneedles for the Delivery of Anticancer Agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef]
- Ross, S.; Scoutaris, N.; Lamprou, D.; Mallinson, D.; Douroumis, D. Inkjet Printing of Insulin Microneedles for Transdermal Delivery. Drug Deliv. Transl. Res. 2015, 5, 451–461. [Google Scholar] [CrossRef]
- Ihalainen, P.; Määttänen, A.; Sandler, N. Printing Technologies for Biomolecule and Cell-Based Applications. Int. J. Pharm. 2015, 494, 585–592. [Google Scholar] [CrossRef]
- Zheng, Q.; Lu, J.; Chen, H.; Huang, L.; Cai, J.; Xu, Z. Application of Inkjet Printing Technique for Biological Material Delivery and Antimicrobial Assays. Anal. Biochem. 2011, 410, 171–176. [Google Scholar] [CrossRef]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of Printing Technologies for Oral Film Formulations. Int. J. Pharm. 2015, 494, 578–584. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef]
- Thabet, Y.; Sibanc, R.; Breitkreutz, J. Printing Pharmaceuticals by Inkjet Technology: Proof of Concept for Stand-Alone and Continuous in-Line Printing on Orodispersible Films. J. Manuf. Process. 2018, 35, 205–215. [Google Scholar] [CrossRef]
- Visser, J.C.; Wibier, L.; Kiefer, O.; Orlu, M.; Breitkreutz, J.; Woerdenbag, H.J.; Taxis, K. A Pediatrics Utilization Study in The Netherlands to Identify Active Pharmaceutical Ingredients Suitable for Inkjet Printing on Orodispersible Films. Pharmaceutics 2020, 12, 164. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.; Bermak, A. Smart Manufacturing Technologies for Printed Electronics. Hybrid Nanomater. Flex. Electron. Mater. 2019. [Google Scholar] [CrossRef]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of Inkjet Printing to Tissue Engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet Printing Biomaterials for Tissue Engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet Printing of Polymers: State of the Art and Future Developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Komuro, N.; Takaki, S.; Suzuki, K.; Citterio, D. Inkjet Printed (Bio)Chemical Sensing Devices. Anal. Bioanal. Chem. 2013, 405, 5785–5805. [Google Scholar] [CrossRef]
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet Printing of Proteins. Soft Matter 2009, 5, 4866–4877. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet Printing as a Deposition and Patterning Tool for Polymers and Inorganic Particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef]
- Moya, A.; Gabriel, G.; Villa, R.; Javier del Campo, F. Inkjet-Printed Electrochemical Sensors. Curr. Opin. Electrochem. 2017, 3, 29–39. [Google Scholar] [CrossRef]
- Wickström, H.; Nyman, J.O.; Indola, M.; Sundelin, H.; Kronberg, L.; Preis, M.; Rantanen, J.; Sandler, N. Colorimetry as Quality Control Tool for Individual Inkjet-Printed Pediatric Formulations. AAPS PharmSciTech 2017, 18, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Vakili, H.; Wickström, H.; Desai, D.; Preis, M.; Sandler, N. Application of a Handheld NIR Spectrometer in Prediction of Drug Content in Inkjet Printed Orodispersible Formulations Containing Prednisolone and Levothyroxine. Int. J. Pharm. 2017, 524, 414–423. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing—Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Lee, W.H.; Lee, H.S.; Lee, J.H.; Park, Y.D.; Cho, K. Self-Organization of Ink-Jet-Printed Triisopropylsilylethynyl Pentacene via Evaporation-Induced Flows in a Drying Droplet. Adv. Funct. Mater. 2008, 18, 229–234. [Google Scholar] [CrossRef]
- Farid, M. A New Approach to Modelling of Single Droplet Drying. Chem. Eng. Sci. 2003, 58, 2985–2993. [Google Scholar] [CrossRef]
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M.R.; Clasen, C.; Van den Mooter, G. Unraveling Particle Formation: From Single Droplet Drying to Spray Drying and Electrospraying. Pharmaceutics 2020, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray Drying Formulation of Amorphous Solid Dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Mezhericher, M.; Levy, A.; Borde, I. Modelling the Morphological Evolution of Nanosuspension Droplet in Constant-Rate Drying Stage. Chem. Eng. Sci. 2011, 66, 884–896. [Google Scholar] [CrossRef]
- de Souza Lima, R.; Ré, M.I.; Arlabosse, P. Drying Droplet as a Template for Solid Formation: A Review. Powder Technol. 2020, 359, 161–171. [Google Scholar] [CrossRef]
- Mezhericher, M.; Levy, A.; Borde, I. Modelling of Particle Breakage during Drying. Chem. Eng. Process. Process Intensif. 2008, 47, 1404–1411. [Google Scholar] [CrossRef]
- Perdana, J.; Bereschenko, L.; Fox, M.B.; Kuperus, J.H.; Kleerebezem, M.; Boom, R.M.; Schutyser, M.A.I. Dehydration and Thermal Inactivation of Lactobacillus Plantarum WCFS1: Comparing Single Droplet Drying to Spray and Freeze Drying. Food Res. Int. 2013, 54, 1351–1359. [Google Scholar] [CrossRef]
- Mezhericher, M.; Levy, A.; Borde, I. Spray Drying Modelling Based on Advanced Droplet Drying Kinetics. Chem. Eng. Process. Process Intensif. 2010, 49, 1205–1213. [Google Scholar] [CrossRef]
- Machekposhti, S.A.; Movahed, S.; Narayan, R.J. Physicochemical Parameters That Underlie Inkjet Printing for Medical Applications. Biophys. Rev. 2020, 1, 011301. [Google Scholar] [CrossRef]
