Practice Transformation Driven through Academic Partnerships
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dalton, K.; Byrne, S. Role of the pharmacist in reducing healthcare costs: Current insights. Int. Pharm. Regul. Programme 2017, 6, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Duedahl, T.H.; Hansen, W.B.; Kjeldsen, L.J.; Graabæk, T. Pharmacist-led interventions improve quality of medicine-related healthcare service at hospital discharge. Eur. J. Hosp. Pharm. 2018, 25, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Livet, M.; Haines, S.T.; Curran, G.M.; Seaton, T.L.; Ward, C.S.; Sorensen, T.D.; Roth McClurg, M. Implementation Science to Advance Care Delivery: A Primer for Pharmacists and Other Health Professionals. Pharmacotherapy 2018, 38, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Seaton, T.L. Dissemination and implementation sciences in pharmacy: A call to action for professional organizations. Res. Soc. Adm. Pharm. 2017, 13, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Draugalis, J.R.; Plaza, C.M. Preparing graduate students for teaching and service roles in pharmacy education. Am. J. Pharm. Educ. 2007, 71, 105. [Google Scholar] [CrossRef]
- Embedding Pharmacists with Physicians | AACP. Available online: https://www.aacp.org/article/embedding-pharmacists-physicians (accessed on 25 May 2020).
- Bansal, N.; Tai, W.-T.; Chen, L.-C. Implementation of an innovative surgical pharmacy service to improve patient outcomes-Twelve-month outcomes of the Enhanced Surgical Medicines Optimization Service. J. Clin. Pharm. Ther. 2019, 44, 904–911. [Google Scholar] [CrossRef]
- Weir, N.M.; Newham, R.; Dunlop, E.; Bennie, M. Factors influencing national implementation of innovations within community pharmacy: A systematic review applying the Consolidated Framework for Implementation Research. Implement. Sci. 2019, 14, 21. [Google Scholar] [CrossRef]
- Meisenberg, B.; Ness, J.; Rao, S.; Rhule, J.; Ley, C. Implementation of solutions to reduce opioid-induced oversedation and respiratory depression. Am. J. Health Syst. Pharm. 2017, 74, 162–169. [Google Scholar] [CrossRef]
- Dissemination & Implementation Models. Available online: https://dissemination-implementation.org/ (accessed on 10 May 2020).
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef]
- McNabb, J.; Ross, J.W.; Abriola, K.; Turley, C.; Nightingale, C.H.; Nicolau, D.P. Adherence to highly active antiretroviral therapy predicts virologic outcome at an inner-city human immunodeficiency virus clinic. Clin. Infect. Dis. 2001, 33, 700–705. [Google Scholar] [CrossRef]
- Wagner, G.J.; Kanouse, D.E.; Koegel, P.; Sullivan, G. Adherence to HIV Antiretrovirals among Persons with Serious Mental Illness. AIDS Patient Care STDs 2003, 17, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bangsberg, D.R.; Hecht, F.M.; Charlebois, E.D.; Zolopa, A.R.; Holodniy, M.; Sheiner, L.; Bamberger, J.D.; Chesney, M.A.; Moss, A. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. Acquir. Immune Defic. Syndr. 2000, 14, 357–366. [Google Scholar] [CrossRef] [PubMed]
