Systematic Review of l-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Sources and Search Strategy
- (arginine or lady prelox or ArginMax or Stronvivo or Ristela) and
- (hypoactive sexual desire disorder or female sexual interest arousal disorder
- or female sexual arousal disorder or (exp sexual dysfunctions, psychological)
- or sexual dysfunction or sexual behavior or dyspareunia or libido)
2.3. Study Selection
2.4. Data Collection
2.5. Risk of Bias Analysis
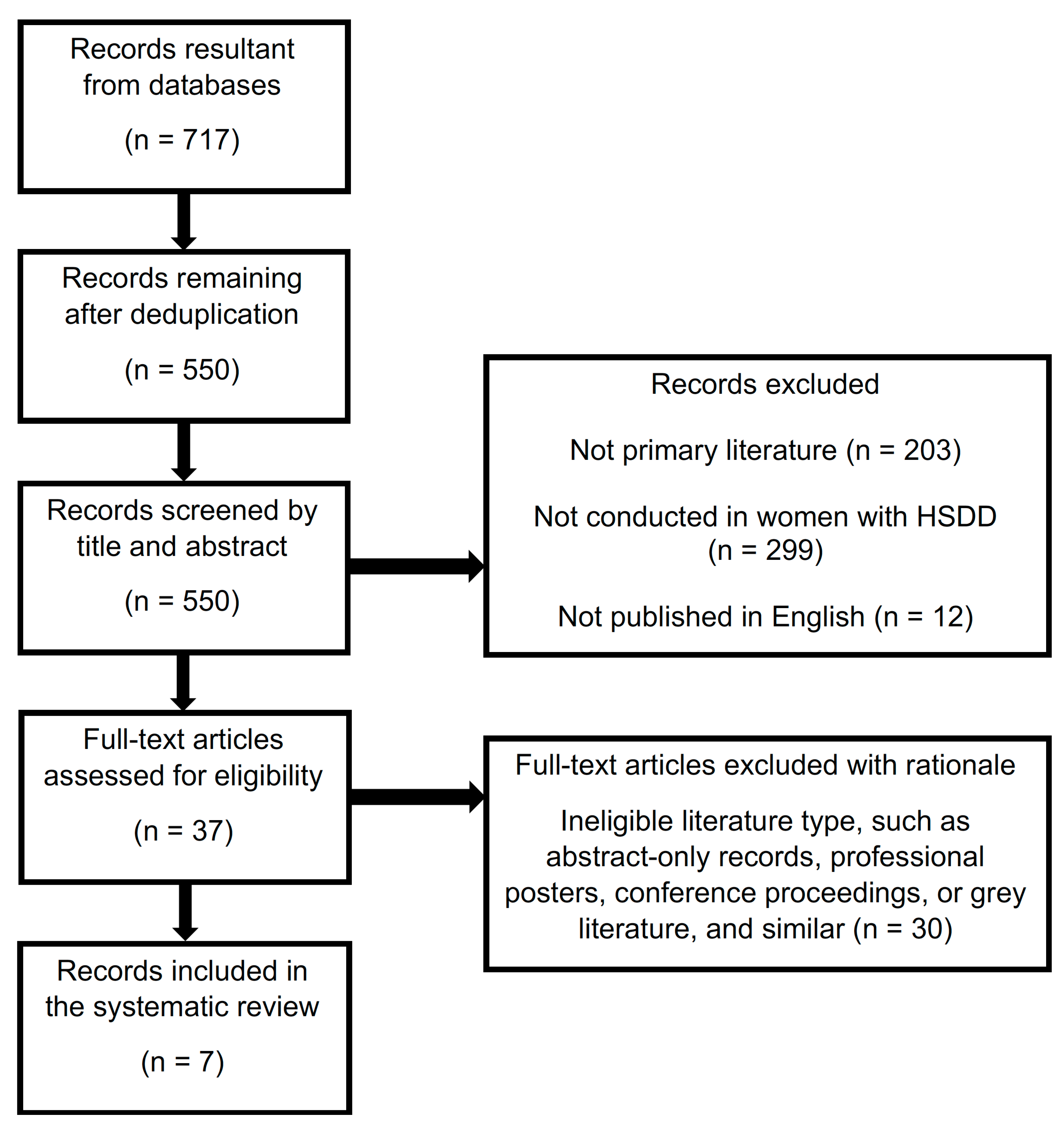
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Revised; (DSM-4-TR); American Psychiatric Association: Washington, DC, USA, 2003. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Clayton, A.H.; Goldstein, I.; Kim, N.N.; Althof, S.E.; Faubion, S.S.; Faught, B.M.; Parish, S.J.; Simon, J.A.; Vignozzi, L.; Christiansen, K.; et al. The International Society for the Study of Women’s Sexual Health Process of Care for Management of Hypoactive Sexual Desire Disorder in Women. Mayo Clin. Proc. 2018, 93, 467–487. [Google Scholar] [CrossRef]
- Balon, R.; Clayton, A.H. Further commentary on DSM-5 FSIAD diagnosis. J. Sex. Med. 2015, 12, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.H.; Derogatis, L.R.; Rosen, R.C. Intended or Unintended Consequences? The Likely Implications of Raising the Bar for Sexual Dysfunction Diagnosis in the Proposed DSM-V Revisions: 2. For Women with Loss of Subjective Sexual Arousal. J. Sex Med. 2012, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Parish, S.J.; Hahn, S.R. Hypoactive Sexual Desire Disorder: A Review of Epidemiology, Biopsychology, Diagnosis, and Treatment. Sex. Med. Rev. 2016, 4, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.K.; West, S.L.; D’Aloisio, A.A.; Wheeler, S.B.; Borisov, N.N.; Thorp, J. Hypoactive Sexual Desire Disorder in Postmenopausal Women: Quality of Life and Health Burden. Value Heal. 2009, 12, 763–772. [Google Scholar] [CrossRef]