- Sohrabi, S.; Liu, Y. Modeling Thermal Inkjet and Cell Printing Process Using Modified Pseudopotential and Thermal Lattice Boltzmann Methods. Phys. Rev. E 2018, 97, 033105. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, H.C.; Kuk, K.; Oh, Y.S. A Monolithic Inkjet Print Head: DomeJet. Sens. Actuators A Phys. 2002, 95, 114–119. [Google Scholar] [CrossRef]
- Kholghi Eshkalak, S.; Chinnappan, A.; Jayathilaka, W.A.D.M.; Khatibzadeh, M.; Kowsari, E.; Ramakrishna, S. A Review on Inkjet Printing of CNT Composites for Smart Applications. Appl. Mater. Today 2017, 9, 372–386. [Google Scholar] [CrossRef]
- Yin, Z.P.; Huang, Y.A.; Bu, N.B.; Wang, X.M.; Xiong, Y.L. Inkjet Printing for Flexible Electronics: Materials, Processes and Equipments. Chin. Sci. Bull. 2010, 55, 3383–3407. [Google Scholar] [CrossRef]
- Andò, B.; Baglio, S.; Bulsara, A.R.; Emery, T.; Marletta, V.; Pistorio, A. Low-Cost Inkjet Printing Technology for the Rapid Prototyping of Transducers. Sensors 2017, 17, 748. [Google Scholar] [CrossRef]
- Matsuda, Y.; Shibayama, S.; Uete, K.; Yamaguchi, H.; Niimi, T. Electric Conductive Pattern Element Fabricated Using Commercial Inkjet Printer for Paper-Based Analytical Devices. Anal. Chem. 2015, 87, 5762–5765. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, W.; Xu, Q.; Tan, R.; Song, W. Properties of Polyacrylic Acid-Coated Silver Nanoparticle Ink for Inkjet Printing Conductive Tracks on Paper with High Conductivity. Mater. Chem. Phys. 2014, 147, 550–556. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, W.; Xu, Q.; Tan, R.; Song, W. Room-Temperature Sintering of Conductive Ag Films on Paper. Mater. Lett. 2014, 123, 124–127. [Google Scholar] [CrossRef]
- Garcia, A.; Hanifi, N.; Jousselme, B.; Jégou, P.; Palacin, S.; Viel, P.; Berthelot, T. Polymer Grafting by Inkjet Printing: A Direct Chemical Writing Toolset. Adv. Funct. Mater. 2013, 23, 3668–3674. [Google Scholar] [CrossRef]
- Moon, S.J.; Robin, M.; Wenlin, K.; Yann, M.; Bae, B.S.; Mohammed-Brahim, T.; Jacques, E.; Harnois, M. Morphological Impact of Insulator on Inkjet-Printed Transistor. Flex. Print. Electron. 2017, 2, 035008. [Google Scholar] [CrossRef]
- Ando, B.; Baglio, S. All-Inkjet Printed Strain Sensors. IEEE Sens. J. 2013, 13, 4874–4879. [Google Scholar] [CrossRef]
- Salaoru, I.; Zhou, Z.; Morris, P.; Gibbons, G.J. Inkjet-Printed Polyvinyl Alcohol Multilayers. J. Vis. Exp. 2017, 2017, 55093. [Google Scholar] [CrossRef]
- Genina, N.; Fors, D.; Vakili, H.; Ihalainen, P.; Pohjala, L.; Ehlers, H.; Kassamakov, I.; Haeggström, E.; Vuorela, P.; Peltonen, J.; et al. Tailoring Controlled-Release Oral Dosage Forms by Combining Inkjet and Flexographic Printing Techniques. Eur. J. Pharm. Sci. 2012, 47, 615–623. [Google Scholar] [CrossRef]
- Genina, N.; Janßen, E.M.; Breitenbach, A.; Breitkreutz, J.; Sandler, N. Evaluation of Different Substrates for Inkjet Printing of Rasagiline Mesylate. Eur. J. Pharm. Biopharm. 2013, 85, 1075–1083. [Google Scholar] [CrossRef]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet Printing of Drug Substances and Use of Porous Substrates-towards Individualized Dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef]
- McManus, D.; Vranic, S.; Withers, F.; Sanchez-Romaguera, V.; Macucci, M.; Yang, H.; Sorrentino, R.; Parvez, K.; Son, S.K.; Iannaccone, G.; et al. Water-Based and Biocompatible 2D Crystal Inks for All-Inkjet-Printed Heterostructures. Nat. Nanotechnol. 2017, 12, 343–350. [Google Scholar] [CrossRef]
- Reis, N.; Ainsley, C.; Derby, B. Ink-Jet Delivery of Particle Suspensions by Piezoelectric Droplet Ejectors. J. Appl. Phys. 2005, 97, 094903. [Google Scholar] [CrossRef]
- Derby, B. Inkjet Printing Ceramics: From Drops to Solid. J. Eur. Ceram. Soc. 2011, 31, 2543–2550. [Google Scholar] [CrossRef]
- Jang, D.; Kim, D.; Moon, J. Influence of Fluid Physical Properties on Ink-Jet Printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef]
- Wijshoff, H. Drop Dynamics in the Inkjet Printing Process. Curr. Opin. Colloid Interface Sci. 2018, 36, 20–27. [Google Scholar] [CrossRef]
- Van Der Bos, A.; Van Der Meulen, M.J.; Driessen, T.; Van Den Berg, M.; Reinten, H.; Wijshoff, H.; Versluis, M.; Lohse, D. Velocity Profile inside Piezoacoustic Inkjet Droplets in Flight: Comparison between Experiment and Numerical Simulation. Phys. Rev. Appl. 2014, 1, 014004. [Google Scholar] [CrossRef]
- Liu, Y.; Derby, B. Experimental Study of the Parameters for Stable Drop-on-Demand Inkjet Performance. Phys. Fluids 2019, 31, 032004. [Google Scholar] [CrossRef]
- Choi, H.W.; Zhou, T.; Singh, M.; Jabbour, G.E. Recent Developments and Directions in Printed Nanomaterials. Nanoscale 2015, 7, 3338–3355. [Google Scholar] [CrossRef]
- Padilla-Martinez, J.P.; Ramirez-San-Juan, J.C.; Berrospe-Rodriguez, C.; Korneev, N.; Aguilar, G.; Zaca-Moran, P.; Ramos-Garcia, R. Controllable Direction of Liquid Jets Generated by Thermocavitation within a Droplet. Appl. Opt. 2017, 56, 7167. [Google Scholar] [CrossRef]
