Precision of Medication Therapy Problem Identification and Classification amongst Primary Care Clinic Pharmacists
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Evaluation Strategy and Data Collection
2.3. Outcomes
2.4. Case Vignettes
2.5. Statistical Analysis
3. Results
3.1. Participant Demographics
3.2. Primary Outcomes
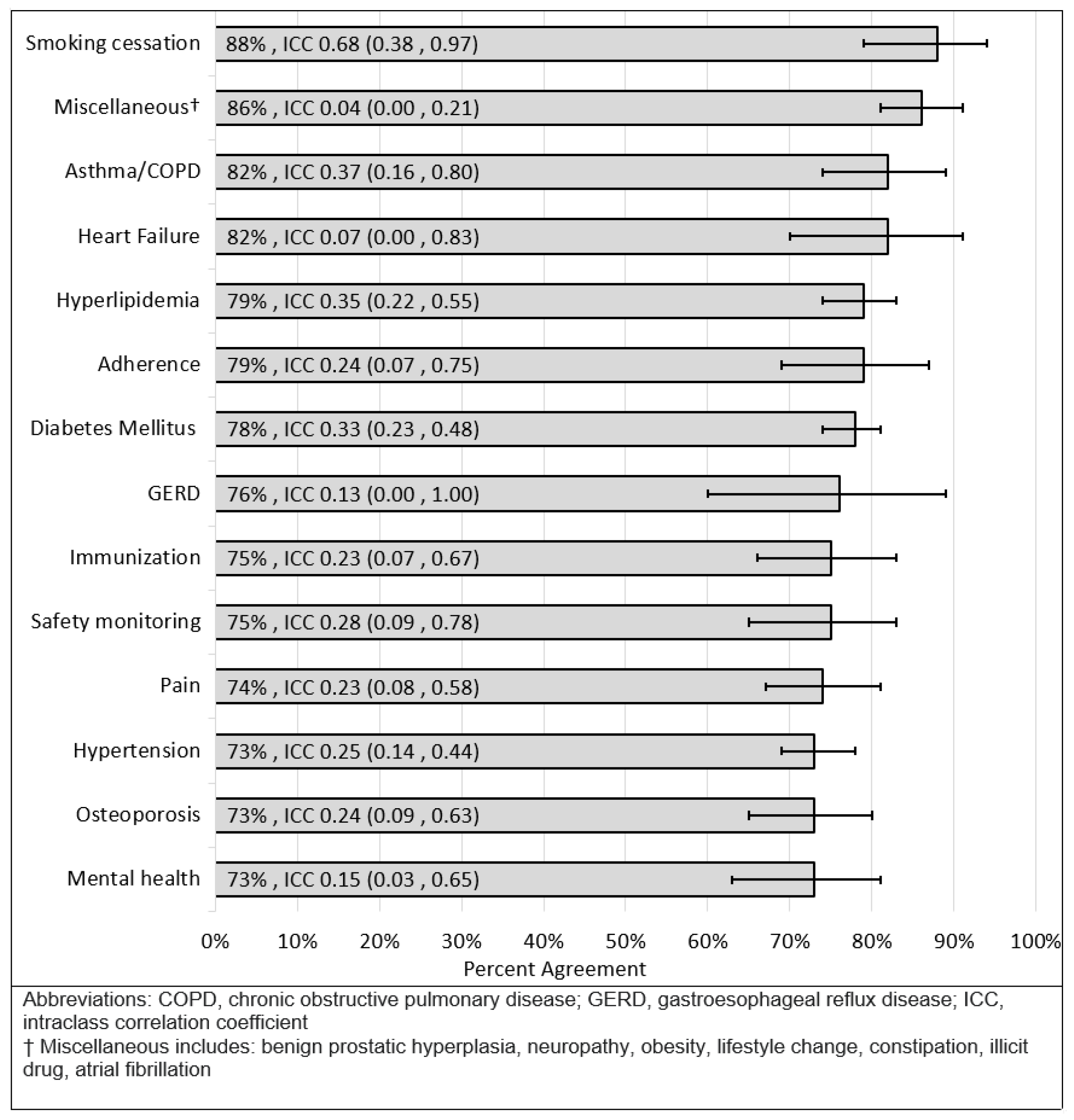
3.3. Secondary Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Informing the Future: Critical Issues in Health, 4th ed.; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Isetts, B.J.; Brummel, A.R.; de Oliveira, D.R.; Moen, D.W. Managing Drug-related Morbidity and Mortality in the Patient-centered Medical Home. Med. Care 2012, 50, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.H.; McInnis, T.; Hirsch, J.D. Cost of Prescription Drug–Related Morbidity and Mortality. Ann. Pharmacother. 2018, 52, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.E. What Safety Events Are Reported For Ambulatory Care? Analysis of Incident Reports from a Patient Safety Organization. Jt. Comm. J. Qual. Patient Saf. 2021, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- American College of Clinical Pharmacy. Comprehensive Medication Management in Team-Based Care. Available online: https://www.accp.com/docs/positions/misc/CMM%20Brief.pdf (accessed on 9 September 2019).
- Ramalho de Oliveira, D.; Brummel, A.R.; Miller, D.B. Medication Therapy Management: 10 Years of Experience in a Large Integrated Health Care System. J. Manag. Care Pharm. 2010, 16, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolle, R.J.; Strand, L.M.; Morley, P.C. Pharmaceutical Care Practice: The Patient-Centered Approach to Medication Management, 3rd ed.; Mcgraw-Hill: New York, NY, USA, 2012; p. 157. [Google Scholar]
- PQA. MTP Categories Framework. Pharmacy Quality Alliance. Available online: https://www.pqaalliance.org/assets/Measures/PQA_MTP_Categories_Framework.pdf (accessed on 30 March 2021).
- McClurg, M.R. CMM in Primary Care Research Team Co-Principal Investigators. Available online: https://www.accp.com/docs/positions/misc/CMM_Care_Process.pdf (accessed on 9 September 2019).
