Study of Structural, Strength, and Thermophysical Properties of Li2+4xZr4−xO3 Ceramics
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
- The proposed conditions to obtain Li2ZrO3 ceramics with different contents of the initial components xLiClO4·3H2O and (1−x)ZrO2 (x = 0.1–0.5) at an annealing temperature of 1500 °C make it possible to obtain highly ordered pure ceramics with a monoclinic phase (Li2ZrO3) and a density close to the theoretical value (95–97%).
- The decrease in the crystal lattice parameters for the Li2ZrO3 monoclinic phase is due to the substitution of zirconium ions (atomic radius = 13.9 Å) by lithium ions (atomic radius = 7.1 Å), and it indicates an increase in lithium concentration in the ceramic structure. At the same time, changes in the crystal lattice parameters depending on the X component concentration are anisotropic.
- A change in the X component content leads to an increase in the dislocation density, a change in which leads to the strengthening of ceramics and an increase in resistance to mechanical stress and cracking.
- An increase in the density of ceramics, as well as a decrease in density, leads to an increase in the thermal conductivity coefficient of 3.5–7%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Tu, J.; Yang, X.; Gui, N. A review of pebble flow study for pebble bed high temperature gas-cooled reactor. Exp. Comput. Multiph. Flow 2019, 1, 159–176. [Google Scholar] [CrossRef]
- Piazza, G.; Reimann, J.; Knitter, E.; Roux, N.; Lulewicz, J.D. Characterisation of ceramic breeder materials for the helium cooled pebble bed blanket. J. Nucl. Mater. 2002, 307, 811–816. [Google Scholar] [CrossRef]