- Oktavianty, O.; Haruyama, S.; Ishii, Y. Enhancing Droplet Quality of Edible Ink in Single and Multi-Drop Methods by Optimization the Waveform Design of DoD Inkjet Printer. Processes 2022, 10, 91. [Google Scholar] [CrossRef]
- Saleh, E.; Woolliams, P.; Clarke, B.; Gregory, A.; Greedy, S.; Smartt, C.; Wildman, R.; Ashcroft, I.; Hague, R.; Dickens, P.; et al. 3D Inkjet-Printed UV-Curable Inks for Multi-Functional Electromagnetic Applications. Addit. Manuf. 2017, 13, 143–148. [Google Scholar] [CrossRef]
- Barlow, N.E.; Kusumaatmaja, H.; Salehi-Reyhani, A.; Brooks, N.; Barter, L.M.C.; Flemming, A.J.; Ces, O. Measuring Bilayer Surface Energy and Curvature in Asymmetric Droplet Interface Bilayers. J. R. Soc. Interface 2018, 15, 20180610. [Google Scholar] [CrossRef]
- Mypati, S.; Dhanushkodi, S.R.; McLaren, M.; Docoslis, A.; Peppley, B.A.; Barz, D.P.J. Optimized Inkjet-Printed Silver Nanoparticle Films: Theoretical and Experimental Investigations. RSC Adv. 2018, 8, 19679–19689. [Google Scholar] [CrossRef]
- Pandiyan, S.; El-Kharouf, A.; Steinberger-Wilckens, R. Formulation of Spinel Based Inkjet Inks for Protective Layer Coatings in SOFC Interconnects. J. Colloid Interface Sci. 2020, 579, 82–95. [Google Scholar] [CrossRef]
- Abdolmaleki, H.; Agarwala, S. PVDF-BaTiO3 Nanocomposite Inkjet Inks with Enhanced β-Phase Crystallinity for Printed Electronics. Polymers 2020, 12, 2430. [Google Scholar] [CrossRef]
- Xu, C.; An, C.; Long, Y.; Li, Q.; Guo, H.; Wang, S.; Wang, J. Inkjet Printing of Energetic Composites with High Density. RSC Adv. 2018, 8, 35863–35869. [Google Scholar] [CrossRef]
- Zhu, Z.; Gong, Z.; Qu, P.; Li, Z.; Rasaki, S.A.; Liu, Z.; Wang, P.; Liu, C.; Lao, C.; Chen, Z. Additive Manufacturing of Thin Electrolyte Layers via Inkjet Printing of Highly-Stable Ceramic Inks. J. Adv. Ceram. 2021, 10, 279–290. [Google Scholar] [CrossRef]
- Li, C.; Shi, H.; Ran, R.; Su, C.; Shao, Z. Thermal Inkjet Printing of Thin-Film Electrolytes and Buffering Layers for Solid Oxide Fuel Cells with Improved Performance. Int. J. Hydrogen Energy 2013, 38, 9310–9319. [Google Scholar] [CrossRef]
- Kolakovic, R.; Viitala, T.; Ihalainen, P.; Genina, N.; Peltonen, J.; Sandler, N. Printing Technologies in Fabrication of Drug Delivery Systems. Expert Opin. Drug Deliv. 2013, 10, 1711–1723. [Google Scholar] [CrossRef]
- Lau, G.K.; Shrestha, M. Ink-Jet Printing of Micro-Electro-Mechanical Systems (MEMS). Micromachines 2017, 8, 194. [Google Scholar] [CrossRef]
- Kumar, P.; Ebbens, S.; Zhao, X. Inkjet Printing of Mammalian Cells—Theory and Applications. Bioprinting 2021, 23, e00157. [Google Scholar] [CrossRef]
- Alamán, J.; Alicante, R.; Peña, J.I.; Sánchez-Somolinos, C. Inkjet Printing of Functional Materials for Optical and Photonic Applications. Materials 2016, 9, 910. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Calvert, P. Materials Science. Printing Cells. Science 2007, 318, 208–209. [Google Scholar] [CrossRef]
- Gaisford, S. 3D Printed Pharmaceutical Products. 3D Print. Med. 2017, 155–166. [Google Scholar] [CrossRef]
- Özkol, E.; Ebert, J.; Uibel, K.; Wätjen, A.M.; Telle, R. Development of High Solid Content Aqueous 3Y-TZP Suspensions for Direct Inkjet Printing Using a Thermal Inkjet Printer. J. Eur. Ceram. Soc. 2009, 29, 403–409. [Google Scholar] [CrossRef]
- Mannerbro, R.; Ranlöf, M.; Robinson, N.; Forchheimer, R. Inkjet Printed Electrochemical Organic Electronics. Synth. Met. 2008, 158, 556–560. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Uzun, S.; Schelling, M.; Hantanasirisakul, K.; Mathis, T.S.; Askeland, R.; Dion, G.; Gogotsi, Y. Additive-Free Aqueous MXene Inks for Thermal Inkjet Printing on Textiles. Small 2021, 17, 2006376. [Google Scholar] [CrossRef]
- Lee, S.; Byun, D.; Jung, D.; Choi, J.; Kim, Y.; Yang, J.H.; Son, S.U.; Tran, S.B.Q.; Ko, H.S. Pole-Type Ground Electrode in Nozzle for Electrostatic Field Induced Drop-on-Demand Inkjet Head. Sensors Actuators A Phys. 2008, 141, 506–514. [Google Scholar] [CrossRef]
- Khan, A.; Rahman, K.; Hyun, M.-T.; Kim, D.-S.; Choi, K.-H.; Khan, A.; Rahman, K.; Hyun, M.; Choi, K.; Kim, D. Multi-Nozzle Electrohydrodynamic Inkjet Printing of Silver Colloidal Solution for the Fabrication of Electrically Functional Microstructures. Appl. Phys. A 2011, 104, 1113–1120. [Google Scholar] [CrossRef]
- Khan, A.; Rahman, K.; Kim, D.S.; Choi, K.H. Direct Printing of Copper Conductive Micro-Tracks by Multi-Nozzle Electrohydrodynamic Inkjet Printing Process. J. Mater. Process. Technol. 2012, 212, 700–706. [Google Scholar] [CrossRef]
- Wijshoff, H. The Dynamics of the Piezo Inkjet Printhead Operation. Phys. Rep. 2010, 491, 77–177. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.J.; Gilmore, K.G.; Wallace, G.G.; In Het Panhuis, M. Biofabrication: An Overview of the Approaches Used for Printing of Living Cells. Appl. Microbiol. Biotechnol. 2013, 97, 4243–4258. [Google Scholar] [CrossRef]
- Dodoo, C.C.; Alomari, M.; Basit, A.W.; Stapleton, P.; Gaisford, S. A Thermal Ink-Jet Printing Approach for Evaluating Susceptibility of Bacteria to Antibiotics. J. Microbiol. Methods 2019, 164, 105660. [Google Scholar] [CrossRef]