- Foley, K.; Foley, D.; Johnson, B.H. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J. Med. Econ. 2010, 13, 583–590. [Google Scholar] [CrossRef]
- Warnock, J.; Jill, K. Female Hypoactive Sexual Desire Disorder: Epidemiology, diagnosis and treatment. CNS Drugs 2002, 16, 745–753. [Google Scholar] [CrossRef]
- Ito, T.Y.; Trant, A.S.; Polan, M.L. A Double-Blind Placebo-Controlled Study of ArginMax, a Nutritional Supplement for Enhancement of Female Sexual Function. J. Sex Marital. Ther. 2001, 27, 541–549. [Google Scholar] [CrossRef]
- Munarriz, R.; Kim, N.N.; Goldstein, I.; Traish, A.M. Biology of female sexual function. Urol. Clin. North Am. 2002, 29, 685–693. [Google Scholar] [CrossRef]
- Revicki, D.A.; Margolis, M.K.; Bush, E.N.; Derogatis, L.R.; Hanes, V. Content Validity of the Female Sexual Function Index (FSFI) in Pre- and Postmenopausal Women with Hypoactive Sexual Desire Disorder (HSDD). J. Sex. Med. 2011, 8, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Bottari, A.; Belcaro, G.; Ledda, A.; Luzzi, R.; Cesarone, M.R.; Dugall, M. Lady Prelox® improves sexual function in generally healthy women of reproductive age. Minerva Ginecol. 2013, 65, 435–444. [Google Scholar]
- Hatch, J.P. Vaginal photoplethysmography: Methodological considerations. Arch. Sex. Behav. 1979, 8, 357–374. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS): Addyi (filbanserin). Available online: https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=350 (accessed on 13 January 2021).