- Moscato, I.; Barucca, L.; Ciattaglia, S.; D’Aleo, F.; Di Maio, P.A.; Federici, G.; Tarallo, A. Progress in the design development of EU DEMO helium-cooled pebble bed primary heat transfer system. Fusion Eng. Des. 2019, 146, 2416–2420. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, J.; Tang, G.; Gledenov, Y.M.; Sedysheva, M.; Khuukhenkhuu, G. Measurement of differential and angle-integrated cross sections of the 6Li (n, t) 4He reaction in the MeV neutron energy range. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 566, 615–621. [Google Scholar] [CrossRef]
- Biersack, J.P.; Fink, D. Observation of the blocking effect after 6Li (n, t) 4He reactions with thermal neutrons. Nucl. Instrum. Methods 1973, 108, 397–399. [Google Scholar] [CrossRef]
- Dang, C.; Yang, M.; Gong, Y.; Feng, L.; Wang, H.; Shi, Y.; Shi, Q.; Qi, J.; Lu, T. A promising tritium breeding material: Nanostructured 2Li2TiO3-Li4SiO4 biphasic ceramic pebbles. J. Nucl. Mater. 2018, 500, 265–269. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, Y.; Mi, Y.; Xiang, M.; Zhang, Y. Fabrication and characterization of Li2TiO3 core–shell pebbles with enhanced lithium density. J. Nucl. Mater. 2014, 445, 111–116. [Google Scholar] [CrossRef]
- Chen, R.; Yang, M.; Shi, Y.; Wang, H.; Guo, H.; Zeng, Y.; Qi, J.; Shi, Q.; Liao, Z.; Lu, T. Development of an advanced core-shell ceramic pebble with Li4TiO4 pure phase core and Li2TiO3 nanostructured shell by a physical coating method. J. Nucl. Mater. 2019, 520, 252–257. [Google Scholar] [CrossRef]
- Xiao, C.; Gao, X.; Kobayashi, M.; Kawasaki, K.; Uchimura, H.; Toda, K.; Kang, C.; Chen, X.; Wang, H.; Peng, S.; et al. Tritium release kinetics in lithium orthosilicate ceramic pebbles irradiated with low thermal-neutron fluence. J. Nucl. Mater. 2013, 438, 46–50. [Google Scholar] [CrossRef]