- Isetts, B.J.; Schondelmeyer, S.W.; Artz, M.B.; Lenarz, L.A.; Heaton, A.H.; Wadd, W.B.; Brown, L.M.; Cipolle, R.J. Clinical and economic outcomes of medication therapy management services: The Minnesota experience. J. Am. Pharm. Assoc. 2008, 48, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, M.; Kahwati, L.C.; Golin, C.E.; Blalock, S.J.; Coker-Schwimmer, E.; Posey, R.; Lohr, K.N. Medication Therapy Management Interventions in Outpatient Settings: A Systematic Review and Meta-analysis. JAMA Int. Med. 2015, 175, 76. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.; Buu, J.; Kuzel, M.; Van Wagoner, E.; Berrett, G. Early Implementation of Comprehensive Medication Management within an Academic Medical Center Primary Care Pharmacy Team. Innov. Pharm. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, C.; Livet, M.; Ward, C.; Sorge, L.; Sorensen, T.D.; McClurg, M.R. The Active Implementation Frameworks: A roadmap for advancing implementation of Comprehensive Medication Management in Primary care. Res. Soc. Adm. Pharm. 2017, 13, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Livet, M.; Blanchard, C.; Frail, C.; Sorensen, T.; McClurg, M.R. Ensuring effective implementation: A fidelity assessment system for comprehensive medication management. J. Am. Coll. Clin. Pharm. 2020, 3, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Livet, M.; Blanchard, C.; Sorensen, T.D.; Roth McClurg, M. An implementation system for medication optimization: Operationalizing comprehensive medication management delivery in primary care. J. Am. Coll. Clin. Pharm. 2018, 1, 14–20. [Google Scholar] [CrossRef]
- LaFleur, J.; Larson, B.S.; Gunning, K.M.; Stoddard, G.J.; Madden, C.; Oderda, L.; Steinvoort, C.; Oderda, G.M. Agreement between Pharmacists for Problem Identification: An Initial Quality Measurement of Cognitive Services. Ann. Pharmacother. 2009, 43, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- The Data Collection for This Paper Was Generated Using Qualtrics Software, Version 10/2019 of Qualtrics. Copyright© 2021 Qualtrics. Qualtrics and All Other Qualtrics Product or Service Names Are Registered Trademarks or Trademarks of Qualtrics, Provo, UT, USA. Available online: https://www.Qualtrics.Com (accessed on 10 February 2021).
