Intentions to “Reuse” Medication in the Future Modelled and Measured Using the Theory of Planned Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Compliance with Ethical Standards
2.2. Questionnaire Development
2.3. Questionnaire Distribution
2.4. Questionnaire Sample
2.5. Analysis of Survey Results
3. Results
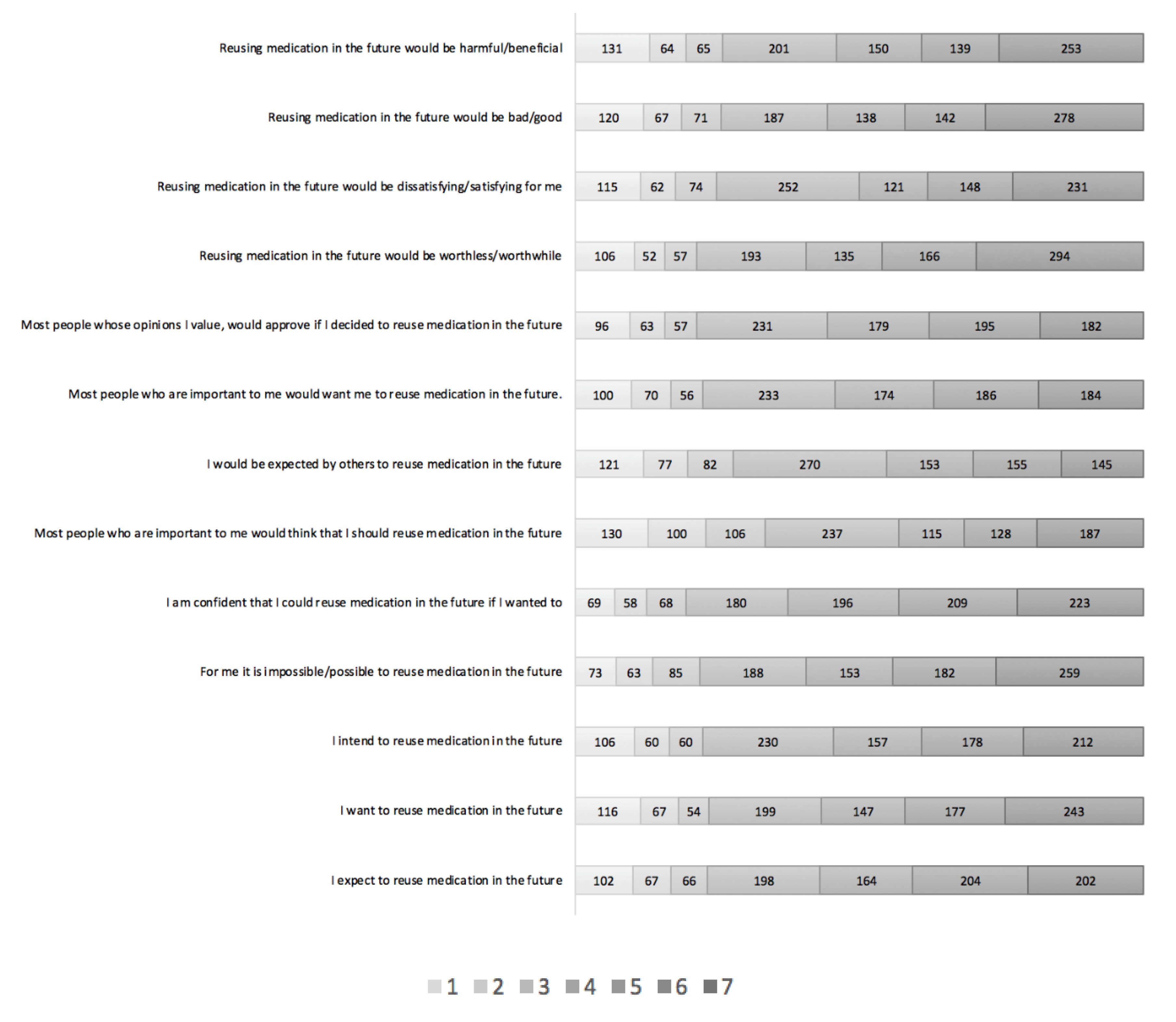
