Validation Assessment of a Pain Interference Questionnaire among Student Pharmacists
Abstract
1. Introduction
2. Materials and Methods
2.1. Pain Interference Scale
2.2. Content Validity
2.3. Construct Validity
2.4. Scale Functioning
2.5. Reliability
3. Results
3.1. Content Validity
3.2. Construct Validity
3.3. Scale Function
3.4. Reliability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine (US) Committie on Advancing Pain Research, Care and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research; National Academics Press: Washington, DC, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK91497/ (accessed on 25 August 2021).
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Preva-lence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States. Morb. Mortal. Wkly. Rep. 2016, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, C.E.; Dahlhamer, J.M.; Lucas, J.W.; Connor, E.M. Chronic Pain and High-impact Chronic Pain Among U.S. Adults, 2019. NCHS Data Brief 2020, 390, 1–8. [Google Scholar]
- Karayannis, N.; Sturgeon, J.; Chih-Kao, M.; Cooley, C.; Mackey, S.C. Pain interference and physical function demonstrate poor longitudinal association in people living with pain: A PROMIS investigation. Pain 2017, 158, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Bann, C.; Dodd, S.L.; Schein, J.; Mendoza, T.R.; Cleeland, C.S. Validity of the Brief Pain Inventory for Use in Documenting the Outcomes of Patients with Noncancer Pain. Clin. J. Pain 2004, 20, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.I.; Schink, J.; Bass, M.; Patel, S.; Diaz, M.V.; Rothrock, N.; Pearman, T.; Gershon, R.; Penedo, F.J.; Rosen, S.; et al. Bringing PROMIS to practice: Brief and precise symptom screening in ambulatory cancer care. Cancer 2015, 121, 927–934. [Google Scholar] [CrossRef]
- Taylor, A.M.; Phillips, K.; Patel, K.V.; Turk, D.C.; Dworkin, R.H.; Beaton, D.; Clauw, D.J.; Gignac, M.A.; Markman, J.D.; Williams, D.A.; et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain 2016, 157, 1836–1850. [Google Scholar] [CrossRef]
- Zhao, X.; Shah, D.; Gandhi, K.; Wei, W.; Dwibedi, N.; Webster, L.; Sambamoorthi, U. The association of pain interference and opioid use with healthcare utilization and costs, and wage loss among adults with osteoarthritis in the United States. J. Med. Econ. 2019, 22, 1192–1201. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A. For the Clinical Guidelines Committee of the American College of Physicians Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef]
- Axon, D.R.; Bhattacharjee, S.; Warholak, T.L.; Slack, M.K. Xm2Scores for Estimating Total Exposure to Multimodal Strategies Identified by Pharmacists for Managing Pain: Validity Testing and Clinical Relevance. Pain Res. Manag. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Axon, D.R.; Patel, M.J.; Martin, J.; Slack, M.K. Use of multidomain management strategies by community dwelling adults with chronic pain: Evidence from a systematic review. Scand. J. Pain 2019, 19, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Axon, D.R.; Hernandez, C.; Lee, J.; Slack, M. An Exploratory Study of Student Pharmacists’ Self-Reported Pain, Management Strategies, Outcomes, and Implications for Pharmacy Education. Pharmacy 2018, 6, 11. [Google Scholar] [CrossRef]
- Askew, R.L.; Cook, K.F.; Revicki, D.A.; Cella, D.; Amtmann, D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J. Clin. Epidemiol. 2016, 73, 103–111. [Google Scholar] [CrossRef]
- Johnson, A.M.; Smith, S.M. A review of general pain measurement tools and instruments for consideration of use in COPD clinical practice. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Matza, L.S.; Fallowfield, L.; Chung, K.C.; Currie, B.M.; Van Brunt, K.; Patrick, D.L. Patient-reported outcome instruments used to assess pain and functioning in studies of bisphosphonate treatment for bone metastases. Support. Care Cancer 2012, 20, 657–677. [Google Scholar] [CrossRef] [PubMed]
- Mchorney, C.A.; Johne, W.; Anastasiae, R. The MOS 36-Item Short-Form Health Survey (SF-36). Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R.; Cairney, J. Health Measurement Scales, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287. [Google Scholar] [CrossRef]
- Wright, B.D.; Masters, G.N. Rating Scale Analysis (Rasch Measurement Series), 1st ed.; Pluribus Press: Chicago, IL, USA, 1982. [Google Scholar]
- A User’s Guide to Winsteps. Available online: https://www.winsteps.com/winman/copyright.htm (accessed on 25 August 2021).