- Lodise, N.M. Hypoactive Sexual Desire Disorder in Women: Treatment Options beyond Testosterone and Approaches to Communicating with Patients on Sexual Health. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Klotz, T.; Mathers, M.; Braun, M.; Bloch, W.; Engelmann, U. Effectiveness of oral L-arginine in first-line treatment of erectile dysfunction in a controlled crossover study. Urol. Int. 1999, 63, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Stanislavov, R.; Rohdewald, P. PACR (Pine Bark Extract, L Arginine, L Citrulline, Rose Hip Extract) Improves Emotional, Physical Health and Sexual Function in Peri-Menopausal Women. J. Women’s Heal. Care 2014, 3. [Google Scholar] [CrossRef]
- Bottari, A.; Belcaro, G.; Ledda, A.; Cesarone, M.R.; Vinciguerra, G.; Di Renzo, A.; Stuard, S.; Dugall, M.; Pellegrini, L.; Errichi, S.; et al. Lady Prelox® improves sexual function in post-menopausal women. Panminerva Med. 2012, 54 (Suppl. 1), 3–9. [Google Scholar] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Scipione, C.; Scipione, V.; Dugall, M.; Hu, S.; Cotellese, R.; Feragalli, B.; Ledda, A. Prevention of vaginal dryness in perimenopausal women. Supplementation with Lady Prelox®. Minerva Ginecol. 2020, 71, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Kellogg Spad, S.; Dweck, A. 083 The effect of Ristela supplementation on Female Sexual Function in women taking antide-pressants: An open label trial. J. Sex Med. 2020, 17, S259–S260. [Google Scholar] [CrossRef]
- Smetanka, A.; Stara, V.; Farsky, I.; Tonhajzerova, I.; Ondrejka, I. Pycnogenol supplementation as an adjunct treatment for antidepressant-induced sexual dysfunction. Physiol. Int. 2019, 106, 59–69. [Google Scholar] [CrossRef]
- West, E.; Krychman, M. Natural Aphrodisiacs—A Review of Selected Sexual Enhancers. Sex. Med. Rev. 2015, 3, 279–288. [Google Scholar] [CrossRef]
- PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses. PRISMA Statement. Available online: http://www.prisma-statement.org/PRISMAStatement/ (accessed on 11 November 2020).
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evidence-Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Meston, C.M.; Worcel, M. The effects of yohimbine plus L-arginine glutamate on sexual arousal in postmenopausal women with sexual arousal disorder. Arch. Sex. Behav. 2002, 31, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.Y.; Polan, M.L.; Whipple, B.; Trant, A.S. The Enhancement of Female Sexual Function with ArginMax, a Nutritional Supplement, Among Women Differing in Menopausal Status. J. Sex Marital. Ther. 2006, 32, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, E.; Amato, M.; Izzo, A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000, 71, S1–S5. [Google Scholar] [CrossRef]
- Rector, T.S.; Bank, A.J.; Mullen, K.A.; Tschumperlin, L.K.; Sih, R.; Pillai, K.; Kubo, S.H. Randomized, Double-Blind, Placebo-Controlled Study of Supplemental Oral l -Arginine in Patients with Heart Failure. Circulation 1996, 93, 2135–2141. [Google Scholar] [CrossRef]
- ConsumerLab.com. L-Arginine Supplements Review. ConsumerLab.com. 19 October 2019. Available online: https://www.consumerlab.com/reviews/l-arginine-supplements-review/arginine/ (accessed on 7 December 2020).