- Leys, J.M.; Zarins, A.; Cipa, J.; Baumane, L.; Kizane, G.; Knitter, R. Radiation-induced effects in neutron-and electron-irradiated lithium silicate ceramic breeder pebbles. J. Nucl. Mater. 2020, 540, 152347. [Google Scholar] [CrossRef]
- Shlimas, D.I.; Zdorovets, M.V.; Kozlovskiy, A.L. Synthesis and resistance to helium swelling of Li2TiO3 ceramics. J. Mater. Sci. Mater. Electron. 2020, 3, 12903–12912. [Google Scholar] [CrossRef]
- Blynskiy, P.; Chikhray, Y.; Kulsartov, T.; Gabdullin, M.; Zaurbekova, Z.; Kizane, G.; Kenzhin, Y.; Tolenova, A.; Nesterov, E.; Shaimerdenov, A. Experiments on tritium generation and yield from lithium ceramics during neutron irradiation. Int. J. Hydrogen Energy 2021, 46, 9186–9192. [Google Scholar] [CrossRef]
- Kulsartov, T.; Tazhibayeva, I.; Gordienko, Y.; Chikhray, E.; Tsuchiya, K.; Kawamura, H.; Kulsartova, A. Study of tritium and helium release from irradiated lithium ceramics Li2TiO3. Fusion Sci. Technol. 2011, 60, 1139–1142. [Google Scholar] [CrossRef]
- Ba, J.; Zeng, R.; Yan, X.; Li, R.; Wu, W.; Li, F.; Xiang, X.; Meng, D.; Tang, T. Long-term helium bubble evolution in sequential He+ and H+ irradiated Li4SiO4. Ceram. Int. 2021, 47, 32310–32317. [Google Scholar] [CrossRef]
- Zhao, M.; Memon, M.Z.; Ji, G.; Yang, X.; Vuppaladadiyama, A.K.; Song, Y. Alkali metal bifunctional catalyst sorbents enabled biomass pyrolysis for enhanced hydrogen production. In this study, Li2ZrO3, Li4SiO4, and Na2ZrO3 were selected as catalystsorbent bifunctional materials to enhance the pyrolysis of sawdust. TIDEE TERI Inf. Dig. Energy Environ. 2021, 20, 52. [Google Scholar]
- Roux, N.; Abassin, J.J.; Briec, M.; Cruz, D.; Flament, T.; Schuster, I. Compatibility behavior of beryllium with LiAlO2 and Li2ZrO3 ceramics, with 316 L and 1.4914 steels in SIBELIUS. J. Nucl. Mater. 1992, 191, 168–172. [Google Scholar] [CrossRef]