- Prasad, L.K.; Smyth, H. 3D Printing Technologies for Drug Delivery: A Review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of Warfarin Therapy Using Thermal Ink-Jet Printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef]
- Buanz, A.B.M.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of Personalized-Dose Salbutamol Sulphate Oral Films with Thermal Ink-Jet Printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef]
- Alper, J. Biology and the Inkjets. Science 2004, 305, 1895. [Google Scholar] [CrossRef]
- Wilson, W.C.; Boland, T. Cell and Organ Printing 1: Protein and Cell Printers. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272, 491–496. [Google Scholar] [CrossRef]
- Lemmo, A.V.; Rose, D.J.; Tisone, T.C. Inkjet Dispensing Technology: Applications in Drug Discovery. Curr. Opin. Biotechnol. 1998, 9, 615–617. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing of Viable Mammalian Cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, P.A.; Kane, K.M.; Ashvar, C.S.; Albrecht, M.; Smith, P.A. Thermal Inkjet Application in the Preparation of Oral Dosage Forms: Dispensing of Prednisolone Solutions and Polymorphic Characterization by Solid-State Spectroscopic Techniques. J. Pharm. Sci. 2008, 97, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Young, V. A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue. Cell Biochem. Biophys. 2016, 74, 93–98. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex Heterogeneous Tissue Constructs Containing Multiple Cell Types Prepared by Inkjet Printing Technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef]
- Gao, G.; Cui, X. Three-Dimensional Bioprinting in Tissue Engineering and Regenerative Medicine. Biotechnol. Lett. 2016, 38, 203–211. [Google Scholar] [CrossRef]
- Zhou, H.; Gué, A.M. Simulation Model and Droplet Ejection Performance of a Thermal-Bubble Microejector. Sens. Actuators B Chem. 2010, 145, 311–319. [Google Scholar] [CrossRef]
- Setti, L.; Piana, C.; Bonazzi, S.; Ballarin, B.; Frascaro, D.; Fraleoni-Morgera, A.; Giuliani, S. Thermal Inkjet Technology for the Microdeposition of Biological Molecules as a Viable Route for the Realization of Biosensors. Anal. Lett. 2007, 37, 1559–1570. [Google Scholar] [CrossRef]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell Damage Evaluation of Thermal Inkjet Printed Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149. [Google Scholar] [CrossRef]
- Agarwal, S.; Saha, S.; Balla, V.K.; Pal, A.; Barui, A.; Bodhak, S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration–A Review. Front. Mech. Eng. 2020, 6, 589171. [Google Scholar] [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D Bioprinting of Cells, Tissues and Organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Ayan, B.; Undieh, A.A.; Yang, Y.P.; Huang, N.F. Advances in Three-Dimensional Bioprinted Stem Cell-Based Tissue Engineering for Cardiovascular Regeneration. J. Mol. Cell. Cardiol. 2022, 169, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion Bioprinting of Soft Materials: An Emerging Technique for Biological Model Fabrication. Appl. Phys. Rev. 2019, 6, 011310. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.W.; Lee, K.X.A.; Yeong, W.Y.; Shen, Y.F. Vat Polymerization-Based Bioprinting—Process, Materials, Applications and Regulatory Challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Fon, D.; Li, X.; Tian, J.; Forsythe, J.; Garnier, G.; Shen, W. Biosurface Engineering through Ink Jet Printing. Colloids Surf. B. Biointerfaces 2010, 75, 441–447. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform Inkjet Printing of Cellular Structures with Bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Setti, L.; Fraleoni-Morgera, A.; Ballarin, B.; Filippini, A.; Frascaro, D.; Piana, C. An Amperometric Glucose Biosensor Prototype Fabricated by Thermal Inkjet Printing. Biosens. Bioelectron. 2005, 20, 2019–2026. [Google Scholar] [CrossRef]
- Ng, W.L.; Huang, X.; Shkolnikov, V.; Goh, G.L.; Suntornnond, R.; Yeong, W.Y. Controlling Droplet Impact Velocity and Droplet Volume: Key Factors to Achieving High Cell Viability in Sub-Nanoliter Droplet-Based Bioprinting. Int. J. Bioprinting 2021, 8, 424. [Google Scholar] [CrossRef]
- Park, J.A.; Lee, H.R.; Park, S.Y.; Jung, S. Self-Organization of Fibroblast-Laden 3D Collagen Microstructures from Inkjet-Printed Cell Patterns. Adv. Biosyst. 2020, 4, 1900280. [Google Scholar] [CrossRef] [PubMed]
- Suntornnond, R.; Ng, W.L.; Huang, X.; Yeow, C.H.E.; Yeong, W.Y. Improving Printability of Hydrogel-Based Bio-Inks for Thermal Inkjet Bioprinting Applications via Saponification and Heat Treatment Processes. J. Mater. Chem. B 2022, 10, 5989–6000. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, S.; Zhang, Y.; Liu, M.; Yi, X.; Hu, J. Insights into the Saponification Process of Di(2-Ethylhexyl) Phosphoric Acid Extractant: Thermodynamics and Structural Aspects. J. Mol. Liq. 2019, 280, 252–258. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol Production and Its Applications as a Raw Material: A Review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Yoon, S.; Park, J.A.; Lee, H.R.; Yoon, W.H.; Hwang, D.S.; Jung, S. Inkjet–Spray Hybrid Printing for 3D Freeform Fabrication of Multilayered Hydrogel Structures. Adv. Healthc. Mater. 2018, 7, 1800050. [Google Scholar] [CrossRef]