- Meston, C.M.; Rellini, A.H.; Telch, M.J. Short- and Long-term Effects of Ginkgo Biloba Extract on Sexual Dysfunction in Women. Arch. Sex. Behav. 2008, 37, 530–547. [Google Scholar] [CrossRef]
- Newell, C.; Anderson, L.; Phillipson, J. Herbal Medicines: A Guide for Health-Care Professionals; The Pharmaceutical Press: London, UK, 1996. [Google Scholar]
- Oh, K.-J.; Chae, M.-J.; Lee, H.-S.; Hong, H.-D.; Park, K. Effects of Korean Red Ginseng on Sexual Arousal in Menopausal Women: Placebo-Controlled, Double-Blind Crossover Clinical Study. J. Sex. Med. 2010, 7, 1469–1477. [Google Scholar] [CrossRef]
- Wheatley, D. Triple-blind, placebo-controlled trial ofGinkgo biloba in sexual dysfunction due to antidepressant drugs. Hum. Psychopharmacol. Clin. Exp. 2004, 19, 545–548. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2007, 99, 855–862. [Google Scholar] [CrossRef]
- Andersson, U.; Berger, K.; Hogberg, A.; Landinolsson, M.; Holm, C. Effects of rose hip intake on risk markers of type 2 diabetes and cardiovascular disease: A randomized, double-blind, cross-over investigation in obese persons. Eur. J. Clin. Nutr. 2011, 66, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Packer, L. Flavonoids in Health and Disease; Marcel Dekker, Inc.: Manhattan, NY, USA, 1998. [Google Scholar]
- Anderson, R.; Moffatt, C.E. Ignorance Is Not Bliss: If We Don’t Understand Hypoactive Sexual Desire Disorder, How Can Flibanserin Treat It? Commentary. J. Sex. Med. 2018, 15, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kingsberg, S.A.; Althof, S.; Simon, J.A.; Bradford, A.; Bitzer, J.; Carvalho, J.; Flynn, K.E.; Nappi, R.E.; Reese, J.B.; Rezaee, R.L.; et al. Female Sexual Dysfunction—Medical and Psychological Treatments, Committee 14. J. Sex. Med. 2017, 14, 1463–1491. [Google Scholar] [CrossRef] [PubMed]
- Lexi-Comp Online. Flibanserin; Lexi-Comp, Inc.: Hudson, OH, USA, 5 January 2021. [Google Scholar]
- Clayton, A.H. Original Articles: Epidemiology and Neurobiology of Female Sexual Dysfunction. J. Sex. Med. 2007, 4, 260–268. [Google Scholar] [CrossRef] [PubMed]
- The US National Institutes of Health. ClinicalTrials.gov. Available online: http://www.clinicaltrials.gov/ (accessed on 7 December 2020).
| n | Age (years) | Height (Mean, cm) | Weight (Mean, kg) | Ethnicity | Concurrent Use of Hormonal Therapy | Reported Sexual Dysfunction | Previous Treatment for Sexual Dysfunction | |
|---|---|---|---|---|---|---|---|---|
| Ito et al. RCT (2001) | ||||||||
| 77 | 22–71 | NR | NR | NR | NR | NR | 6 | |
| Meston et al. RCT (2002) | ||||||||
| 24 | 27–69 | 159.5 | 70.7 | Caucasian (88%) Hispanic (4%) African American (4%) Asian (4%) | NR | NR | NR | |
| Ito et al. RCT (2006) | ||||||||
| Total | 108 | 22–73 | NR | NR | NR | ArginMax (n = 18) Placebo (n = 16) | NR | n = 12 |