- Linacre, J.M. Investigating rating scale category utility. J. Outcome Meas. 1999, 3, 103–122. [Google Scholar]
- Brogden, H.E. The rasch model, the law of comparative judgment and additive conjoint measurement. Psychometrika 1977, 42, 631–634. [Google Scholar] [CrossRef]
- National Quality Forum. Draft Acceptable Reliability Thresholds. Available online: https://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=95263 (accessed on 25 August 2021).
- Wright, B.; Panchapakesan, N. A Procedure for Sample-Free Item Analysis. Educ. Psychol. Meas. 1969, 29, 23–48. [Google Scholar] [CrossRef]
- Amtmann, D.; Cook, K.F.; Jensen, M.P.; Chen, W.-H.; Choi, S.; Revicki, D.; Cella, D.; Rothrock, N.; Keefe, F.; Callahan, L.; et al. Development of a PROMIS item bank to measure pain interference. Pain 2010, 150, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kappelman, M.D.; Long, M.D.; Martin, C.; DeWalt, D.; Kinneer, P.M.; Chen, W.; Lewis, J.D.; Sandler, R.S. Evaluation of the Patient-Reported Outcomes Measurement Information System in a Large Cohort of Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 1315-1323.e2. [Google Scholar] [CrossRef] [PubMed]
- Reeve, B.B.; Hays, R.D.; Bjorner, J.B.; Cook, K.F.; Crane, P.K.; Teresi, J.A.; Thissen, D.; Revicki, D.A.; Weiss, D.J.; Hambleton, R.K.; et al. Psychometric Evaluation and Calibration of Health-Related Quality of Life Item Banks. Med. Care 2007, 45, S22–S31. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Choi, S.W.; Junghaenel, D.U.; Schwartz, J.; Stone, A.A. Psychometric characteristics of daily diaries for the Patient-Reported Outcomes Measurement Information System (PROMIS®): A preliminary investigation. Qual. Life Res. 2012, 22, 1859–1869. [Google Scholar] [CrossRef][Green Version]
- Instrument: PROMIS Pain Interference- Short Form 6b v1.0. Available online: https://cde.drugabuse.gov/instrument/0a47fbff-5f72-2281-e050-bb89ad4358ae (accessed on 25 August 2021).

| How Much Does Pain Interfere with Your: | Not At All | A Little Bit | Somewhat | Quite A Bit | Very Much |
|---|---|---|---|---|---|
| Daily activities? | ◯ | ◯ | ◯ | ◯ | ◯ |
| Ability to participate in leisure activites? | ◯ | ◯ | ◯ | ◯ | ◯ |
| Ability to work? | ◯ | ◯ | ◯ | ◯ | ◯ |