- DiMatteo, M.R.; Giordani, P.J.; Lepper, H.S.; Croghan, T.W. Patient adherence and medical treatment outcomes: A meta-analysis. Med. Care 2002, 40, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Mannheimer, S.; Friedland, G.; Matts, J.; Child, C.; Chesney, M.; Terry Beirn Community Programs for Clinical Research on AIDS. The Consistency of Adherence to Antiretroviral Therapy Predicts Biologic Outcomes for Human Immunodeficiency Virus–Infected Persons in Clinical Trials. Clin. Infect. Dis. 2002, 34, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.A.; Neoh, C.F.; Zin, R.M.; Elrggal, M.; Cheema, E. Pharmacovigilance: Pharmacists & rsquo; perspective on spontaneous adverse drug reaction reporting. Int. Pharm. Regul. Programme 2017, 6, 91–98. [Google Scholar] [CrossRef]
- Nivya, K.; Sri Sai Kiran, V.; Ragoo, N.; Jayaprakash, B.; Sonal Sekhar, M. Systemic review on drug related hospital admissions—A pubmed based search. Saudi Pharm. J. 2015, 23, 1–8. [Google Scholar] [CrossRef]
- Jokanovic, N.; Wang, K.N.; Dooley, M.J.; Lalic, S.; Tan, E.CK.; Kirkpatrick, C.M.; Bell, J.S. Prioritizing interventions to manage polypharmacy in Australian aged care facilities. Res. Soc. Adm. Pharm. 2017, 13, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.; Strandell, J.; Melskens, L.; Petersen, P.S.G.; Hansen, E.H. Global Patterns of Adverse Drug Reactions Over a Decade: Analyses of Spontaneous Reports to VigiBaseTM. Drug Saf. 2012, 35, 1171–1182. [Google Scholar] [CrossRef]
- Stausberg, J. International prevalence of adverse drug events in hospitals: An analysis of routine data from England, Germany, and the USA. BMC Health Serv. Res. 2014, 14, 125. [Google Scholar] [CrossRef]
- Marcum, Z.A.; Handler, S.M.; Boyce, R.; Gellad, W.; Hanlon, J.T. Medication misadventures in the elderly: A year in review. Am. J. Geriatr. Pharmacother. 2010, 8, 77–83. [Google Scholar] [CrossRef][Green Version]
- Dugdale, D.C.; Epstein, R.; Pantilat, S.Z. Time and the patient-physician relationship. J. Gen. Intern. Med. 1999, 14, S34–S40. [Google Scholar] [CrossRef] [PubMed]
- Mechanic, D.; McAlpine, D.D.; Rosenthal, M. Are Patients’ Office Visits with Physicians Getting Shorter? N. Engl. J. Med. 2001, 344, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yarnall, K.S.H.; Pollak, K.I.; Østbye, T.; Krause, K.M.; Michener, J.L. Primary Care: Is There Enough Time for Prevention? Am. J. Public Health 2003, 93, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, E.M.; Franks, P.; Kravitz, R.L. Primary Care Visit Length, Quality, and Satisfaction for Standardized Patients with Depression. J. Gen. Intern. Med. 2007, 22, 1641–1647. [Google Scholar] [CrossRef]
- Bressler, R.; Bahl, J.J. Principles of Drug Therapy for the Elderly Patient. Mayo Clin. Proc. 2003, 78, 1564–1577. [Google Scholar] [CrossRef]
- Davies, E.C.; Green, C.F.; Taylor, S.; Williamson, P.R.; Mottram, D.R.; Pirmohamed, M. Adverse Drug Reactions in Hospital In-Patients: A Prospective Analysis of 3695 Patient-Episodes. PLoS ONE 2009, 4, e4439. [Google Scholar] [CrossRef]
- Robins, L.S.; Jackson, J.E.; Green, B.B.; Korngiebel, D.; Force, R.W.; Baldwin, L.-M. Barriers and Facilitators to Evidence-based Blood Pressure Control in Community Practice. J. Am. Board Fam. Med. 2013, 26, 539–557. [Google Scholar] [CrossRef]
- Shoemaker, S.J.; Curran, G.M.; Swan, H.; Teeter, B.S.; Thomas, J. Application of the Consolidated Framework for Implementation Research to community pharmacy: A framework for implementation research on pharmacy services. Res. Soc. Adm. Pharm. 2017, 13, 905–913. [Google Scholar] [CrossRef]