| Pre-menopausal | 59 | 22–48 | NR | NR | NR | NR | NR | n = 12 |
| Perimenopausal | 20 | 36–57 | NR | NR | NR | 3 | NR | NR |
| Postmenopausal | 29 | 42–73 | NR | NR | NR | 18 | NR | NR |
| Bottari et al. RCT (2012) | ||||||||
| 80 | 45–55 | NR | NR | NR | Exclusion criteria | Moderate sexual dysfunction | NR | |
| Bottari et al. NRCT (2013) | ||||||||
| 100 | 37–45 | NR | NR | NR | All women reported taking oral contraceptives | NR | NR | |
| Stanislavov et al. RCT (2014) | ||||||||
| 80 | 40–50 | NR | NR | NR | Exclusion criteria | Moderate sexual dysfunction | NR | |
| Cesarone et al. NRCT study (2019) | ||||||||
| Pre-menopausal | 67 | 40–50 | NR | NR | NR | Exclusion criteria | Moderate sexual dysfunction | NR |
| Post-menopausal women | 73 | 50–60 | NR | NR | NR | Exclusion criteria | Moderate sexual dysfunction | NR |
| n | Mean Total Scores | Desire ab | Sexual Arousal | Lubrication 1 | Orgasm | Satisfaction cd | Discomfort and Pain | Frequency of Intercourse | Degree of Clitoral Sensation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ito et al. RCT (2001) 4 | ||||||||||
| ArginMax | 34 | NR | 70.6% a 61.8% b | NR | 52.9% | 47.1% | 73.5% c 61.8% d | 38.2% | 64.7% | 52.9% |
| Placebo | 43 | NR | 41.9% a 34.9% b | NR | 25.6% | 30.2% | 37.2% c 34.9% d | 18.6% | 25.6% | 34.9% |
| p < 0.01 a p = NR b | NR | p < 0.01 | p < 0.07 | p < 0.01 c p < 0.01 d | p < 0.03 | p < 0.01 | p < 0.06 | |||
| Ito et al. RCT (2006) 3 | ||||||||||
| ArginMax Premenopausal | 25 | NR | 72% a 60% b | NR | 40% | 44% | 68% c 52% d | 32% | 56% | 52% |
| Placebo Premenopausal | 34 | NR | 47% a 38% b | NR | 29% | 29% | 35% c 35% d | 24% | 26% | 38% |
| p = 0.03 a p = 0.05 b | NR | p = 0.20 | p = 0.13 | p = 0.007 c p = 0.10 d | p = 0.23 | p = 0.01 | p = 0.15 | |||
| ArginMax Perimenopausal | 14 | NR | 57% a 57% b | NR | 64% | 50% | 71% c 79% d | 43% | 86% | 71% |
| Placebo Perimenopausal | 6 | NR | 50% a 33% b | NR | 17% | 17% | 33% c 33% d | 17% | 17% | 33% |
| p = 0.38 a p = 0.18 b | NR | p = 0.03 | p = 0.10 | p = 0.06 c p = 0.03 d | p = 0.16 | p = 0.002 | p = 0.06 | |||
| ArginMax Postmenopausal | 16 | NR | 50% a 31% b | NR | 25% | 31% | 44% c 50% d | 19% | 38% | 38% |
| Placebo Postmenopausal | 13 | NR | 8% a 23% b | NR | 15% | 31% | 31% c 31% d | 15% | 31% | 15% |
| p = 0.008 a p = 0.33 b | NR | p = 0.29 | p = 0.50 | p = 0.25 c p = 0.16 d | p = 0.43 | p = 0.36 | p = 0.11 | |||
| Bottari et al. RCT (2012) | ||||||||||
| LP Baseline | 40 | 44.6 ± 24.1 | 5.13 ± 2.3 a | 9.2 ± 4.5 | 11.4 ± 5.8 | 6.5 ± 4.1 | 6.1 ± 3.9 c | 6.3 ± 3.5 | NR | NR |
| LP 4 weeks | 40 | 70.9 ± 18.5 (p < 0.05) | 6.6 ± 2.1 a | 14.0 ± 4.5 | 16.0 ± 4.3 | 11.3 ± 2.9 | 11.3 ± 2.9 c | 11.7 ± 1.7 | NR | NR |