- Freeman, S.; Ramos, R.; Alexis Chando, P.; Zhou, L.; Reeser, K.; Jin, S.; Soman, P.; Ye, K. A Bioink Blend for Rotary 3D Bioprinting Tissue Engineered Small-Diameter Vascular Constructs. Acta Biomater. 2019, 95, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Solis, L.H.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.; Boland, T. Thermal Inkjet Bioprinting Triggers the Activation of the VEGF Pathway in Human Microvascular Endothelial Cells in Vitro. Biofabrication 2019, 11, 045005. [Google Scholar] [CrossRef]
- Gao, G.; Hubbell, K.; Schilling, A.F.; Dai, G.; Cui, X. Bioprinting Cartilage Tissue from Mesenchymal Stem Cells and PEG Hydrogel. Methods Mol. Biol. 2017, 1612, 391–398. [Google Scholar] [CrossRef]
- Cui, X.; Breitenkamp, K.; Lotz, M.; D’Lima, D. Synergistic Action of Fibroblast Growth Factor-2 and Transforming Growth Factor-Beta1 Enhances Bioprinted Human Neocartilage Formation. Biotechnol. Bioeng. 2012, 109, 2357–2368. [Google Scholar] [CrossRef]
- Kador, K.E.; Grogan, S.P.; Dorthé, E.W.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; D’Lima, D.D. Control of Retinal Ganglion Cell Positioning and Neurite Growth: Combining 3D Printing with Radial Electrospun Scaffolds. Tissue Eng. Part A 2016, 22, 286–294. [Google Scholar] [CrossRef]
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive Nanoparticles Stimulate Bone Tissue Formation in Bioprinted Three-Dimensional Scaffold and Human Mesenchymal Stem Cells. Biotechnol. J. 2014, 9, 1304–1311. [Google Scholar] [CrossRef]
- Allain, L.R.; Stratis-Cullum, D.N.; Vo-Dinh, T. Investigation of Microfabrication of Biological Sample Arrays Using Piezoelectric and Bubble-Jet Printing Technologies. Anal. Chim. Acta 2004, 518, 77–85. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing for High-Throughput Cell Patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Xu, T.; Rohozinski, J.; Zhao, W.; Moorefield, E.C.; Atala, A.; Yoo, J.J. Inkjet-Mediated Gene Transfection into Living Cells Combined with Targeted Delivery. Tissue Eng. Part A 2008, 15, 95–101. [Google Scholar] [CrossRef]
- Cui, X.; Gao, G.; Qiu, Y. Accelerated Myotube Formation Using Bioprinting Technology for Biosensor Applications. Biotechnol. Lett. 2012, 35, 315–321. [Google Scholar] [CrossRef]
- Klebe, R.J. Cytoscribing: A Method for Micropositioning Cells and the Construction of Two- and Three-Dimensional Synthetic Tissues. Exp. Cell Res. 1988, 179, 362–373. [Google Scholar] [CrossRef]
- Okamoto, T.; Suzuki, T.; Yamamoto, N. Microarray Fabrication with Covalent Attachment of DNA Using Bubble Jet Technology. Nat. Biotechnol. 2000, 18, 438–441. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T. Human Microvasculature Fabrication Using Thermal Inkjet Printing Technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, X.; Shen, W.; Garnier, G. Thermal Stability of Bioactive Enzymatic Papers. Colloids Surf. B Biointerfaces 2010, 75, 239–246. [Google Scholar] [CrossRef]
- Nandi, U.; Trivedi, V.; Ross, S.A.; Douroumis, D. Advances in Twin-Screw Granulation Processing. Pharmaceutics 2021, 13, 624. [Google Scholar] [CrossRef]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced Methodologies for Cocrystal Synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef]
- Preis, M.; Pein, M.; Breitkreutz, J. Development of a Taste-Masked Orodispersible Film Containing Dimenhydrinate. Pharmaceutics 2012, 4, 551. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder Jet 3D Printing—Process Parameters, Materials, Properties, Modeling, and Challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.K.; Chaudhuri, B. Pharmaceutical Applications of Powder-Based Binder Jet 3D Printing Process—A Review. Adv. Drug Deliv. Rev. 2021, 177, 113943. [Google Scholar] [CrossRef]
- Chang, S.Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Nagapudi, K.; Chaudhuri, B.; Ma, A.W.K. Binder-Jet 3D Printing of Indomethacin-Laden Pharmaceutical Dosage Forms. J. Pharm. Sci. 2020, 109, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Charoo, N.A.; Kuttolamadom, M.; Asadi, A.; Khan, M.A. Printing of Personalized Medication Using Binder Jetting 3D Printer. Precis. Med. Investig. Pract. Provid. 2020, 473–481. [Google Scholar] [CrossRef]
- Hong, X.; Han, X.; Li, X.; Li, J.; Wang, Z.; Zheng, A. Binder Jet 3D Printing of Compound LEV-PN Dispersible Tablets: An Innovative Approach for Fabricating Drug Systems with Multicompartmental Structures. Pharmaceutics 2021, 13, 1780. [Google Scholar] [CrossRef]
- Kozakiewicz-Latała, M.; Nartowski, K.P.; Dominik, A.; Malec, K.; Gołkowska, A.M.; Złocińska, A.; Rusińska, M.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Górniak, A.; et al. Binder Jetting 3D Printing of Challenging Medicines: From Low Dose Tablets to Hydrophobic Molecules. Eur. J. Pharm. Biopharm. 2022, 170, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Buanz, A.B.M.; Telford, R.; Scowen, I.J.; Gaisford, S. Rapid Preparation of Pharmaceutical Co-Crystals with Thermal Ink-Jet Printing. CrystEngComm 2013, 15, 1031–1035. [Google Scholar] [CrossRef]