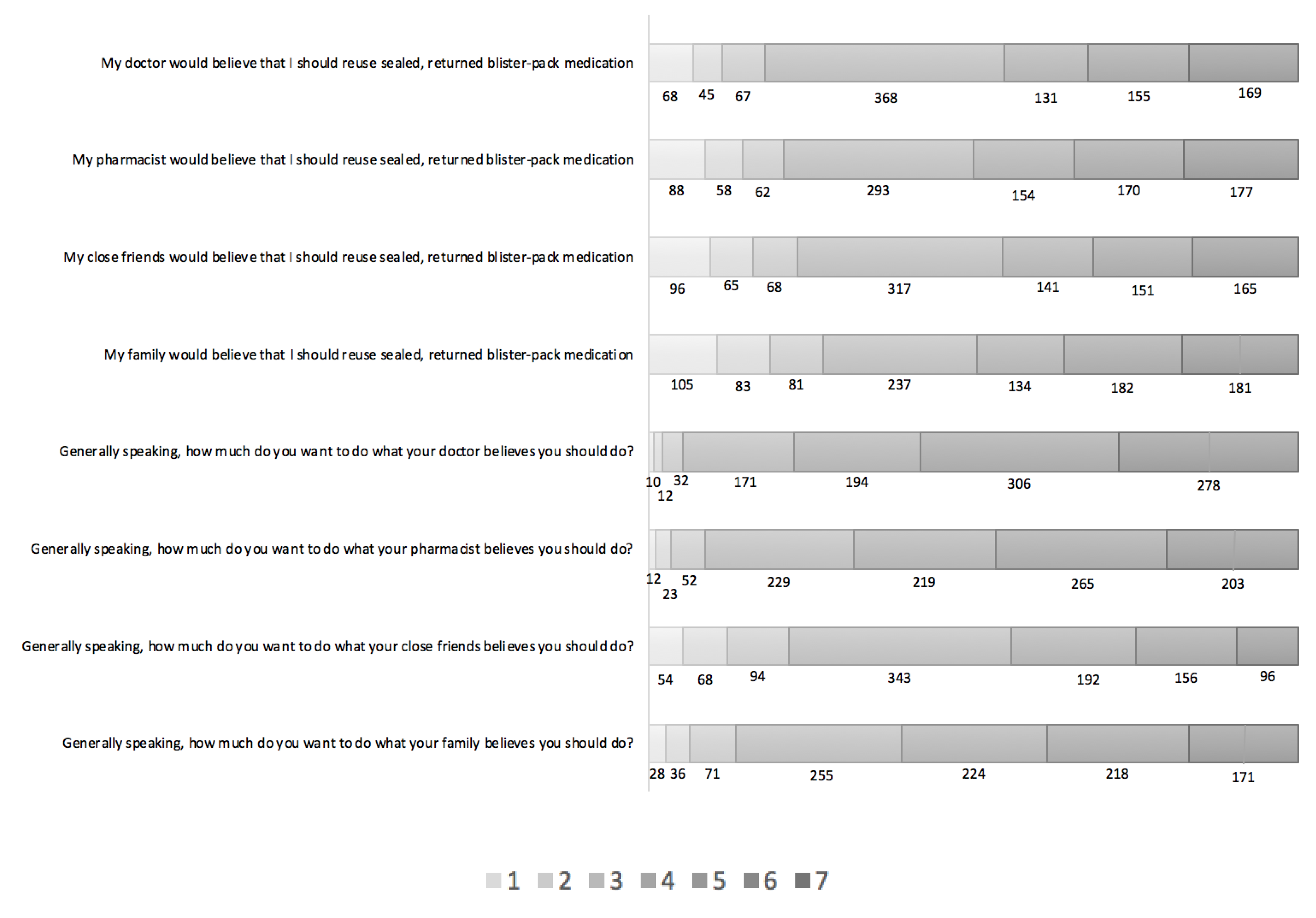
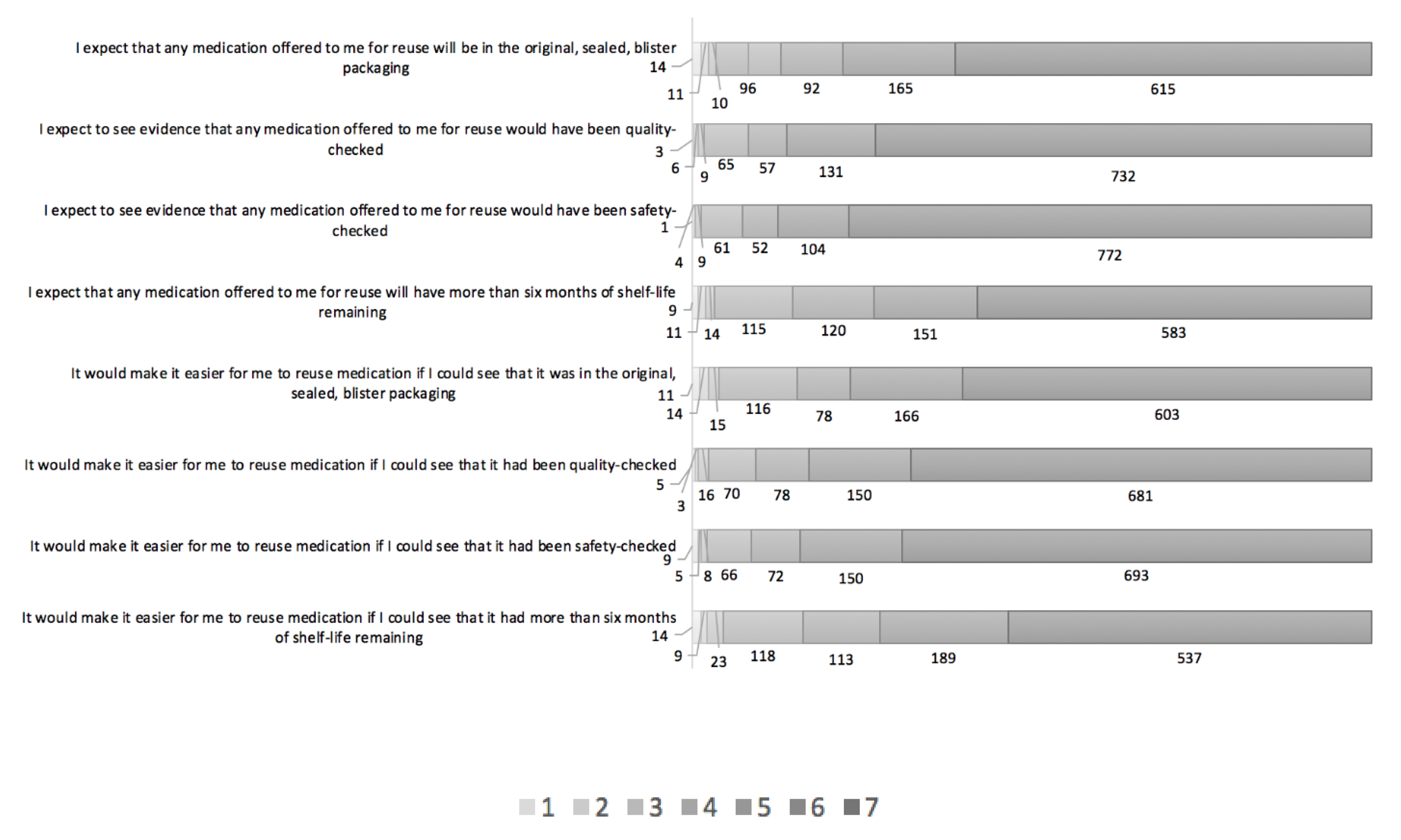
3.1. Description of Findings
3.2. Regression Analysis
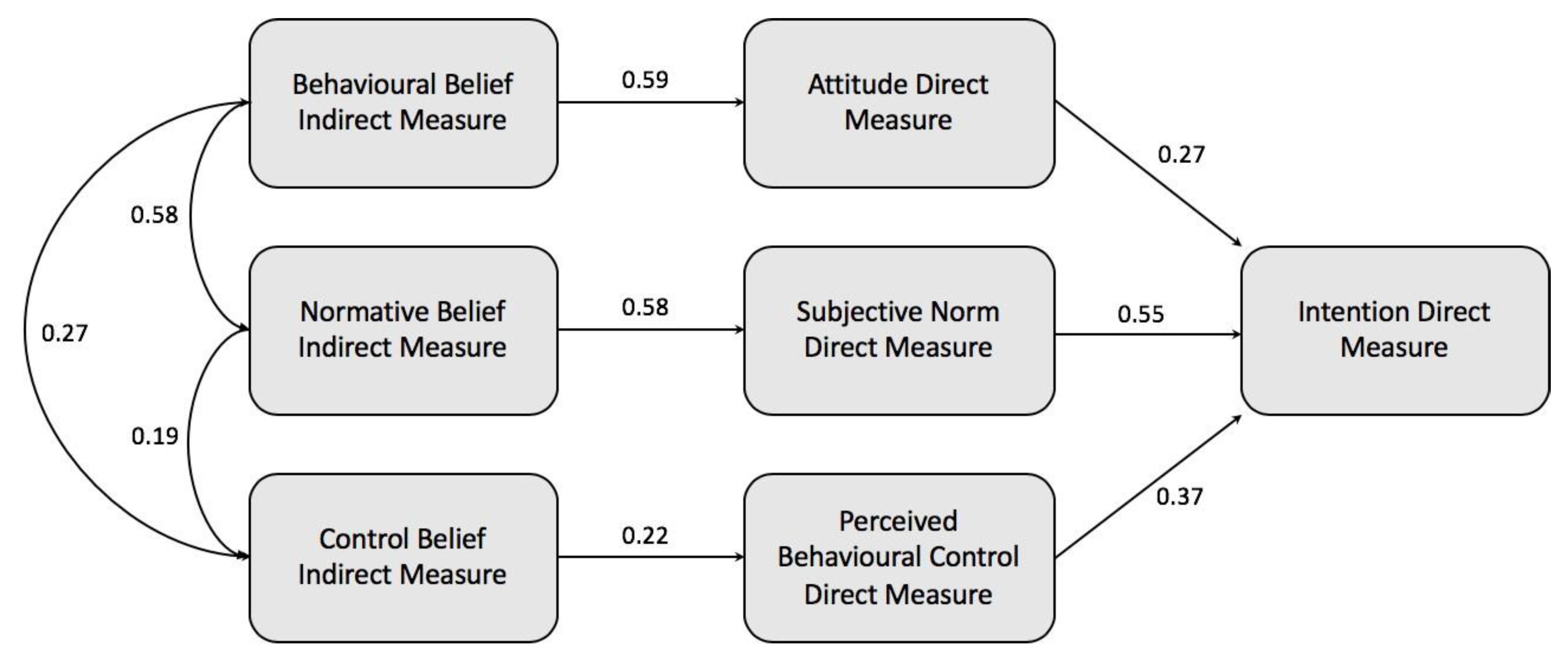
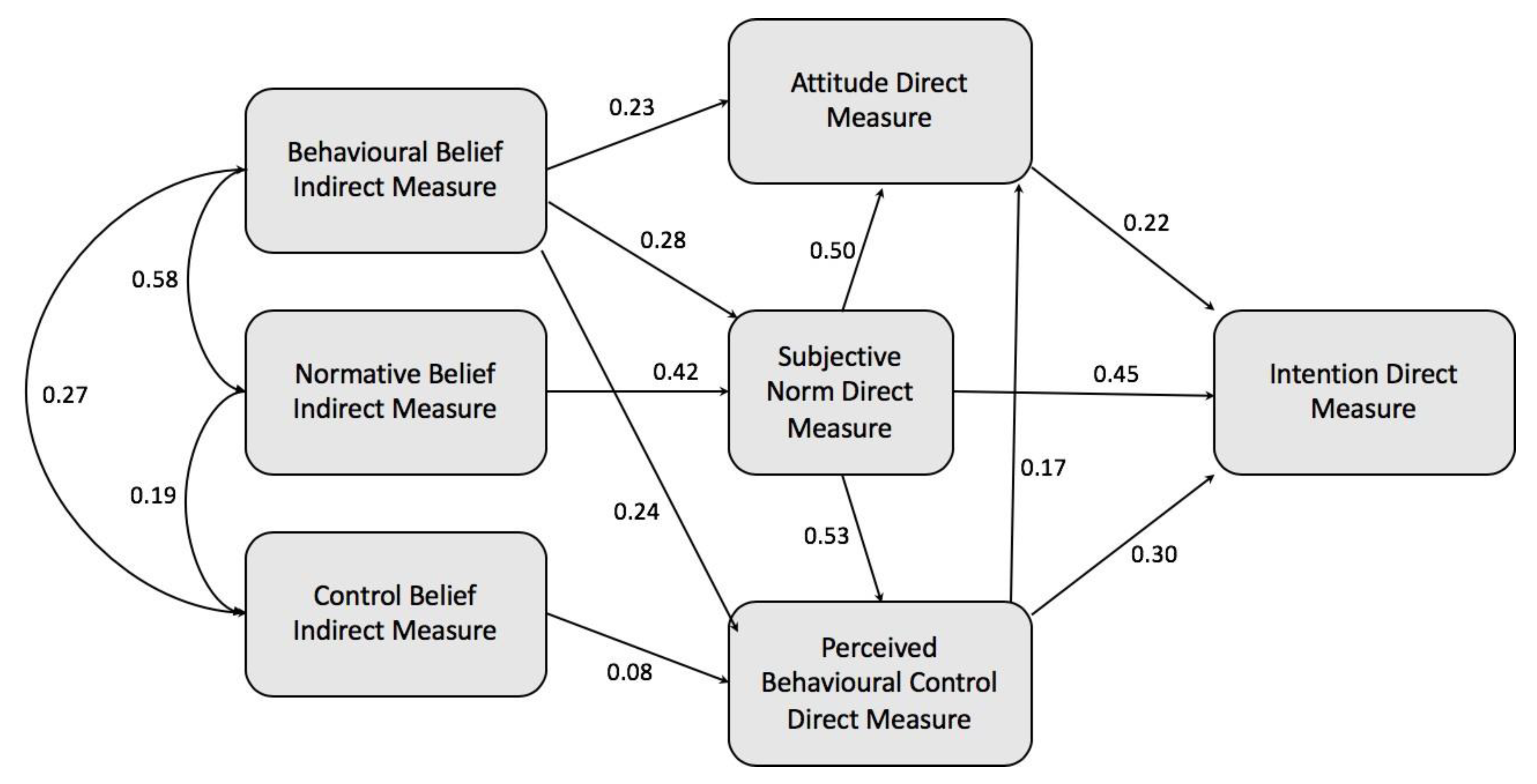
3.3. Construction of a TPB-based Model and Hypothesis Testing
3.4. Model Modification
3.5. Participant Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trueman, P.; Lowson, K.; Blighe, A.; Meszaros, A.; Wright, D.; Glanville, J.; Taylor, D.; Newbould, J.; Bury, M.; Barber, N.; et al. Evaluation of the Scale, Causes and Costs of Waste Medicines Evaluation of the Scale, Causes and Costs of Waste Medicines; YHEC/School of Pharmacy, University of London: London, UK, 2011; Volume 17. [Google Scholar]
- PSNC. Disposal of Unwanted Medicines: PSNC Main site. Available online: https://psnc.org.uk/services-commissioning/essential-services/disposal-of-unwanted-medicines/ (accessed on 4 February 2019).
- BMA. BMA-Dispensed But Unopened Medications. British Medical Association. Available online: https://www.bma.org.uk/collective-voice/committees/patient-liaison-group/resources/dispensed-but-unopened-medications (accessed on 4 February 2019).
- Cauchi, R.; Berg, K. State Prescription Drug Return, Reuse and Recycling Laws. National Conference of State Legislatures. Available online: https://www.ncsl.org/research/health/state-prescription-drug-return-reuse-and-recycling.aspx (accessed on 9 January 2020).