| LP 8 weeks | 40 | 71.7 ± 23.9 (p < 0.05) | 7.0 ± 2.4 a | 14.5 ± 4.5 | 16.6 ± 5.2 | 11.1 ± 3.5 | 11.1 ± 3.5 c | 11.4 ± 3.7 | NR | NR |
| Placebo Baseline | 40 | 44.1 ± 22.8 | 5.2 ± 2.4 a | 9.2 ± 4.9 | 10.9 ± 5.1 | 6.4 ± 3.6 | 6.0 ± 3.4 c | 6.4 ± 3.4 | NR | NR |
| Placebo 4 weeks | 40 | 45.0 ± 21.4 | 5.0 ± 2.4 a | 9.3 ± 4.5 | 11.2 ± 4.5 | 6.7± 4.0 | 6.0 ± 3.7 c | 6.8 ± 3.3 | NR | NR |
| Placebo 8 weeks | 40 | 47.4 ± 21.8 | 5.7 ± 2.4 a | 11.3 ± 4.7 | 10.7 ± 4.4 | 6.6 ± 3.6 | 6.0 ± 3.7 c | 7.1 ± 3.4 | NR | NR |
| Bottari et al. NRCT (2013) 2 | ||||||||||
| LP Baseline | 49 | 14.96 ± 2.68 | 2.0 a 2.0 b | NR | 1.0 | 2.0 | 2.0 c 1.0 d | 1.0 | 1.0 | 2.0 |
| LP 4 weeks | 49 | 28.26 ± 2.35 (p = NR) | 3.0 a 3.0 b | NR | 3.0 | 3.0 | 3.0 c 3.0 d | 3.0 | 3.0 | 3.0 |
| LP 8 weeks | 49 | 33.91 ± 2.7 (p < 0.001) | 4.0 a 4.0 b | NR | 4.0 | 4.0 | 4.0 c 4.0 d | 4.0 | 4.0 | 4.0 |
| BM Baseline | 51 | 17.92 ± 2.32 | 2.0 a 3.0 b | NR | 1.0 | 2.0 | 2.0 c 2.0 d | 2.0 | 1.0 | 2.0 |
| BM 4 weeks | 51 | 23.45 ± 1.82 | 3.0 a 4.0 b | NR | 2.0 | 3.0 | 2.0 c 3.0 d | 2.0 | 2.0 | 2.0 |
| BM 8 weeks | 51 | 23.52 ± 2.2 | 3.0 a 3.5 b | NR | 3.0 | 3.0 | 2.0 c 3.0 d | 2.0 | 2.0 | 2.0 |
| Stanislavov et al. RCT (2014) | ||||||||||
| LP Baseline | 40 | 16.50 (SD 2.85) | 2.58 (SD 0.43) | 2.85 (SD 0.59) | 2.85 (SD 0.59) | 2.85 (SD 0.59) | 2.85 (SD 0.59) | 2.52 (SD 0.53) | NR | NR |
| LP 1 month | 40 | 21.65 (SD 2.79) (p < 0.001) | 3.50 (SD 0.54) 58% (p < 0.001) | 3.72 (SD 0.53) 62% (p < 0.001) | 3.72 (SD 0.53) 62% (p < 0.001) | 3.69 (SD 0.57) 62% (p < 0.001) | 3.63 (SD 0.64) 61% (p < 0.001) | 3.39 (SD 0.54) 57% (p < 0.001) | NR | NR |
| LP 2 months | 40 | 26.49 (SD 3.28) (p < 0.001) | 4.23 (SD 0.66) 71% (p < 0.001) | 4.47 (SD 0.62) 75% (p < 0.001) | 4.53 (SD 0.62) 76% (p < 0.001) | 4.46 (SD 0.64) 74% (p < 0.001) | 4.47 (SD 0.61) 75% (p < 0.001) | 4.32 (SD 0.65) 72% (p < 0.001) | NR | NR |
| Placebo Baseline | 40 | 12.42 (SD 2.25) | 2.13 (SD 0.51) | 2.13 (SD 0.51) | 2.13 (SD 0.51) | 2.13 (SD 0.51) | 2.13 (SD 0.51) | 1.77 (SD 0.61) | NR | NR |
| Placebo 1 month | 40 | 14.40 (SD 0.82) | 2.13 (SD 0.51) 36% | 2.40 (SD 0.00) 40% | 2.40 (SD 0.00) 40% | 2.40 (SD 0.00) 40% | 2.40 (SD 0.00) 40% | 2.67 (SD 0.51) 45% | NR | NR |
| Placebo 2 months | 40 | 16.62 (SD 2.30) | 2.67 (SD 0.51) 45% | 2.67 (SD 0.51) 45% | 2.76 (SD 0.56) 46% | 2.73 (SD 0.54) 46% | 2.73 (SD 0.54) 46% | 3.06 (SD 0.60) 51% | NR | NR |
| Cesarone et al. NRCT study (2019) | ||||||||||
| Premenopausal LP vs. BM | LP: 34 BM: 33 | NR | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | NR | NR |
| Post-menopausal LP vs. BM | LP: 38 BM: 35 | NR | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | NR | NR |
| Oxidative Stress 1 | Menopausal Symptoms Questionnaire 2,3 | Preclinical Items 4 | Vaginal Pulse Amplitude 5 | Subjective Rating of Sexual Arousal 6 | Hunter’s WHQ | Safety Parameters 7 | |