- Takala, M.; Helkiö, H.; Sundholm, J.; Genina, N.; Kivikuoma, P.; Widmaier, T.; Sandler, N.; Kuosmanen, P. Ink-Jet Printing of Pharmaceuticals. In Proceedings of the 8th International DAAAM Baltic Conference “INDUSTRIAL ENGINEERING”, Tallinn, Estonia, 19–21 April 2012; Volume 6. [Google Scholar]
- Wilts, E.M.; Ma, D.; Bai, Y.; Williams, C.B.; Long, T.E. Comparison of Linear and 4-Arm Star Poly(Vinyl Pyrrolidone) for Aqueous Binder Jetting Additive Manufacturing of Personalized Dosage Tablets. ACS Appl. Mater. Interfaces 2019, 11, 23938–23947. [Google Scholar] [CrossRef]
- Montenegro-Nicolini, M.; Reyes, P.E.; Jara, M.O.; Vuddanda, P.R.; Neira-Carrillo, A.; Butto, N.; Velaga, S.; Morales, J.O. The Effect of Inkjet Printing over Polymeric Films as Potential Buccal Biologics Delivery Systems. AAPS PharmSciTech 2018, 19, 3376–3387. [Google Scholar] [CrossRef]
- Montenegro-Nicolini, M.; Miranda, V.; Morales, J.O. Inkjet Printing of Proteins: An Experimental Approach. AAPS J. 2016, 19, 234–243. [Google Scholar] [CrossRef]
- Tam, C.H.; Alexander, M.; Belton, P.; Qi, S. Drop-on-Demand Printing of Personalised Orodispersible Films Fabricated by Precision Micro-Dispensing. Int. J. Pharm. 2021, 610, 121279. [Google Scholar] [CrossRef] [PubMed]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-Based 3D Inkjet Printing of an Oral Pharmaceutical Dosage Form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous Inkjet Printing of Enalapril Maleate onto Orodispersible Film Formulations. Int. J. Pharm. 2018, 546, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Mueannoom, W.; Buanz, A.B.M.; Taylor, K.M.G.; Gaisford, S. In Vitro Characterisation of Terbutaline Sulphate Particles Prepared by Thermal Ink-Jet Spray Freeze Drying. Int. J. Pharm. 2013, 447, 165–170. [Google Scholar] [CrossRef]
- Mueannoom, W.; Srisongphan, A.; Taylor, K.M.G.; Hauschild, S.; Gaisford, S. Thermal Ink-Jet Spray Freeze-Drying for Preparation of Excipient-Free Salbutamol Sulphate for Inhalation. Eur. J. Pharm. Biopharm. 2012, 80, 149–155. [Google Scholar] [CrossRef]
- Lion, A.; Wildman, R.D.; Alexander, M.R.; Roberts, C.J. Customisable Tablet Printing: The Development of Multimaterial Hot Melt Inkjet 3D Printing to Produce Complex and Personalised Dosage Forms. Pharmaceutic 2021, 13, 1679. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nat. Rev. Microbiol. 2020, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.M.; Hu, Y. Novel Approaches to Developing New Antibiotics for Bacterial Infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef]
- Mohamed, A.; Menon, H.; Chulkina, M.; Yee, N.S.; Pinchuk, I.V. Drug–Microbiota Interaction in Colon Cancer Therapy: Impact of Antibiotics. Biomedicines 2021, 9, 259. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The Microbiome, Cancer, and Cancer Therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Roy, S.; Trinchieri, G. Microbiota: A Key Orchestrator of Cancer Therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Kumari, S.; Deshmukh, R. β-Lactam Antibiotics to Tame down Molecular Pathways of Alzheimer’s Disease. Eur. J. Pharmacol. 2021, 895, 173877. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Yimer, E.M.; Hishe, H.Z.; Tuem, K.B. Repurposing of the β-Lactam Antibiotic, Ceftriaxone for Neurological Disorders: A Review. Front. Neurosci. 2019, 13, 2260–2271. [Google Scholar] [CrossRef]
- Spellberg, B.; Gilbert, D.N. The Future of Antibiotics and Resistance: A Tribute to a Career of Leadership by John Bartlett. Clin. Infect. Dis. 2014, 59, S71–S75. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Cars, O.; Chandy, S.J.; Mpundu, M.; Peralta, A.Q.; Zorzet, A.; So, A.D. Resetting the Agenda for Antibiotic Resistance through a Health Systems Perspective. Lancet Glob. Health 2021, 9, e1022–e1027. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef]
- Nguyen, M.; Brettin, T.; Long, S.W.; Musser, J.M.; Olsen, R.J.; Olson, R.; Shukla, M.; Stevens, R.L.; Xia, F.; Yoo, H.; et al. Developing an in Silico Minimum Inhibitory Concentration Panel Test for Klebsiella Pneumoniae. Sci. Rep. 2018, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Carret, G.; Flandrois, J.P.; Delignette-Muller, M.L. How Does Susceptibility Prevalence Impact on the Performance of Disk Diffusion Susceptibility Testing? Diagn. Microbiol. Infect. Dis. 2004, 49, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Kelman, T.; Pitesky, M. Overview of Quantitative Methodologies to Understand Antimicrobial Resistance via Minimum Inhibitory Concentration. Animals 2020, 10, 1405. [Google Scholar] [CrossRef]
- Derby, B. Bioprinting: Inkjet Printing Proteins and Hybrid Cell-Containing Materials and Structures. J. Mater. Chem. 2008, 18, 5717–5721. [Google Scholar] [CrossRef]

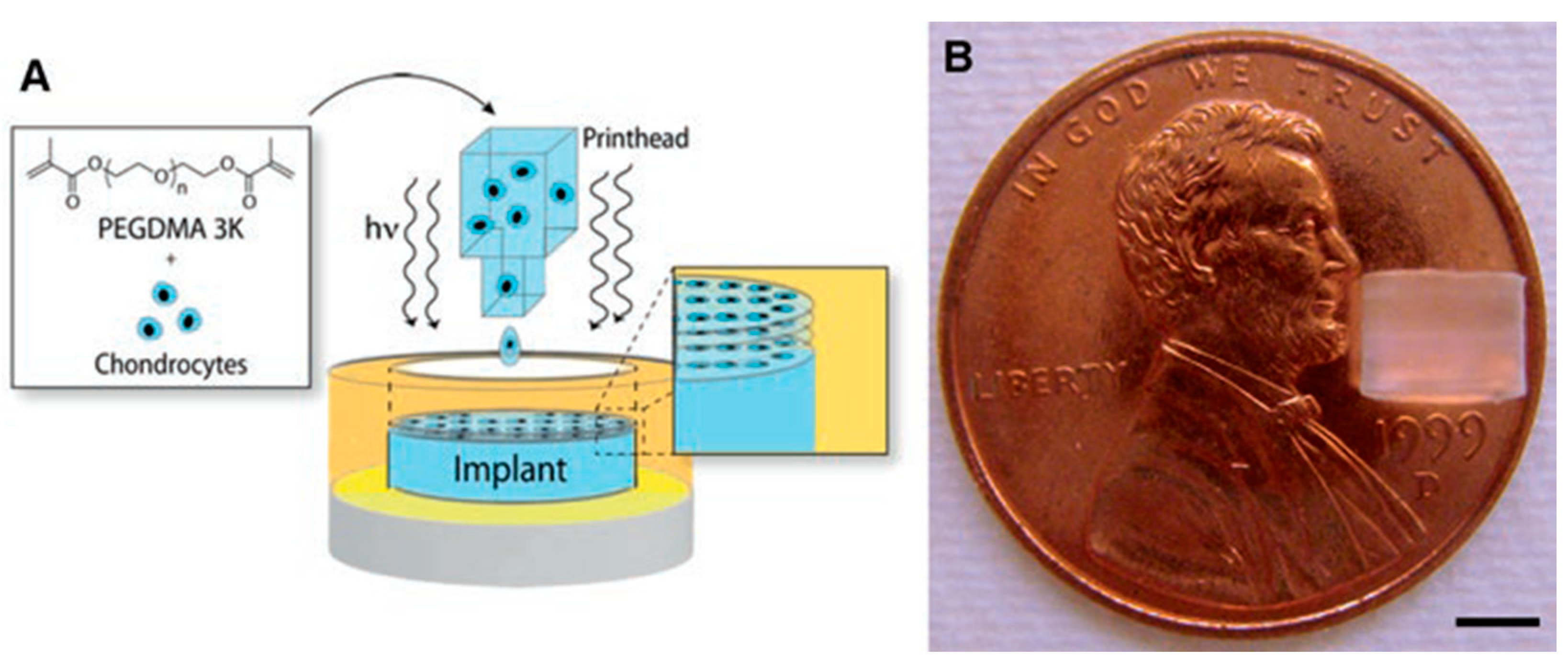
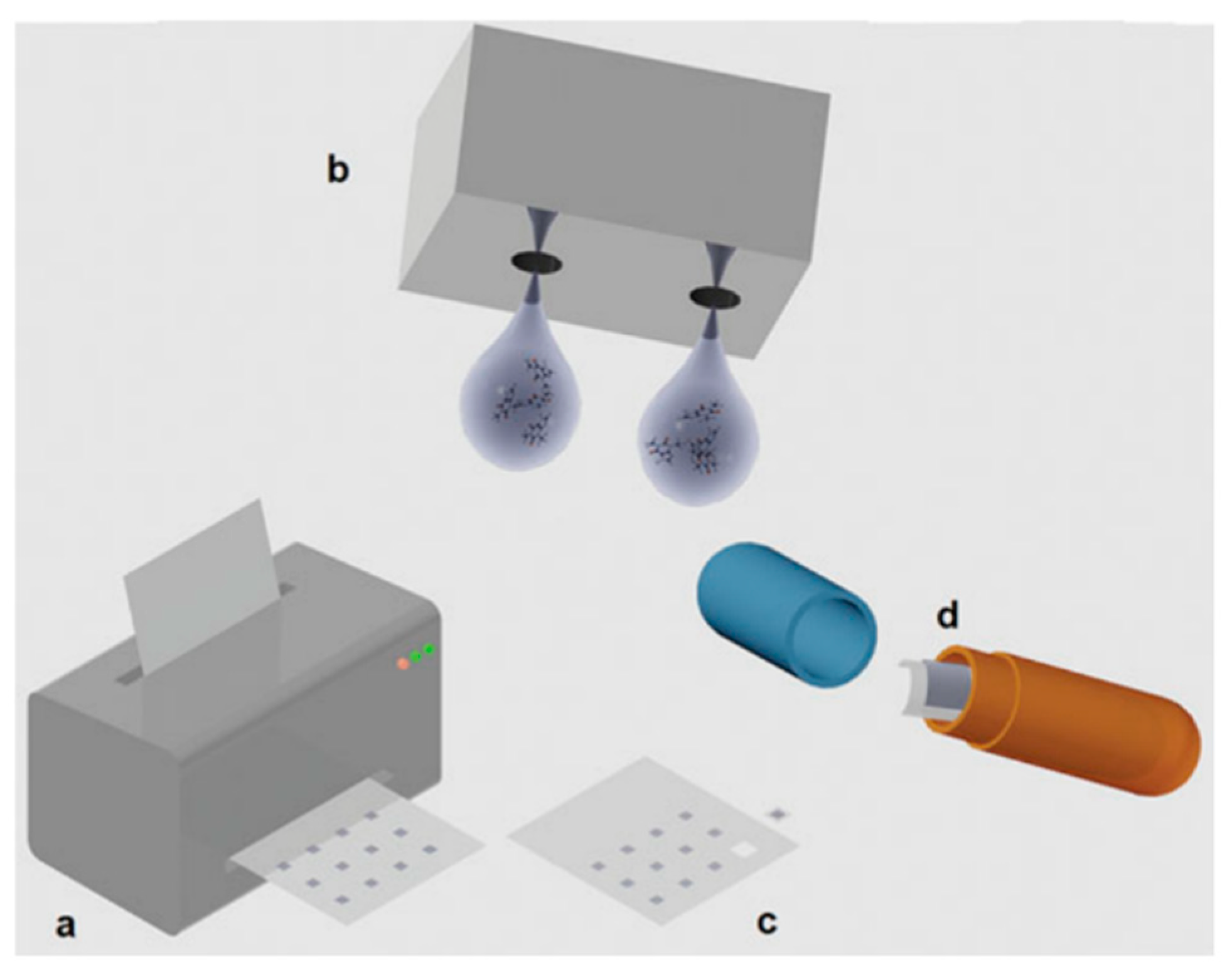
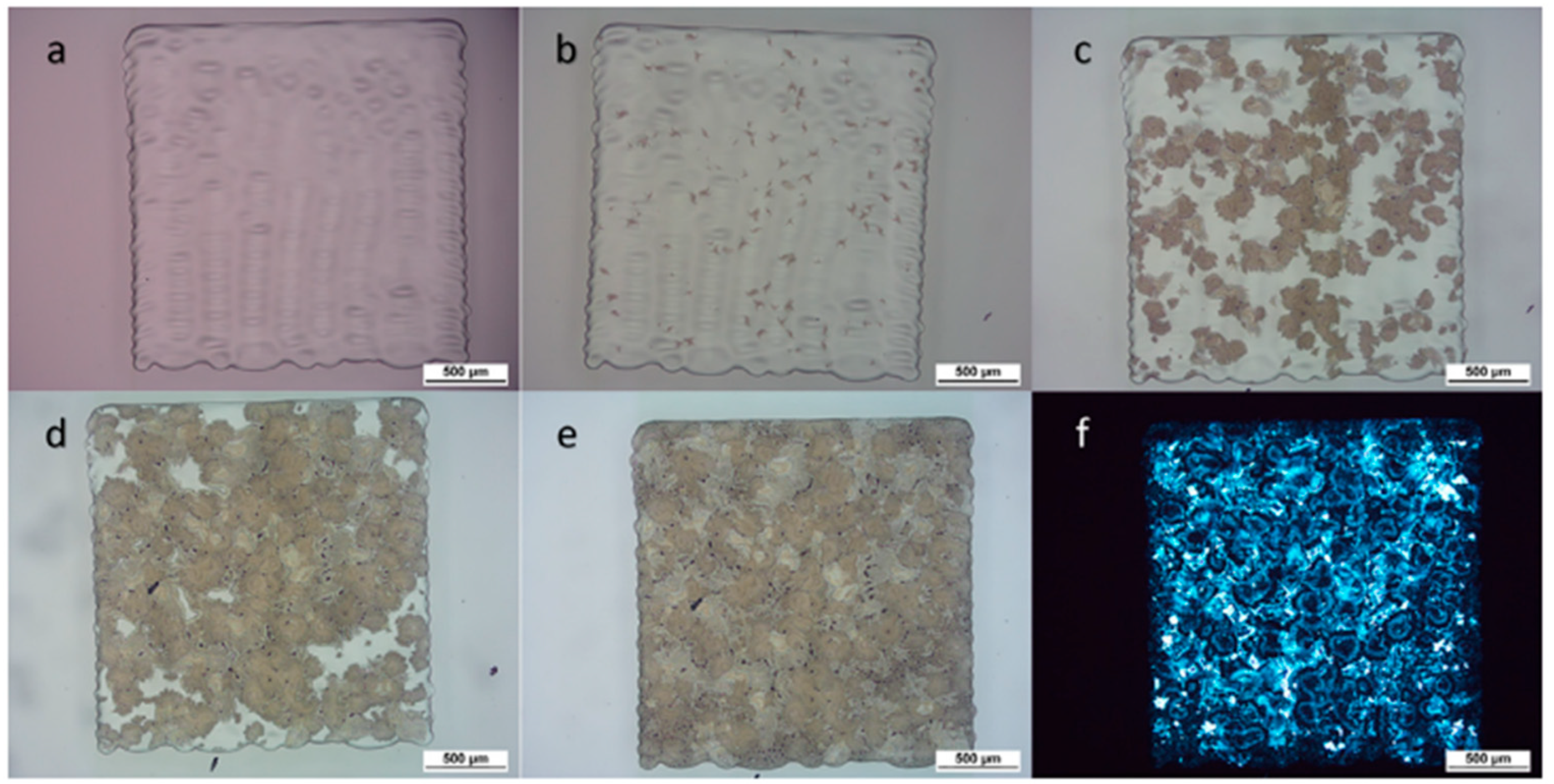
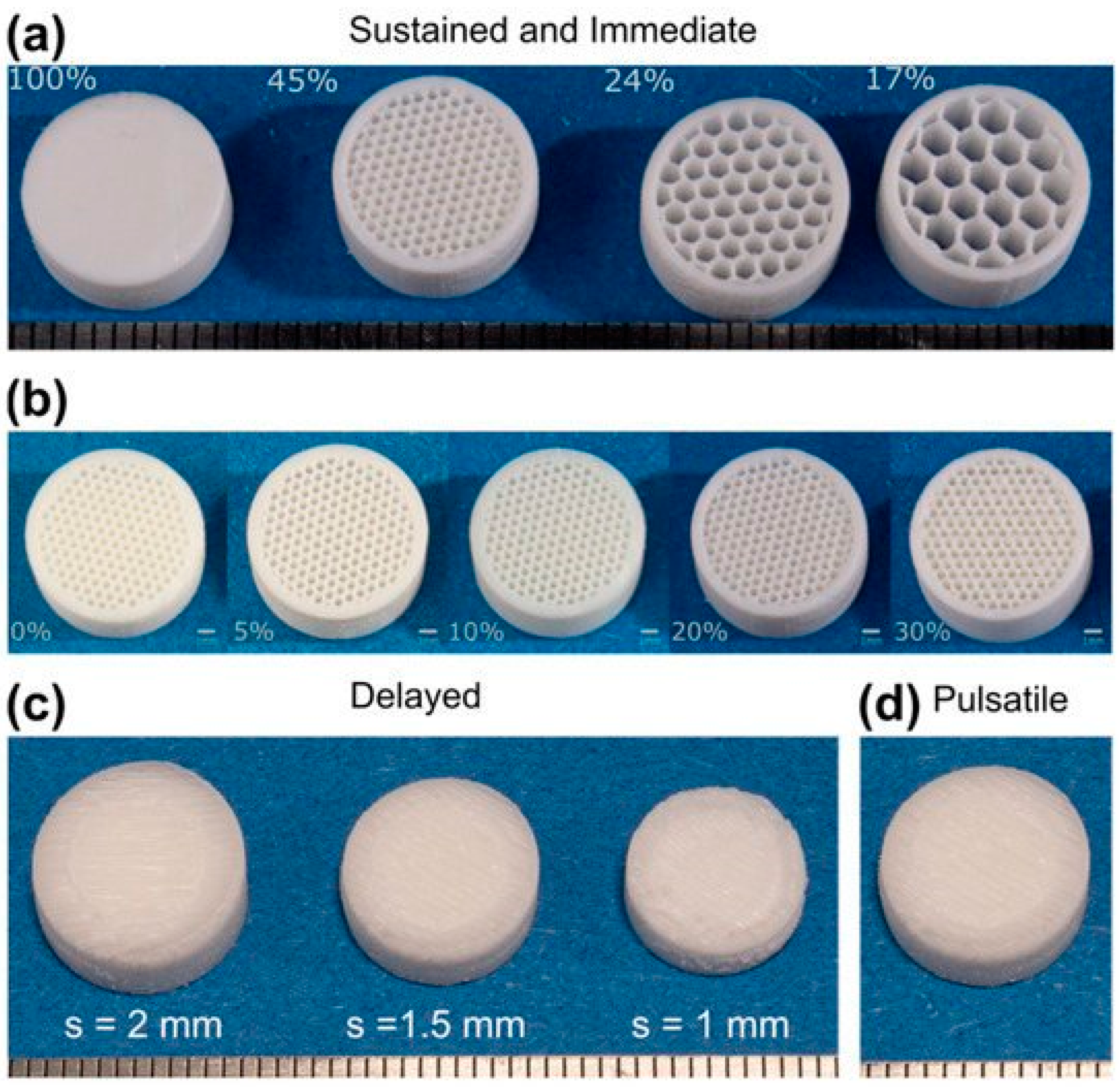
| SN | Properties | Photolithography | Micro-Contact Printing | Shadow Mask | Inkjet Printing |
|---|---|---|---|---|---|