- GIVMED. Available online: https://givmed.org/en/ (accessed on 9 January 2020).
- Mackridge, A.J.; Marriott, J.F. Returned medicines: Waste or a wasted opportunity? J. Public Health 2007, 29, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.L.; van den Bemt, B.J.F.; Egberts, A.C.G.; Bouvy, M.L.; Gardarsdottir, H. Patient and medication factors associated with preventable medication waste and possibilities for redispensing. Int. J. Clin. Pharm. 2018, 40, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.R.; Chew, L. Turning waste medicines to cost savings: A pilot study on the feasibility of medication recycling as a solution to drug wastage. Palliat Med. 2017, 31, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lenzer, J. US could recycle 10 million unused prescription drugs a year, report says. BMJ 2014, 349, g7677. [Google Scholar] [CrossRef] [PubMed]
- Connelly, D. Should pharmacists be allowed to reuse medicines? Pharm. J. 2018, 301. [Google Scholar] [CrossRef]
- Alhamad, H.; Patel, N.; Donyai, P. How do people conceptualise the reuse of medicines? An interview study. Int. J. Pharm. Pr. 2018, 26, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Why Green and Sustainable Pharmacy? Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Paut Kusturica, M.; Tomas, A.; Sabo, A. Disposal of unused drugs: Knowledge and behavior among people around the world. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Germany, 2017; Volume 240, pp. 71–104. [Google Scholar] [CrossRef]
- Bound, J.P.; Voulvoulis, N. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environ. Health Perspect. 2005, 113, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Bound, J.P.; Kitsou, K.; Voulvoulis, N. Household disposal of pharmaceuticals and perception of risk to the environment. Environ. Toxicol. Pharm. 2006, 21, 301–307. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Brandt-Rauf, P. Pharmaceutical residues in the drinking water supply: Modeling residue concentrations in surface waters of drugs prescribed in the United States. Puerto Rico Health Sci. J. 2008, 27, 236–240. [Google Scholar]
- NHS. NHS Carbon Footprint Measuring Carbon Footprint NHS Requirements Sustainable Development Unit. Available online: https://www.sduhealth.org.uk/policy-strategy/reporting/nhs-carbon-footprint.aspx (accessed on 4 February 2019).
- Xie, Y.; Breen, L. Who cares wins? A comparative analysis of household waste medicines and batteries reverse logistics systems: The case of the NHS (UK). Supply Chain Manag. 2014, 19, 455–474. [Google Scholar] [CrossRef]
- NHS Business Services Authority. Help with NHS Prescription Costs. Available online: https://www.nhsbsa.nhs.uk/help-nhs-prescription-costs (accessed on 4 February 2020).
- The Pharmaceutical Journal. Council Approves Use of Patient-Returned and Date-Expired Medicines in the Event of Pandemic Flu. Available online: https://www.pharmaceutical-journal.com/news-and-analysis/news/council-approves-use-of-patient-returned-and-date-expired-medicines-in-the-event-of-pandemic-flu/10036098.article (accessed on 4 February 2019).
- Department of Health & Social Care. Update: EpiPen and EpiPen Junior (Adrenaline Auto-Injector Devices)–Supply Disruption. Available online: https://www.sps.nhs.uk/articles/shortage-of-epipen/ (accessed on 4 February 2019).
- Department of Health & Social Care. A Guide to Managing Medicines Supply and Shortages. Available online: https://www.england.nhs.uk/wp-content/uploads/2019/11/a-guide-to-managing-medicines-supply-and-shortages-2.pdf (accessed on 4 February 2019).