|---|---|---|---|---|---|---|---|
| Ito et al. RCT (2001) | |||||||
| NR | NR | NR | NR | NR | NR | No AE’s reported | |
| Meston et al. RCT (2002) | |||||||
| L-Arg + YH 30 min | NR | NR | NR | 2.50 ± 3.9 | NR | NR | No AE’s reported |
| L-Arg + YH 60 min | NR | NR | NR | 2.66 ± 3.1 | NR | NR | No AE’s reported |
| L-Arg + YH 90 min | NR | NR | NR | 2.61 ± 4.3 | NR | NR | No AE’s reported |
| Placebo (p = 0.01), YH (p = 0.19) | No AE’s reported | ||||||
| YH 30 min | NR | NR | NR | 2.54 ± 2.7 | NR | NR | No AE’s reported |
| YH 60 min | NR | NR | NR | 1.56 ± 2.6 | NR | NR | No AE’s reported |
| YH 90 min | NR | NR | NR | 2.12 ± 2.6 | NR | NR | No AE’s reported |
| Placebo (p = 0.21) | No AE’s reported | ||||||
| Placebo 30 min | NR | NR | NR | 1.70 ± 3.6 | NR | NR | No AE’s reported |
| Placebo 60 min | NR | NR | NR | 1.03 ± 2.1 | NR | NR | No AE’s reported |
| Placebo 90 min | NR | NR | NR | 2.00 ± 3.5 | NR | NR | No AE’s reported |
| Overall Group Differences | NR | NR | NR | NR | Physical Sexual arousal (p = 0.68) Mental sexual arousal (p = 0.69) Autonomic Arousal (p = 0.82) Positive Affect (p = 0.56) Negative affect (p = 0.44) | NR | No AE’s reported |
| Ito et al. RCT (2006) | |||||||
| NR | NR | NR | NR | NR | NR | Minor gastric disturbances (n = 4) Heavier bleeding during menstruation (n = 2) Increased headache (n = 1) | |
| Bottari et al. RCT (2012) | |||||||
| NR | NR | NR | NR | NR | NR | No AE’s reported | |
| Bottari et al. NRCT (2013) | |||||||
| LP Baseline | 388.29 | NR | NR | NR | NR | NR | No AE’s reported |
| LP 4 weeks | 344.28 (p < 0.05) | NR | NR | NR | NR | NR | No AE’s reported |
| LP 8 weeks | 332.31 (p < 0.05) | NR | NR | NR | NR | NR | No AE’s reported |
| BM Baseline | 389.33 | NR | NR | NR | NR | NR | No AE’s reported |
| BM 4 weeks | 377.32 (p < 0.05) | NR | NR | NR | NR | NR | No AE’s reported |
| BM 8 weeks | 365.33 (p < 0.05) | NR | NR | NR | NR | NR | No AE’s reported |
| Stanislavov et al. RCT (2014) 1 | |||||||
| LP Baseline | NR | Total score different at LP baseline compared to placebo baseline | NR | NR | NR | NR | No AE’s reported |
| LP 1 month | NR | Total score significantly different (p < 0.001) at LP 1 month compared to placebo 1 month; significantly different from baseline | NR | NR | NR | Significantly different (p < 0.001) at LP 1 month compared to placebo 1 month in all parameters except attractiveness | No AE’s reported |
| LP 2 months | NR | Total score significantly different (p < 0.001) at LP 2 month compared to placebo 2 month; significantly different from baseline | NR | NR | NR | Significantly different (p < 0.001) at LP 1 month compared to placebo 1 month in all parameters | No AE’s reported |
| Placebo Baseline | NR | NR | NR | NR | No AE’s reported | ||