- Segal, E.M.; Bates, J.; Fleszar, S.L.; Holle, L.M.; Kennerly-Shah, J.; Rockey, M.; Jeffers, K.D. Demonstrating the value of the oncology pharmacist within the healthcare team. J. Oncol. Pharm. Pract. 2019, 25, 1945–1967. [Google Scholar] [CrossRef]
- Roman, C.; Edwards, G.; Dooley, M.; Mitra, B. Roles of the emergency medicine pharmacist: A systematic review. Am. J. Health-Syst. Pharm. 2018, 75, 796–806. [Google Scholar] [CrossRef]
- Milosavljevic, A.; Aspden, T.; Harrison, J. Community pharmacist-led interventions and their impact on patients’ medication adherence and other health outcomes: A systematic review. Int. J. Pharm. Pract. 2018, 26, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, G.D.; Ryan, K.; Patel, N.; Stretch, G. Capturing pharmacists’ impact in general practice: An e-Delphi study to attempt to reach consensus amongst experts about what activities to record. BMC Fam. Pract. 2019, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- McNicol, M.; Kuhn, C.; Sebastian, S. Standardized documentation workflow within an electronic health record to track pharmacists’ interventions in pediatric ambulatory care clinics. J. Am. Pharm. Assoc. 2019, 59, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, B.S.; Cheng, W.-H.; Lee, J.C.; Uppuluri, E.M.; Nutescu, E.A. Quality of Pharmacist-Managed Anticoagulation Therapy in Long-Term Ambulatory Settings: A Systematic Review. Ann. Pharmacother. 2017, 51, 1122–1137. [Google Scholar] [CrossRef] [PubMed]
- Benedict, A.W.; Spence, M.M.; Sie, J.L.; Chin, H.A.; Ngo, C.D.; Salmingo, J.F.; Vidaurreta, A.T.; Rashid, N. Evaluation of a Pharmacist-Managed Diabetes Program in a Primary Care Setting Within an Integrated Health Care System. J. Manag. Care Spec. Pharm. 2018, 24, 114–122. [Google Scholar] [CrossRef]
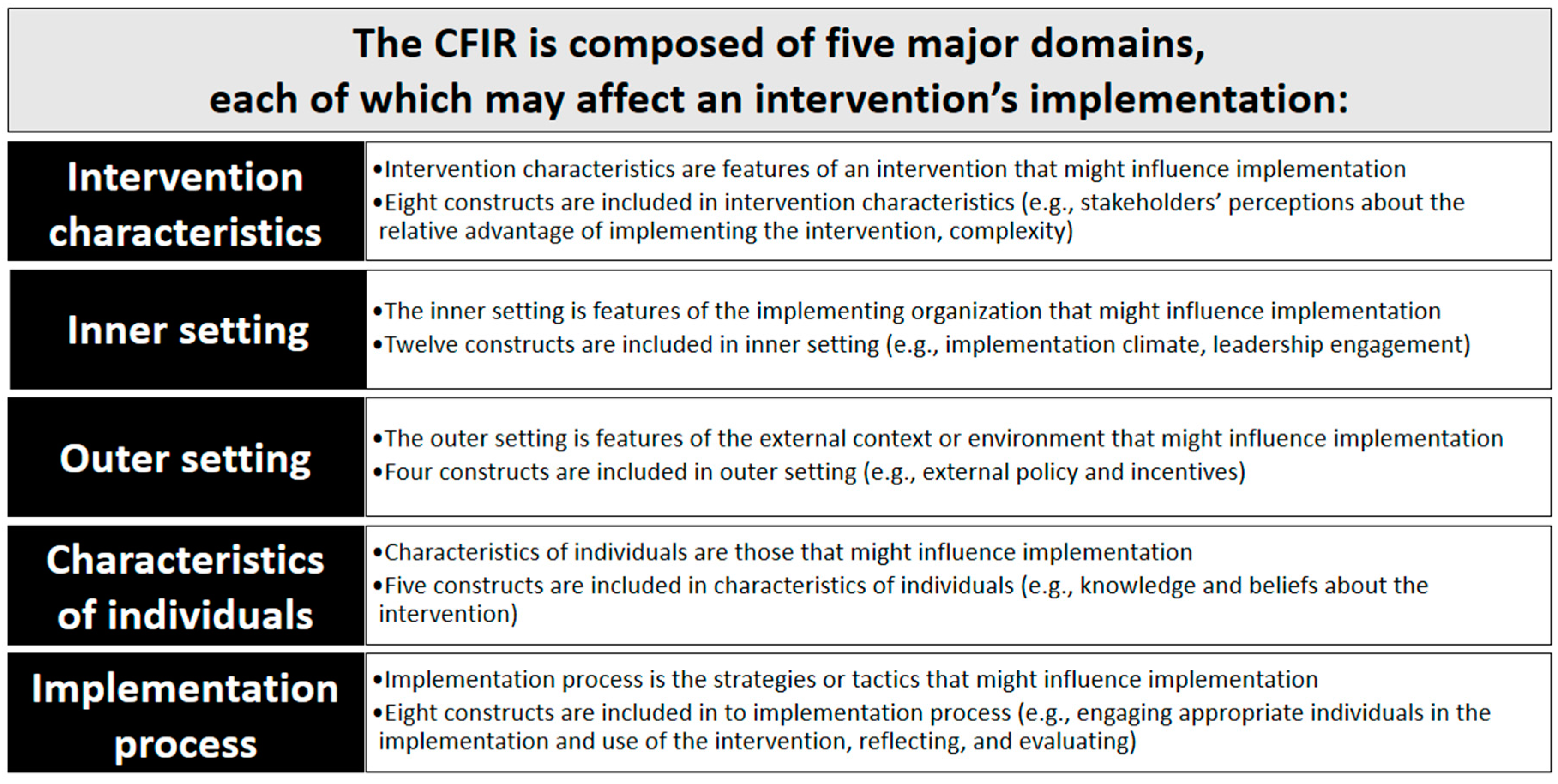
| CFIR Domain | CPC Components | Embedded Pharmacist Experiences: Practice Site Findings | Embedded Pharmacist Perceptions: Contributing Factors to Readiness |
|---|---|---|---|
| Intervention characteristics | Care Management Processes
| Practices faced challenges documenting, coding, and billing for services within established EHR systems. | University subsidized pharmacist and student time served as an incubator for trialing processes, expansion of programs, and establishment of evaluation metrics. |
Access and Continuity
| Access to educational, training, and grant resources improved incorporation of evidence-based practice in rural communities and improved access to necessary clinical and payment support resources. | University and grant subsidized pharmacist and student time served as an incubator for development and trialing and expanding of clinical pharmacists’ programs for underserved and under-resources communities (e.g., rural residents). | |
Planned Chronic and Preventative Care
| Access to educational and training resources improved incorporation of evidence-based practice in rural communities. Better understanding of necessary coding and billing processes were required to provide care and sustain embedded pharmacist service delivery. | University subsidized pharmacist and student time as well as available education and training resources supported embedded pharmacist program management, complex regimen management, and service expansion. | |