- Ma, J.; Fu, Z.; Liu, P.; Tang, X. Microwave dielectric properties of low-fired Li2ZrO3–ZnO composite ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 232–236. [Google Scholar] [CrossRef]
- Avila, R.E.; Peña, L.A.; Jiménez, J.C. Surface desorption and bulk diffusion models of tritium release from Li2TiO3 and Li2ZrO3 pebbles. J. Nucl. Mater. 2010, 405, 244–251. [Google Scholar] [CrossRef]
- Rasneur, B.; Thevenot, G.; Bouilloux, Y. Irradiation behavior of LiAlO2 and Li2ZrO3 ceramics in the ALICE 3 experiment. J. Nucl. Mater. 1992, 191–194, 243–247. [Google Scholar] [CrossRef]
- Hoshino, T. Pebble fabrication of super advanced tritium breeders using a solid solution of Li2+xTiO3+y with Li2ZrO3. Nucl. Mater. Energy 2016, 9, 221–226. [Google Scholar] [CrossRef]
- Yasnó, J.P.; Conconi, S.; Visintin, A.; Suárez, G. Non-isothermal reaction mechanism and kinetic analysis for the synthesis of monoclinic lithium zirconate (m-Li2ZrO3) during solid-state reaction. J. Anal. Sci. Technol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Yang, M.; Gong, Y.; Ran, G.; Wang, H.; Chen, R.; Huang, Z.; Shi, Q.; Chen, X.; Lu, T.; Xiao, C. Tritium release behavior of Li4SiO4 and Li4SiO4+ 5 mol% TiO2 ceramic pebbles with small grain size. J. Nucl. Mater. 2019, 514, 284–289. [Google Scholar] [CrossRef]
- Yang, M.; Ran, G.; Wang, H.; Dang, C.; Huang, Z.; Chen, X.; Lu, T.; Xiao, C. Fabrication and tritium release property of Li2TiO3-Li4SiO4 biphasic ceramics. J. Nucl. Mater. 2018, 503, 151–156. [Google Scholar] [CrossRef]
- Kulsartov, T.; Zaurbekova, Z.; Knitter, R.; Shaimerdenov, A.; Chikhray, Y.; Askerbekov, S.; Akhanov, A.; Kenzhina, I.; Kizane, G.; Kenzhin, Y.; et al. Studies of two-phase lithium ceramics Li4SiO4-Li2TiO3 under conditions of neutron irradiation. Nucl. Mater. Energy 2022, 30, 101129. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, J.; Zhou, Q.; Zhang, Y.; Zhao, M.; Gu, S.; Nakata, M.; Zhou, H.; Oya, Y.; Luo, G.-N. Comparison of tritium release behavior in Li2TiO3 and promising core-shell Li2TiO3–Li4SiO4 biphasic ceramic pebbles. J. Nucl. Mater. 2020, 539, 152330. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, L.; Ran, G.; Gong, Y.; Wang, H.; Peng, S.; Xiao, C.; Lu, T. Tritium release behavior of Li2TiO3 and 2Li2TiO3-Li4SiO4 biphasic ceramic pebbles fabricated by microwave sintering. Fusion Eng. Des. 2021, 168, 112390. [Google Scholar] [CrossRef]
- Zhou, Q.; Togari, A.; Nakata, M.; Zhao, M.; Sun, F.; Xu, Q.; Oya, Y. Release kinetics of tritium generation in neutron irradiated biphasic Li2TiO3–Li4SiO4 ceramic breeder. J. Nucl. Mater. 2019, 522, 286–293. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Kozlovskiy, A.L.; Abyshev, B.; Yansepbayev, T.A.; Uzbekgaliyev, R.U.; Shlimas, D.I. Study of Phase Formation Processes in Li2ZrO3 Ceramics Obtained by Mechanochemical Synthesis. Crystals 2022, 12, 21. [Google Scholar] [CrossRef]
- Kuzovkov, V.N.; Popov, A.I.; Kotomin, E.A.; Monge, M.A.; Gonzales, R.; Chen, Y. Kinetics of nanocavity formation based on F-center aggregation in thermochemically reduced MgO single crystals. Phys. Rev. B 2001, 64, 064102. [Google Scholar] [CrossRef]
- Lushchik, A.; Feldbach, E.; Kotomin, E.A.; Kudryvtseva, I.; Kuzovkov, V.N.; Popov, A.I.; Seeman, V.; Shablonin, E. Distinctive features of diffusion-controlled radiation defect recombination in stoichiometric magnesium aluminate spinel single crystals and transparent polycrystalline ceramics. Sci. Rep. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Kuzovkov, V.N.; Popov, A.I. The kinetics of defect aggregation and metal colloid formation in ionic solids under irradiation. Radiat. Eff. Defects Solids 2001, 155, 113–125. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A.I. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MgAl2O4 Ceramics. Nanomaterials 2021, 11, 3373. [Google Scholar] [CrossRef] [PubMed]