| Ability to attend school? | ◯ | ◯ | ◯ | ◯ | ◯ |
| Relationships with other people? | ◯ | ◯ | ◯ | ◯ | ◯ |
| Test | Threshold | Pain Interference Items | |||||
|---|---|---|---|---|---|---|---|
| Daily Activities | Leisure Activities | Work | School | Relationships | |||
| Reliability | Cronbach’s Alpha | >0.7 [20] | 0.92 | ||||
| Item Separation and Reliability | Separation/Reliability >2/>0.8 [21] | 2.61/0.87 | |||||
| Item Infit/Outfit | 0.6 < MNSQ < 1.4 −2 < ZSTD < 2 [22] | (1.23/1.16) (1.60/1.16) | (0.94/0.94) (0.37/1.16) | (0.70/0.72) (−2.39/−2.13) | (0.94/0.86) −0.40/−0.87) | (1.30/1.04) (1.85/0.28) | |
| Dimensionality and Item correlation | Raw variance explained by measures | >60% [23] | 64.2% | ||||
| The difference between modeled and empirical raw variance explained by measures | ≈0% [23] | 0.6% | |||||
| Eigen value for the unexplained variance in the 1st contrast | >2 [23] | 2.0 | |||||
| Point-biserial correlations between x and y | >0.2 and positive [23] | 0.84 | 0.85 | 0.90 | 0.83 | 0.77 | |
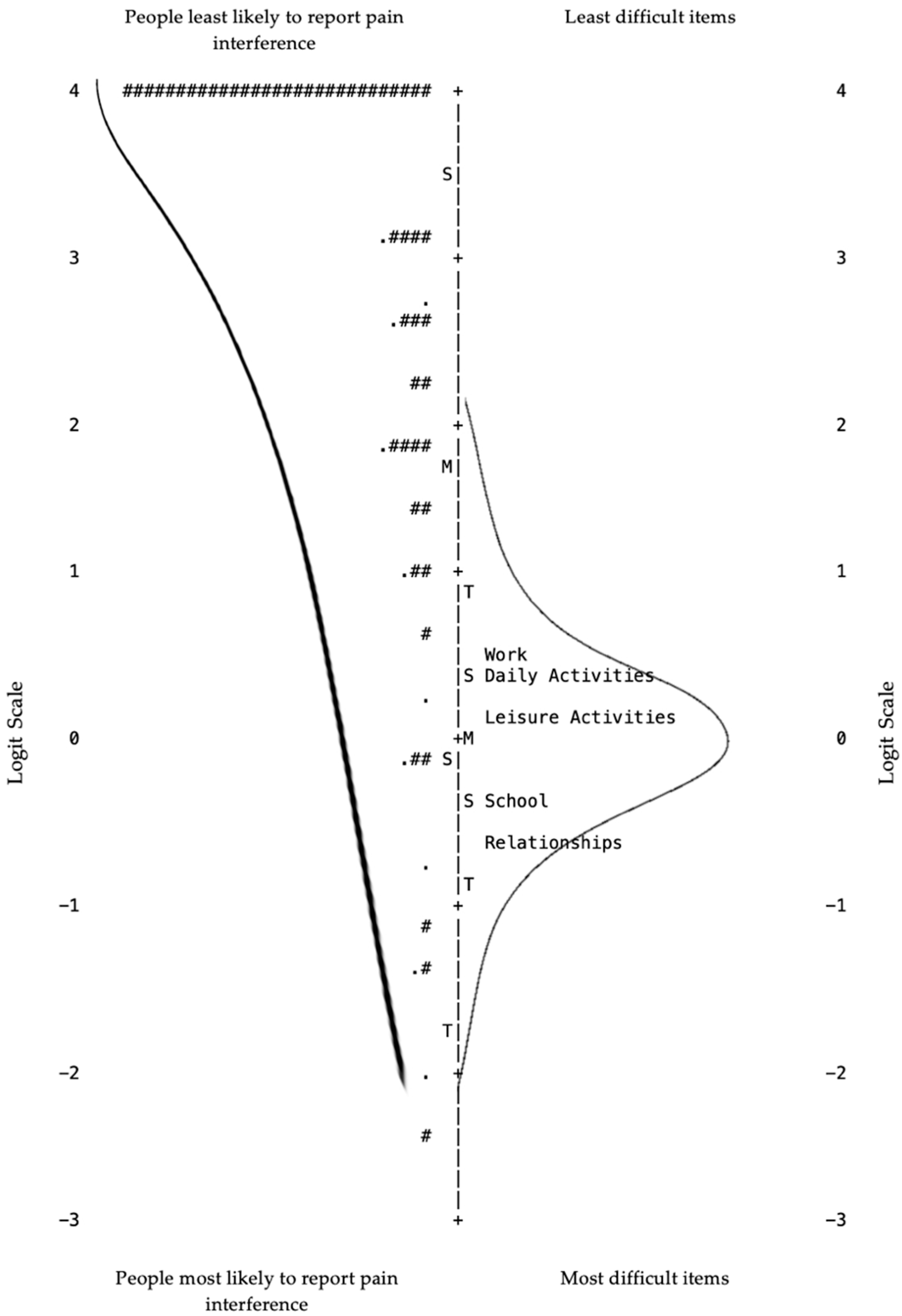
| Content and Criterion Validity | Item-Person Map | Person ability and item difficulty “mirror” each other; assess for ceiling or floor effects [24] | Ceiling effect (See Figure 1. “Item-Person Map”) | ||||
| Redundancy | Items with the same difficulty level [24] | No redundancy (See Figure 1. “Item-Person Map”) | |||||
| Item difficulty gaps | Z-value <2 [24] | Large high difficulty gap (See Figure 1. “Item-Person Map”) | |||||
| Response Options | |||||||
| Very much | Quite a bit | Somewhat | A little bit | Not at all | |||
| Scale function | Frequency response category used | ≥10 per category and regular increase across categories [22] | 21 | 38 | 113 | 153 | 530 |
| Observed average and expected | Advances monotonically [22] | −1.63 | −1.10 | 0.46 | 2.01 | 3.11 | |
| Andrich threshold | Moves from − to 0 to + [22] | REF | −2.05 | −1.33 | 0.97 | 2.41 | |
| Coherence with rating which imply measures and measures which imply ratings | Measures imply ratings/ratings imply measures >40% per category [22] | (33%/10%) | (47%/58%) | (63%/49%) | (49%/68%) | (77%/65%) | |
| Anchor MNSQ | (Infit/Outfit) Anchors (“Very much” and “Not at all”) have largest MNSQ [22] | (1.33/1.35) | (0.93/0.94) | (0.78/0.72) | (0.97/0.87) | (1.20/1.13) | |