- McRae, D.; Allman, M.; James, D. The redistribution of medicines: Could it become a reality? Int. J. Pharm. Pract. 2016, 24, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kotchen, M.; Kallaos, J.; Wheeler, K.; Wong, C.; Zahller, M. Pharmaceuticals in wastewater: Behavior, preferences, and willingness to pay for a disposal program. J. Environ. Manag. 2009, 90, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Sabelström, E.; Gunnarsson, B. Handling of unused prescription drugs–knowledge, behaviour and attitude among Swedish people. Environ. Int. 2009, 35, 771–774. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Koger, S.M.; Winter, D.D.N.; Winter, D.D.N. The Psychology of Environmental Problems: Psychology for Sustainability, 3rd ed.; Psychology Press: London, UK, 2010. [Google Scholar]
- de Leeuw, A.; Valois, P.; Ajzen, I.; Schmidt, P. Using the theory of planned behavior to identify key beliefs underlying pro-environmental behavior in high-school students: Implications for educational interventions. J. Environ. Psychol. 2015, 42, 128–138. [Google Scholar] [CrossRef]
- Pakpour, A.H.; Zeidi, I.M.; Emamjomeh, M.M.; Asefzadeh, S.; Pearson, H. Household waste behaviours among a community sample in Iran: An application of the theory of planned behaviour. Waste Manag. 2014, 34, 980–986. [Google Scholar] [CrossRef]
- Davis, G.; Morgan, A. Using the Theory of Planned Behaviour to determine recycling and waste minimisation behaviours: A case study of Bristol City, UK. Aust. Community Psychol. 2008, 20, 105–117. [Google Scholar]
- Armitage, C.J.; Conner, M. Efficacy of the theory of planned behaviour: A meta-analytic review. Br. J. Soc. Psychol. 2001, 40, 471–499. [Google Scholar] [CrossRef]
- Francis, J.; Eccles, M.P.; Johnston, M.; Walker, A.E.; Grimshaw, J.M.; Foy, R.; Kaner, E.F.S.; Smith, L.; Bonetti, D. Constructing Questionnaires Based on the Theory of Planned Behaviour: A Manual for Health Services Researchers; Centre for Health Services Research: Newcastle upon Tyne, UK, 2004; pp. 1–42. ISBN 9540161-5-7. [Google Scholar]
- Hindi, A.; Parkhurst, C.; Rashidi, Y.; Ho, S.Y.; Patel, N.; Donyai, P. Development and utilisation of the medicines use review patient satisfaction questionnaire. Patient Prefer. Adherence 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Jöreskog, K.; Sörbom, D. LISREL 8.72: A guide to the Program and Applications; SPSS Inc.: Illinois, MS, USA, 2005. [Google Scholar]
- Bansal, H.S.; Taylor, S.F. Investigating interactive effects in the theory of planned behavior in a service-provider switching context. Psychol. Mark. 2002, 19, 407–425. [Google Scholar] [CrossRef]
- Powpaka, S. Factors affecting managers’ decision to bribe: An empirical investigation. J. Bus. Ethics. 2002, 40, 227–246. [Google Scholar] [CrossRef]
- The European Parlament and the Council of the European Union. Directive 2011/62/eu of the European Parliament and of the Council of 8 June 2011. Off. J. Eur. Union 2011, 1, 74–87. [Google Scholar]
- Ajzen, I. The theory of planned behavior. In Handbook of Theories of Social Psychology; SAGE Publications: London, UK, 2012; Volume 1, pp. 438–454. [Google Scholar]
- Bekker, C.; Van Den Bemt, B.; Egberts, T.C.G.; Bouvy, M.; Gardarsdottir, H. Willingness of patients to use unused medication returned to the pharmacy by another patient: A cross-sectional survey. BMJ Open 2019, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Oluka, O.C.; Nie, S.; Sun, Y. Quality assessment of TPB-based questionnaires: A systematic review. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef]