- Klym, H.; Ingram, A.; Shpotyuk, O.; Hadzaman, I.; Solntsev, V.; Hotra, O.; Popov, A.I. Positron annihilation characterization of free volume in micro-and macro-modified Cu0.4Co0.4Ni0.4Mn1.8O4 ceramics. Low Temp. Phys. 2016, 42, 601–605. [Google Scholar] [CrossRef][Green Version]
- Heiba, Z.; El-Sayed, K. Structural and anisotropic thermal expansion correlation of Li2ZrO3 at different temperatures. J. Appl. Crystallogr. 2002, 35, 634–636. [Google Scholar] [CrossRef]
- Rao, J.G.; Mazumder, R.; Bhattacharyya, S.; Chaudhuri, P. Fabrication of Li4SiO4-Li2ZrO3 composite pebbles using extrusion and spherodization technique with improved crush load and moisture stability. J. Nucl. Mater. 2018, 514, 321–333. [Google Scholar]
- Leys, O.; Kolb, M.H.H.; Pucci, A.; Knitter, R. Study of lithium germanate additions to advanced ceramic breeder pebbles. J. Nucl. Mater. 2019, 518, 234–240. [Google Scholar] [CrossRef]
- Enoeda, M.; Ohara, Y.; Roux, N.; Ying, A.; Pizza, G.; Malang, S. Effective Thermal Conductivity Measurement of the Candidate Ceramic Breeder Pebble Beds by the Hot Wire Method. Fusion Technol. 2001, 39, 612–616. [Google Scholar] [CrossRef]

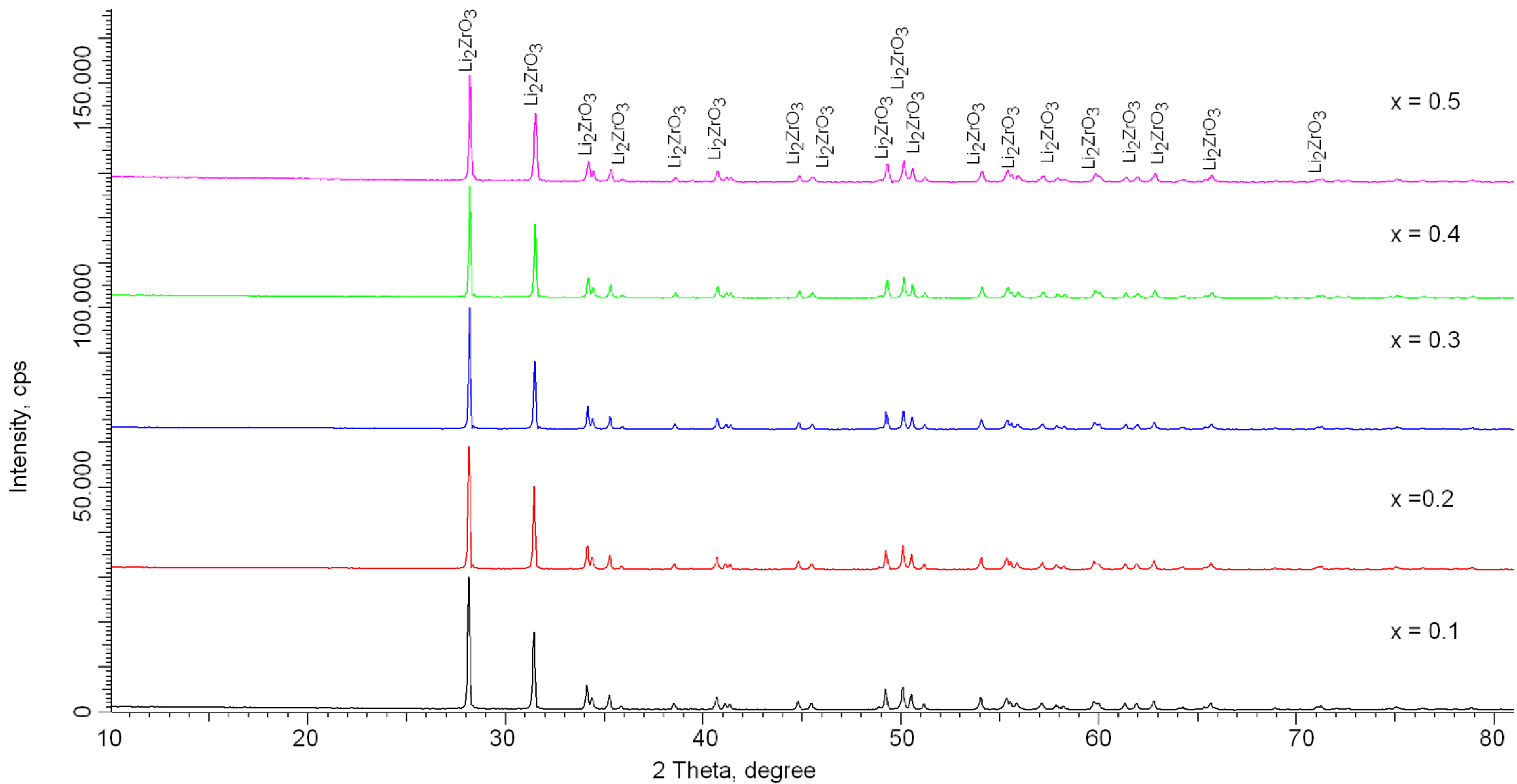



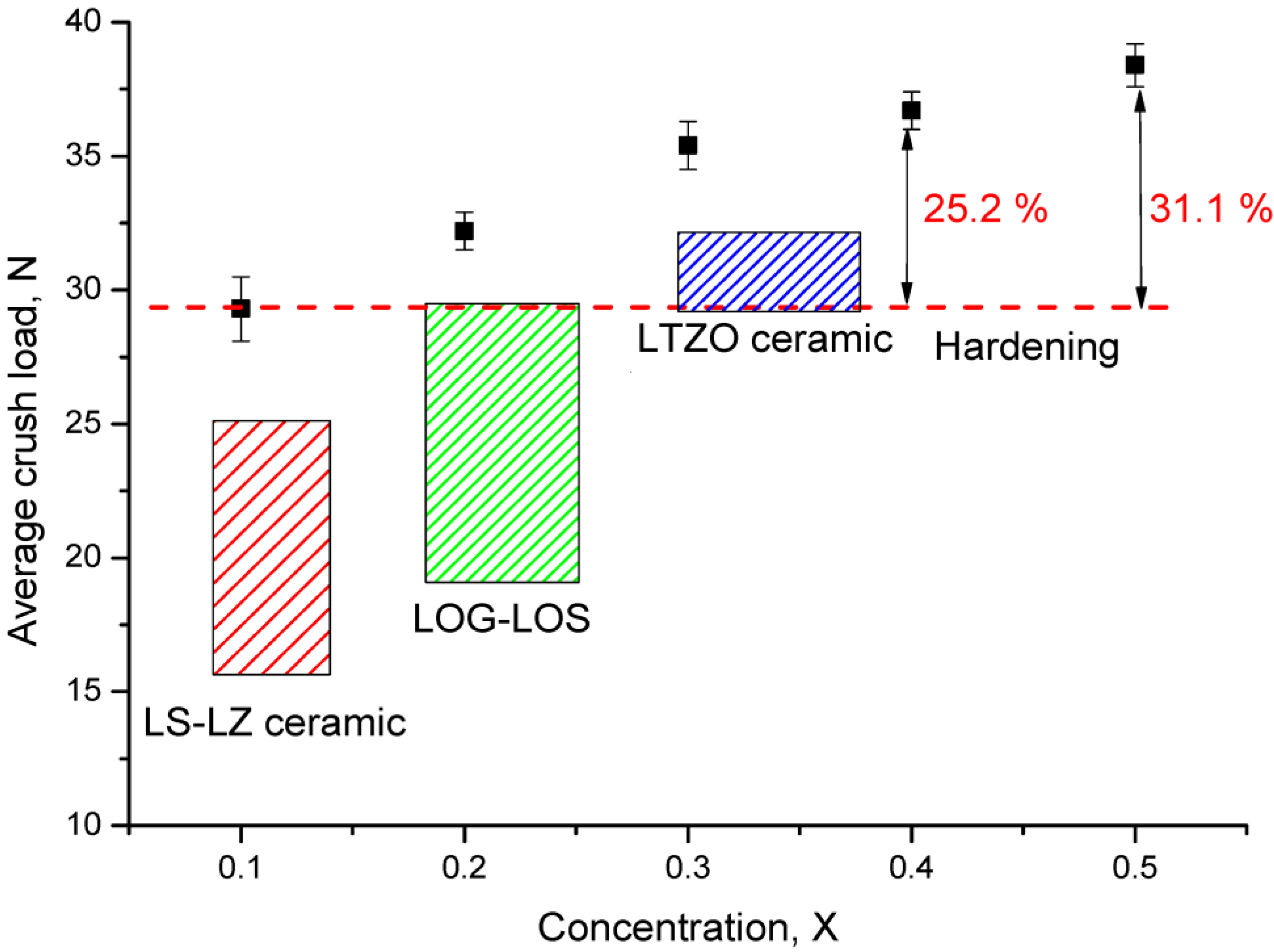
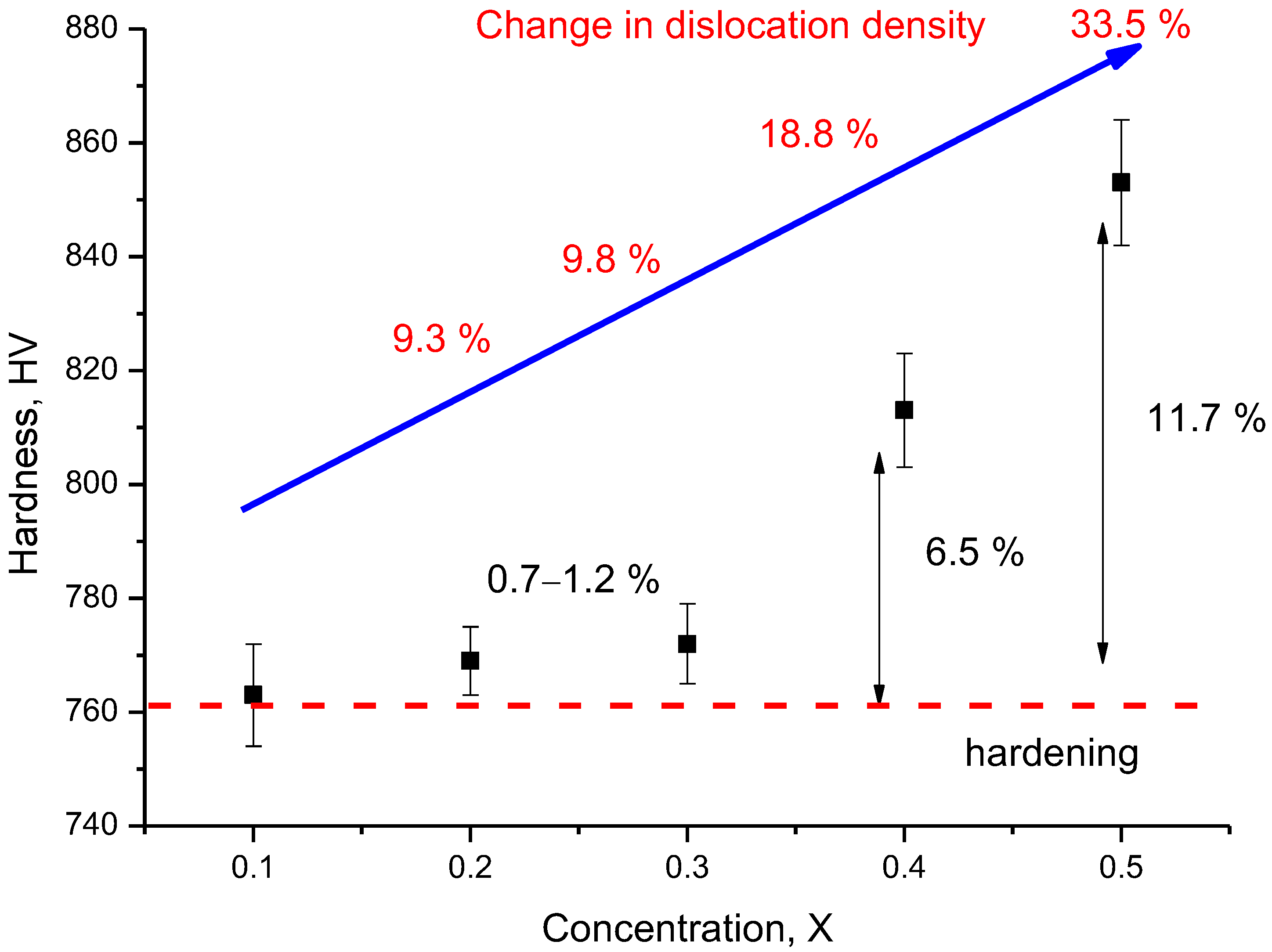

| Parameter | Content of LiClO4·3H2O Component | ||||