Patient Engagement
| Practice members perceived engagement with patients as vital to improve patient outcomes, self-management, and treatment adherence. | University subsidized pharmacist and student time resulted in increased patient engagement and supported chronic disease self-management. | |
Care Coordination
| Perceived coordination within the patient centered medical home (PCMH) ensured patient follow-up, improved adherence, reduced adverse events, and supported improved patient outcomes. | University subsidized pharmacist and student time reduced clinician burden, improved clinical problem identification, and supported optimization of therapy. | |
| Outer setting | Care Management Processes
| Effectively and efficiently addressing patient needs was a common challenge, this included personnel time and economic resources. | Time and resources required to meet patient needs were addressed by embedded pharmacists and students in the primary care clinic. Connecting individuals with medication assistance programs and grant supports. |
| Access and Continuity | Site health information technology systems and billing infrastructure were unable to support state documentation, coding, and billing requirements required for service provision and sustainability. | Sharing of technology resources, EHR templates, CPAs, policies, and training materials across sites. | |
Patient Engagement
| Help patients in rural and underserved communities manage chronic health conditions and access necessary supports. | Using the PCMH model, patients in rural and underserved communities received more attention from the healthcare system. | |
| Inner setting | Care Management Processes
| Health information technology systems and billing infrastructure were unable to support documentation, coding, and billing requirements to support service provision, expansion of services, and/or sustainability. | University subsidized pharmacist and grant funding to support training, infrastructure development, advocacy, and improve EHR and billing infrastructure. |
Access and Continuity
| Embedded pharmacists and students often functioned on a consultant basis and were not present within the primary care clinic due to teaching requirements, resulting in limited access, missed interventions, and sporadic engagement with the team. | Practices with embedded pharmacists’ offices centrally located were more accessible to providers and patients and utilized more by the PCMH team. | |
| Characteristics of individuals | Care Management Processes
| Primary care provider groups who worked with embedded pharmacists in the past were more likely to work with pharmacists and students to improve/optimize drug therapy. | Primary care practices that worked with embedded pharmacists to establish and modify workflows were more likely to successfully impact patient outcomes, improve medication adherence, identify and reduce adverse drug events. |
Access and Continuity
| Practices that believed in the value of embedded pharmacists worked with healthcare systems to incorporate them into the PCMH and daily practice. | Practices tended to utilize embedded pharmacists and students that were physically available, that rounded with the team, and that participated in clinical activities (e.g., lunch and learns, grand rounds). | |