| Predictor Variable | B | SE | Beta (β) | t | p |
|---|---|---|---|---|---|
| Direct Measures | |||||
| Attitude | 0.212 | 0.025 | 0.217 | 8.545 | <0.001 |
| Subjective norm | 0.497 | 0.029 | 0.445 | 16.900 | <0.001 |
| PBC | 0.326 | 0.025 | 0.296 | 12.941 | <0.001 |
| N = 1003 participants, F = 920.645, df = 3, p < 0.001, R = 0.857, R2 = 0.734, Adjusted R2 = 0.734 | |||||
| Indirect Measures | |||||
| Behavioral belief………Attitudes F = 512.301, df = 1, p < 0.001, R = 0.582, R2 = 0.339, Adjusted R2 = 0.339 | 0.024 | 0.001 | 0.591 | 2.18 | <0.001 |
| Normative beliefs………Subjective norms F = 512.301, df = 1, p < 0.001, R = 0.591, R2 = 0.349, Adjusted R2 = 0.349 | 0.027 | 0.001 | 0.582 | 22.634 | <0.001 |
| Control beliefs………PBC F = 50.507, df = 1, p < 0.001, R = 0.219, R2 = 0.048, Adjusted R2 = 0.047 | 0.013 | 0.002 | 0.219 | 7.107 | <0.001 |
| Hypotheses | Standardized Path Coefficient |
|---|---|
| H1 | 0.27 (p < 0.001, n = 1003) |
| H2 | 0.55 (p < 0.001, n = 1003) |
| H3 | 0.37 (p < 0.001, n = 1003) |
| H4 | 0.59 (p < 0.001, n = 1003). |
| H5 | 0.58 (p < 0.001, n = 1003) |
| H6 | 0.22 (p < 0.001, n = 1003) |
| TEST | RECOMMENDED VALUE | MODEL VALUE | DEGREE OF MODEL FIT |
|---|---|---|---|
| Chi-square Chi-square/df | p ≥ 0.05 ≤5 | 1298.857 * 108.238 | Poor fit |
| RMSEA | ≤0.08 | 0.327 | Poor fit |
| NFI | ≥0.9 | 0.676 | Poor fit |
| TLI | ≥0.9 | 0.435 | Poor fit |
| CFI | ≥0.9 | 0.677 | Poor fit |
| The New Relationships between the Constructs | MI | ||
|---|---|---|---|
| Normative belief |  | PBC | 191.137 |
| Behavioral belief |  | PBC | 241.787 |
| Subjective norm |  | PBC | 430.755 |
| Attitude |  | PBC | 372.591 |
| Behavioral belief |  | Subjective norm | 53.964 |
| PBC |  | Subjective norm | 238.809 |
| Attitude |  | Subjective norm | 312.129 |
| Normative belief |  | Attitude | 37.007 |
| PBC |  | Attitude | 156.050 |
| Subjective norm |  | Attitude | 288.170 |
| Normative belief |  | Intention | 7.701 |
| TEST | RECOMMENDED VALUE | MODEL VALUE | DEGREE OF MODEL FIT |
|---|---|---|---|
| Chi-square Chi-square/df | p ≥ 0.05 ≤5 | * 16.755 108.238 | Good fit (considering a large sample) |
| RMSEA | ≤0.08 | 0.037 | Good fit |
| NFI | ≥0.9 | 0.996 | Good fit |
| TLI | ≥0.9 | 0.993 | Good fit |
| CFI | ≥0.9 | 0.998 | Good fit |
| HYPOTHESES | STATISTIC |
|---|---|
| H7 | t = −1.506, df = 1001, p = 0.132 |
| H8 | F = 0.971, df = 13, 1002, p = 0.478 |
| H9 | F = 0.954, df = 17, 1002, p = 0.509 |
| H10 | F = 1.665, df = 16, 1002, p = 0.480 |
| H11 | F = 0.989, df = 12, 1002, p = 0.457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamad, H.; Donyai, P. Intentions to “Reuse” Medication in the Future Modelled and Measured Using the Theory of Planned Behavior. Pharmacy 2020, 8, 213. https://doi.org/10.3390/pharmacy8040213
Alhamad H, Donyai P. Intentions to “Reuse” Medication in the Future Modelled and Measured Using the Theory of Planned Behavior. Pharmacy. 2020; 8(4):213. https://doi.org/10.3390/pharmacy8040213
Chicago/Turabian StyleAlhamad, Hamza, and Parastou Donyai. 2020. "Intentions to “Reuse” Medication in the Future Modelled and Measured Using the Theory of Planned Behavior" Pharmacy 8, no. 4: 213. https://doi.org/10.3390/pharmacy8040213
APA StyleAlhamad, H., & Donyai, P. (2020). Intentions to “Reuse” Medication in the Future Modelled and Measured Using the Theory of Planned Behavior. Pharmacy, 8(4), 213. https://doi.org/10.3390/pharmacy8040213