| Placebo 1 month | NR | NR | NR | NR | No AE’s reported | ||
| Placebo 2 months | NR | NR | NR | NR | No AE’s reported | ||
| Cesarone et al. NRCT study (2019) 2 | |||||||
| Premenopausal LP vs. BM | p < 0.05 vs. baseline | NR | All improved significantly versus best management (p < 0.05) | NR | NR | NR | No AE’s reported |
| Post-menopausal LP vs. BM | p < 0.05 vs. baseline | Significant effect for LP on all symptoms at 8 weeks (p < 0.05) 7 | All improved significantly versus best management (p < 0.05) | NR | NR | NR | No AE’s reported |
| Randomization Process | Confounding Factors | Selection of Participants | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of Reported Result | Overall Bias | |
|---|---|---|---|---|---|---|---|---|---|
| ROB2 1 | |||||||||
| Ito et al. RCT (2001) | Low risk | N/A | N/A | N/A | Low risk | Low risk | Low risk | Low risk | Low risk |
| Meston et al. RCT (2002) | Low risk | N/A | N/A | N/A | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ito et al. RCT (2006) | Low risk | N/A | N/A | N/A | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bottari et al. RCT (2012) | Low risk | N/A | N/A | N/A | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Stanislavov et al. RCT (2014) | Some concerns | N/A | N/A | N/A | Low risk | Low risk | Low risk | Low risk | Some concerns |
| ROBINS-I 2 | |||||||||
| Bottari et al. NRCT (2013) | N/A | Serious concerns | Low risk | Moderate risk | Low risk | Some concerns | Serious concerns | Low Risk | Serious risk |
| Cesarone et al. NRCT (2019) | N/A | Serious concerns | Low risk | Moderate risk | Low risk | Low risk | Serious concerns | Low Risk | Serious risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieri-Hutcherson, N.E.; Jaenecke, A.; Bahia, A.; Lucas, D.; Oluloro, A.; Stimmel, L.; Hutcherson, T.C. Systematic Review of l-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women. Pharmacy 2021, 9, 71. https://doi.org/10.3390/pharmacy9020071
Cieri-Hutcherson NE, Jaenecke A, Bahia A, Lucas D, Oluloro A, Stimmel L, Hutcherson TC. Systematic Review of l-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women. Pharmacy. 2021; 9(2):71. https://doi.org/10.3390/pharmacy9020071
Chicago/Turabian StyleCieri-Hutcherson, Nicole E., Andrea Jaenecke, Ajeet Bahia, Debra Lucas, Ann Oluloro, Lora Stimmel, and Timothy C. Hutcherson. 2021. "Systematic Review of l-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women" Pharmacy 9, no. 2: 71. https://doi.org/10.3390/pharmacy9020071
APA StyleCieri-Hutcherson, N. E., Jaenecke, A., Bahia, A., Lucas, D., Oluloro, A., Stimmel, L., & Hutcherson, T. C. (2021). Systematic Review of l-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women. Pharmacy, 9(2), 71. https://doi.org/10.3390/pharmacy9020071