| 1. | Cost | Extremely high | Medium | Low | Low |
| 2. | Efficiency | Low | High | High | High |
| 3. | Resolution | Extremely high | High | Low | High |
| 4. | Compatibility with polymer | Bad | Bad | Good | Excellent |
| 5. | Process | Multi step | Multi step | Multi step | All in one |
| 6. | Mode of action | Noncontact | Contact | Contact | Noncontact |
| 7. | Flexibility | Bad | Bad | Bad | Good, digital lithography |
| 8. | Requirement of environment | Clean rooms, vibration isolation | Medium | Low | Low |
| 9. | Material consumption | High | Low | Medium | Low |
| SN | Ink | Ink Viscosity (mPa·s) | Ink Surface Tension (m Nm−1) | Z Value | Ref. |
|---|---|---|---|---|---|
| 1. | Ethylene glycol | 15.8 | 45.5 | 2.08 | [68] |
| Ethylene Glycol: Water (5/95) | 1.16 | 69.5 | 33.2 | ||
| Ethylene Glycol: Water (10/90) | 1.47 | 68.9 | 26.1 | ||
| Ethylene Glycol: Water (15/85) | 2.32 | 67.7 | 16.5 | ||
| Ethylene Glycol: Water (25/75) | 2.72 | 67.0 | 14.1 | ||
| Ethylene Glycol: Water (50/50) | 5.05 | 46.7 | |||
| Ethylene Glycol: Water (50/50) | 4.39 | 60.3 | 8.40 | ||
| Ethylene Glycol: Water (75/25) | 7.81 | 52.7 | 4.47 | ||
| Ethylene Glycol: Water (85/15) | 10.5 | 50.2 | 3.28 | ||
| 2. | De-ionized water | 1.07 | 72.7 | 36.8 | [68] |
| 3. | Gallium-indium (75/25) | 1.7 | 624 | [46] | |
| 4. | Glycerol-Water | 1–22.5 | 66.4–7.6 | [69] | |
| 5. | CuNO4- Water | ~4.45 | 88 | [70] | |
| 6. | Dowanol | 10.17 | 15.55 | [71] | |
| 7. | Ethyl acetate | 0.452 | 2.367 | [13] | |
| 8. | 5 Fe3O4-95 (nanoparticles + UV Curable matrix resin) | 18.03 | 23.91 | 1.72 | [72] |
| 9. | 10 Fe3O4-90 (nanoparticles + UV Curable matrix resin) | 18.08 | 20.91 | 1.57 | [72] |
| 10. | Hydroxypropyl cellulose:Water (6/94) | 45 | 44.5 | [73] | |
| 11. | Commercial AgNp | 6.8 ± 0.7 | 30 ± 1 | [74] | |
| 12. | Diethylene glycol | 27.1 | 42.7 | 1.17 | [68] |
| 13. | Glycerol | 934.0 | 76.2 | 0.05 | [68] |
| 14. | MnCo2O4 | 10 | 6.17 | [75] | |
| 15. | MnCo1.8Fe0.2O4 | >15 | 4.77 | [75] | |
| 16. | PVDF: BaTiO3 (40/8) | 13.6 | 30.2 | 1.17 | [76] |
| PVDF: BaTiO3 (32/6.4) | 9.7 | 31.7 | 1.72 | [76] | |
| PVDF: BaTiO3 (24/4.8) | 6.0 | 32.4 | 2.79 | [76] | |
| PVDF: BaTiO3 (16/3.2) | 3.7 | 33.5 | 4.59 | [76] | |
| PVDF: BaTiO3 (8/1.6) | 2.1 | 34.8 | 8.23 | [76] | |
| PVDF: BaTiO3 (1/0.2) | 1.3 | 36.0 | 13.56 | [76] | |
| 17. | DNTF: Hexogen (13.86/0) | 1.2 | 23.33 | 36.94 | [77] |
| DNTF: Hexogen (12.47/1.39) | 1.0 | 23.09 | 44.56 | [77] | |
| DNTF: Hexogen (11.09/2.7) | 0.8 | 23.77 | 58.01 | [77] | |
| DNTF: Hexogen (9.70/4.16) | 0.6 | 24.15 | 75.51 | [77] | |
| DNTF: Hexogen (8.32/5.54) | 0.8 | 24.52 | 58.2 | [77] | |
| DNTF: Hexogen (6.93/6.93) | 1.3 | 23.66 | 35.44 | [77] | |
| 18. | 8 mol% Y2O3-stabilized ZrO2 (8YSZ) | 1.5 | 18.8 ± 0.3 | 7.6 | [78] |
| SN | Printer | Bioink | Area of Application | Outcome (Positive, Negative or Both) |
|---|---|---|---|---|
| 1. | HP Deskjet 500 printer (modified) [Hewlett-Packard, Inc., Palo Alto, CA] | Rat tail collagen type I | Cell printing | Around 89% cell viability was reported [111]. |
| 2. | HP DeskJet 550C printer (modified) | hAFSCs cell line | Stem cell printing | Data revealed that printed hAFSCs are capable of forming a firm bony tissue that can withstand high compressive force [107]. |
| 3. | Prototype of thermal inkjet printer combined with amperometric GOD electrode [developed by Lesepidado srl (Bologna, Italy) & supplied by Olivetti Tecnost (Ivrea, Italy)] | Glucose oxidase (GOD) from Aspergillus niger and poly(3,4-ethylene di-oxy thiophene/ polystyrene sulfonic acid) | Biosensor | Approximately 15% decrease in the efficiency of enzyme was noted [120]. |
| 4. | Canon inkjet printer (Pixma ip4500) (modified) | Fluoroscein isothiocyanate-conjugated bovine albumin and horseradish peroxidase | Microfluidic patterned paper | Bioactivity was retained by patterned paper. However, the percentage was not measured [118]. |
| 5. | Hewlett-Packard (HP) Deskjet 560 (Modified) | Herring sperm DNA in pure water, surfactant, alcohol, or a water-soluble polymer | Microarray | Was reported as a dependable printing option [133] |
| 6. | Bubble Jet (BJC-2100, Canon, Tokyo, Japan) | Rat tail collagen solution | Cell patterning | Spatial resolution of around 350 mm was obtained, and adherence of neuronal and smooth muscle cells to the printed area was reported [134]. |
| 7. | BJ F850 (Canon, Tokyo Japan) | Insulin related growth factors | Cell patterning and analysis | Intensified proliferation of cells on patterned area was observed [133]. |
| 8. | HP Deskjet 500 inkjet printer (modified) [Hewlett-Packard, Inc., Palo Alto, CA] | Chinese hamster ovary (CHO) cells | Cell patterning | Cellular viability count of 80% was reported that improved after changing the carrier fluid. Transient membrane damage of cells was observed after printing [111]. |
| 9. | HP DeskJet 692C and 55uC | CHO cells and porcine aortic endothelial | Gene transfection | Transfection rate of 10% and cellular viability of 90% were reported [135]. |
| 10. | HP Desktop printer (HP 55uC) (modified) | Mouse myoblast | Biosensor | Myotube generation alongside printed substrate was demonstrated [136]. |