| Andrich measures | |1.4| < X < |5| [22] | −3.41 | −1.75 | −0.13 | 1.74 | 3.66 | |
| Probability curves | Advancing, distinct and well separated peaks [24] | Response option 1 is not distinct from 0 or 2; response option 3 is minimally distinct from 2 and 4; advances sequentially (see Figure 2) “Probability Curves” | |||||
| How Much Does Pain Interfere with…? | Very Much n (%) | Quite A Bit n (%) | Somewhat n (%) | A Little Bit n (%) | Not At All n (%) |
|---|---|---|---|---|---|
| Daily activities (n = 171) | 1 (1) | 9 (5) | 32 (19) | 40 (23) | 89 (52) |
| Leisure activities (n = 171) | 5 (3) | 7 (4) | 22 (13) | 34 (20) | 103 (60) |
| Work (n = 170) | 4 (2) | 11 (6) | 25 (15) | 37 (22) | 93 (55) |
| School (n = 171) | 5 (3) | 6 (4) | 19 (11) | 24 (14) | 117 (68) |
| Relationships (n = 172) | 6 (3) | 5 (3) | 15 (9) | 18 (10) | 128 (74) |
| Item # | PROMIS Bank v1.1- Pain Interference Item 1 |
|---|---|
| Painin8 | How much did pain interfere with your ability to concentrate? |
| Painin22 | How much did pain interfere with work around the home? |
| Painin14 | How much did pain interfere with doing your tasks away from home (e.g., getting groceries, running errands)? |
| Painin19 | How much did pain make it difficult to fall asleep? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whaley, M.; Awad, N.B.; Warholak, T.; Axon, D.R. Validation Assessment of a Pain Interference Questionnaire among Student Pharmacists. Pharmacy 2021, 9, 170. https://doi.org/10.3390/pharmacy9040170
Whaley M, Awad NB, Warholak T, Axon DR. Validation Assessment of a Pain Interference Questionnaire among Student Pharmacists. Pharmacy. 2021; 9(4):170. https://doi.org/10.3390/pharmacy9040170
Chicago/Turabian StyleWhaley, Megan, Nouf Bin Awad, Terri Warholak, and David Rhys Axon. 2021. "Validation Assessment of a Pain Interference Questionnaire among Student Pharmacists" Pharmacy 9, no. 4: 170. https://doi.org/10.3390/pharmacy9040170
APA StyleWhaley, M., Awad, N. B., Warholak, T., & Axon, D. R. (2021). Validation Assessment of a Pain Interference Questionnaire among Student Pharmacists. Pharmacy, 9(4), 170. https://doi.org/10.3390/pharmacy9040170