|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| Li2ZrO3 monoclinic phase (PDF-01-070-8744) (a0 = 5.42660 Å, b0 = 9.03100 Å, c0 = 5.42270 Å, β0 = 112.720°, V0 = 245.13 Å3) Crystal lattice parameters, Å | a = 5.3915 ± 0.0011, b = 9.0363 ± 0.0014, c = 5.4216 ± 0.0021, β = 112.609° | a = 5.3829 ± 0.0017, b = 9.0291 ± 0.0022, c = 5.4173 ± 0.0024, β = 112.564° | a = 5.3786 ± 0.0018, b = 9.0061 ± 0.0015, c = 5.4056 ± 0.0022, β = 112.474° | a = 5.3691 ± 0.0016, b = 8.9989 ± 0.0013, c = 5.3855 ± 0.0014, β = 112.384° | a = 5.3617 ± 0.0015, b = 8.9901 ± 0.0023, c = 5.3761 ± 0.0017, β = 112.010° |
| Crystal volume, Å3 | 243.84 ± 0.19 | 243.14 ± 0.16 | 241.96 ± 0.12 | 240.61 ± 0.15 | 240.25 ± 0.14 |
| Crystallinity degree, % (structural ordering value) | 90.2 ± 0.4 | 89.3 ± 0.5 | 88.7 ± 0.3 | 88.6 ± 0.5 | 88.0 ± 0.7 |
| Crystallite size, nm (size determined using the Scherer equation) | 80.2 ± 2.5 | 76.4 ± 2.6 | 76.3 ± 2.8 | 72.2 ± 2.4 | 65.5 ± 2.1 |
| Dislocation density, 1010 1/cm2 | 0.155 | 0.171 | 0.172 | 0.191 | 0.233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlovskiy, A.L.; Abyshev, B.; Shlimas, D.I.; Zdorovets, M.V. Study of Structural, Strength, and Thermophysical Properties of Li2+4xZr4−xO3 Ceramics. Technologies 2022, 10, 58. https://doi.org/10.3390/technologies10030058
Kozlovskiy AL, Abyshev B, Shlimas DI, Zdorovets MV. Study of Structural, Strength, and Thermophysical Properties of Li2+4xZr4−xO3 Ceramics. Technologies. 2022; 10(3):58. https://doi.org/10.3390/technologies10030058
Chicago/Turabian StyleKozlovskiy, Artem L., Bauyrzhan Abyshev, Dmitriy I. Shlimas, and Maxim V. Zdorovets. 2022. "Study of Structural, Strength, and Thermophysical Properties of Li2+4xZr4−xO3 Ceramics" Technologies 10, no. 3: 58. https://doi.org/10.3390/technologies10030058
APA StyleKozlovskiy, A. L., Abyshev, B., Shlimas, D. I., & Zdorovets, M. V. (2022). Study of Structural, Strength, and Thermophysical Properties of Li2+4xZr4−xO3 Ceramics. Technologies, 10(3), 58. https://doi.org/10.3390/technologies10030058