| 11. | Hewlett Packard (HP) Deskjet 500 | Mammalian cells | Cell printing | Cellular viability varied 85–95% [112]. |
| 12. | HP-2225C Think Jet ink jet printer 7470A graphics plotter | Fibronectin | Cell patterning | Stickiness of cells to patterned fibronectin was noticed [137]. |
| 13. | BJC-600 (Canon, Tokyo) and BJC-700J printer | 5′-terminal-thiolated oligonucleotides | DNA microarrays | No trouble was encountered by researchers while ejecting DNA solution using bubble jet printer rather than heat generation, which was stated as an added advantage as it provided efficient reaction energy [138]. |
| 14. | Prototype model of TIJ printer from Olivetti Tecnost developed by Lesepidado srl | β-Galactosidase (GAL) from Aspergillus oryzae | Biosensor | Aside from approximately 15% reduction in enzyme activity, TIJP was determined to be a promising option for enzyme or other biological material micro-deposition [110]. |
| 15. | HP60 inkjet printer | Unmentioned cell | Cell printing | Successful concurrent simulation of thermal transfer, interaction between cell and fluid, transition of phase and increased cell viability was reported [47]. |
| 16. | Hewlett Packard Deskjet 500 thermal inkjet printer (modified) [Hewlett– Packard Company (Palo Alto, CA, USA)] | Human microvascular endothelial cells (HMVEC) and fibrin | Cell printing | Printed HMVEC proliferated and the formation of microvascular endothelial cells along with fibrin scaffolding was observed [139]. |
| 17. | Canon inkjet printer (Pixma ip4500) (modified) | Horseradish peroxidase (HRP) and alkaline phosphatase (ALP, from bovine intestinal mucosa) | Enzymatic paper | Bioactivity was retained by patterned enzymatic paper, but the percentage was not mentioned [140]. |
| 18. | Hewlett Packard (HP) 550C printer (modified) | Suspensions were made using embryonic motoneurons of rat and Chinese Hamster Ovary (CHO) | Cell printing | Successful printing of embryonic motoneurons and CHO cells with >90% viability was reported [104]. |
| 19. | Hewlett-Packard (HP) Deskjet thermal inkjet printer | Bone-marrow derived hMSCs | Cell printing | Viability of the printed cells was significantly higher [129]. |
| 20. | HP TIPS print head (Hewlett-Packard Packard, Corvallis | Retinal ganglion cells | Cell printing | Comparatively better cell survival, neurite outgrowth and functional electrophysiological properties of the printed cells were observed [131]. |
| Sl No. | Printer | Dosage Form | Ink Material | Ref. |
|---|---|---|---|---|
| 1. | Hewlett-Packard HP460 Deskjet | Cocrystal | carbamazepine, nicotinamide, benzoic acid, isonicotinamide, theophylline and saccharin | [150] |
| 2. | HP Photosmart B010 | Cocrystal | riboflavin sodium phosphate and paracetamol | [151] |
| 3. | Combination of thermal inkjet printhead HP11 and ZCorp Spectrum Z510 | Tablets | acetaminophen | [152] |
| 4. | Hewlett-Packard 970 Cxi DeskJet | Tablets | prednisolone | [86] |
| 5. | Hewlett Packard Deskjet 1000 | Buccal film | lysozyme | [153] |
| 6. | Hewlett Packard Deskjet 1000 | Buccal film | lysozyme and ribonuclease-A | [154] |
| 7. | HP Deskjet D1660 | Oral film | salbutamol sulphate | [100] |
| 8. | HP 5940 Deskjet | Orodispersible films | warfarin | [99] |
| 9. | TIJ Canon Pixma (unmodified) | Orodispersible films | vitamin B B1, B2, B3, and B6 | [33] |
| 10. | Nanojet Piezo Valve NJ-K-4020 | Orodispersible films | paracetamol | [155] |
| 11. | Fujifilm Dimatix DMP-2850 Series | Tablet | polyvinylpyrrolidone and thiamine hydrochloride | [156] |
| 12. | PIXDRO JS 20 | Orodispersible films | hydrochlorothiazide and enalapril maleate | [157] |
| 13. | TIZ-SFD | Powder particle | terbutaline sulphate | [158] |
| 14. | TIZ-SFD | Powder particle | salbutamol sulphate | [159] |
| SN | Antibiotic | Thermal Inkjet Printed MIC(μg/ mL) | Broth Microdilution MIC(μg/mL) |
|---|---|---|---|
| 1. | Amoxicillin | 0.20 | 0.5 |
| 0.23 | 0.5 | ||
| 0.15 | 0.5 | ||
| 0.19 | 0.5 | ||
| 2. | Ampicillin | 0.12 | 0.25 |
| 0.12 | 0.25 | ||
| 0.15 | 0.25 | ||
| 3. | Doxycycline | 0.29 | 1 |
| 0.31 | 1 | ||
| 0.29 | 1 | ||
| 0.35 | 1 | ||
| 4. | Tetracycline | 0.59 | 2 |
| 0.55 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, M.J.; Hassan, J.; Douroumis, D. Thermal Inkjet Printing: Prospects and Applications in the Development of Medicine. Technologies 2022, 10, 108. https://doi.org/10.3390/technologies10050108
Uddin MJ, Hassan J, Douroumis D. Thermal Inkjet Printing: Prospects and Applications in the Development of Medicine. Technologies. 2022; 10(5):108. https://doi.org/10.3390/technologies10050108
Chicago/Turabian StyleUddin, Md Jasim, Jasmin Hassan, and Dennis Douroumis. 2022. "Thermal Inkjet Printing: Prospects and Applications in the Development of Medicine" Technologies 10, no. 5: 108. https://doi.org/10.3390/technologies10050108
APA StyleUddin, M. J., Hassan, J., & Douroumis, D. (2022). Thermal Inkjet Printing: Prospects and Applications in the Development of Medicine. Technologies, 10(5), 108. https://doi.org/10.3390/technologies10050108