Patient Engagement
| Patients and staff tend to reach out to individuals with similar values and beliefs. | Healthcare administrators and university providers supported students and pharmacists with whom they have relationships, shared mission, vision, and clinical practice goals. | |
| Implementation process | Care Management Processes
| Shared goals, consistent vernacular and tailored training and supports were required for sustainable practice site improvements. | Co-development of policies, procedures, and workflows is required. Consistent and comprehensive informal and formal training with opportunities for hands-on practice is required for program implementation and sustainability. |
| Patient Engagement | Engagement of patients, staff, and providers were required to sustain practice-level improvements. | Healthcare administrators and university providers supported students and pharmacists that add value to the organizations. This is further strengthened when the service is valued by external partners (e.g., insurers). |
| IMPLEMENTATION (Embedded Pharmacist Position, Family Practice Clinic) | ||||
|---|---|---|---|---|
| Characteristics of Intervention a | Inner Setting | Outer Setting | Individuals Involved | Implementation Process |
|
|
|
|
|
| IMPLEMENTATION (Embedded Pharmacist Position, Internal Medicine Clinic) | ||||
| Characteristics of Intervention | Inner Setting | Outer Setting | Individuals Involved | Implementation Process |
|
|
|
|
|
| IMPLEMENTATION (Embedded Pharmacist Position, Rural Health) | ||||
| Characteristics of Intervention | Inner Setting | Outer Setting | Individuals Involved | Implementation Process |
|
|
|
|
|
| IMPLEMENTATION (HIV/AIDs Incubator Project at Community Pharmacy) | ||||
| Characteristics of Intervention | Inner Setting | Outer Setting | Individuals Involved | Implementation Process |
|
|
|
|
|
| IMPLEMENTATION (Embedded Pharmacist Position with Private Medical Group) | ||||
| Characteristics of Intervention | Inner Setting | Outer Setting | Individuals Involved | Implementation Process |
|
|
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, R.; Liday, C.; Burde, A.; Pettinger, T.; Paul, A.; Nguyen, E.; Holmes, J.; Penner, M.; Jaglowicz, A.; Spann, N.; et al. Practice Transformation Driven through Academic Partnerships. Pharmacy 2020, 8, 120. https://doi.org/10.3390/pharmacy8030120
Robinson R, Liday C, Burde A, Pettinger T, Paul A, Nguyen E, Holmes J, Penner M, Jaglowicz A, Spann N, et al. Practice Transformation Driven through Academic Partnerships. Pharmacy. 2020; 8(3):120. https://doi.org/10.3390/pharmacy8030120
Chicago/Turabian StyleRobinson, Renee, Cara Liday, Anushka Burde, Tracy Pettinger, Amy Paul, Elaine Nguyen, John Holmes, Megan Penner, Angela Jaglowicz, Nathan Spann, and et al. 2020. "Practice Transformation Driven through Academic Partnerships" Pharmacy 8, no. 3: 120. https://doi.org/10.3390/pharmacy8030120
APA StyleRobinson, R., Liday, C., Burde, A., Pettinger, T., Paul, A., Nguyen, E., Holmes, J., Penner, M., Jaglowicz, A., Spann, N., Boyle, J., Biddle, M., Buffat, B., Cleveland, K., Powell, B., & Owens, C. (2020). Practice Transformation Driven through Academic Partnerships. Pharmacy, 8(3), 120. https://doi.org/10.3390/pharmacy8030120