- Rao, D.; Gilbert, A.; Strand, L.M.; Cipolle, R.J. Drug therapy problems found in ambulatory patient populations in Minnesota and South Australia. Pharm. World Sci. 2007, 29, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Isetts, B.J.; Brown, L.M.; Schondelmeyer, S.W.; Lenarz, L.A. Quality Assessment of a Collaborative Approach for Decreasing Drug-Related Morbidity and Achieving Therapeutic Goals. Arch. Int. Med. 2003, 163, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1997, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Bonett, D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef] [PubMed]

| Medication Related Need 1 | Medication Therapy Problem (MTP) 1 | Prevalence Observed in Other Populations 2 | Planned Prevalence (Simulated Sample) | Targeted # of Cases with Given MTP (out of 20 Cases) |
|---|---|---|---|---|
| Indication | Unnecessary drug therapy | 5.7–19.8% | 20% | 4 |
| Needs additional drug therapy | 15.9–31.4% | 30% | 6 | |
| Effectiveness | Ineffective medication | 4.7–15.7% | 5% | 1 |
| Dosage too low | 13.7–26.1% | 25% | 5 | |
| Needs additional monitoring | - | 5% | 1 | |
| Safety | Adverse drug reaction | 8.3–17.0% | 15% | 3 |
| Dosage too high | 5.8–6.7% | 5% | 1 | |
| Needs additional monitoring | - | 5% | 1 | |
| Adherence | Adherence | 16.4–31.7% | 20% | 4 |
| Cost | - | 5% | 1 |
| Kappa (ICC) | Interpretation |
|---|---|
| <0 | Poor agreement |
| 0–0.20 | Slight agreement |
| 0.21–0.40 | Fair agreement |
| 0.41–0.60 | Moderate agreement |
| 0.61–0.80 | Substantial agreement |
| 0.81–1.00 | Almost perfect agreement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, N.; Ashby, B.; Winter, B.; Stoddard, G.; LaFleur, J.; Berrett, G.B.; Turner, K. Precision of Medication Therapy Problem Identification and Classification amongst Primary Care Clinic Pharmacists. Pharmacy 2021, 9, 179. https://doi.org/10.3390/pharmacy9040179
Cox N, Ashby B, Winter B, Stoddard G, LaFleur J, Berrett GB, Turner K. Precision of Medication Therapy Problem Identification and Classification amongst Primary Care Clinic Pharmacists. Pharmacy. 2021; 9(4):179. https://doi.org/10.3390/pharmacy9040179
Chicago/Turabian StyleCox, Nicholas, Bryce Ashby, Bradly Winter, Gregory Stoddard, Joanne LaFleur, G. Benjamin Berrett, and Kyle Turner. 2021. "Precision of Medication Therapy Problem Identification and Classification amongst Primary Care Clinic Pharmacists" Pharmacy 9, no. 4: 179. https://doi.org/10.3390/pharmacy9040179
APA StyleCox, N., Ashby, B., Winter, B., Stoddard, G., LaFleur, J., Berrett, G. B., & Turner, K. (2021). Precision of Medication Therapy Problem Identification and Classification amongst Primary Care Clinic Pharmacists. Pharmacy, 9(4), 179. https://doi.org/10.3390/pharmacy9040179






