Interventions Delivered in the Community Pharmacy to Manage Allergic Rhinitis- A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
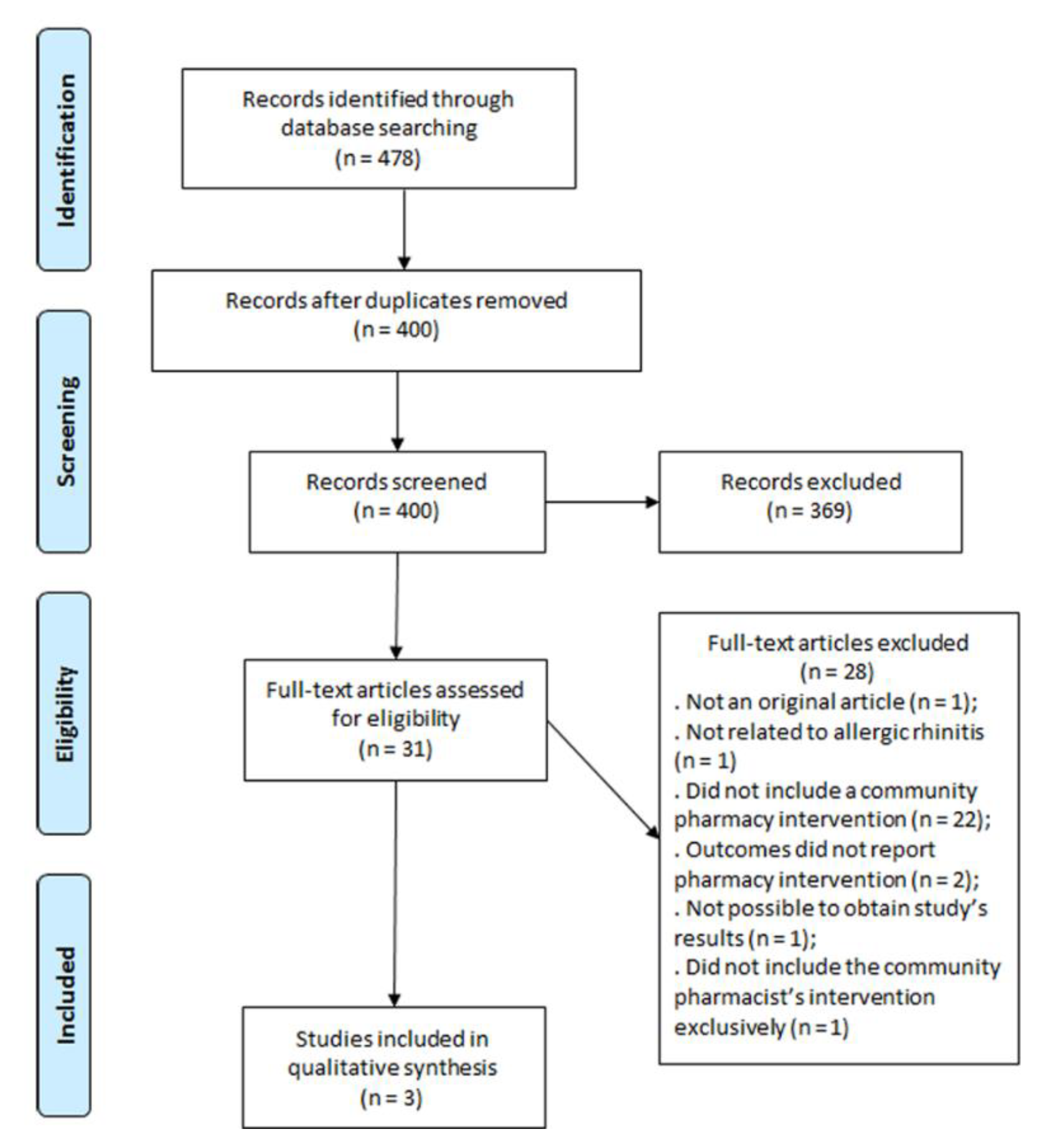
2.2. Study Selection
2.3. Data Synthesis
2.4. Quality Assessment
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hellings, P.W.; Muraro, A.; Fokkens, W.; Mullol, J.; Bachert, C.; Canonica, G.W.; Price, D.; Papadopoulos, N.; Scadding, G.; Rasp, G.; et al. A common language to assess allergic rhinitis control: Results from a survey conducted during EAACI 2013 Congress. Clin. Transl. Allergy 2015, 5, 36. [Google Scholar] [CrossRef]
- Schatz, M. A survey of the burden of allergic rhinitis in the USA. Allergy 2007, 62 (Suppl. 85), 9–16. [Google Scholar] [CrossRef] [PubMed]
- Blaiss, M.S.; Hammerby, E.; Robinson, S.; Kennedy-Martin, T.; Buchs, S. The burden of allergic rhinitis and allergic rhinoconjunctivitis on adolescents: A literature review. Ann. Allergy Asthma Immunol. 2018, 121, 43–52.e3. [Google Scholar] [CrossRef] [PubMed]
- Blaiss, M.S. Allergic rhinitis: Direct and indirect costs. Allergy Asthma Proc. 2010, 31, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Giavina-Bianchi, P.; Aun, M.V.; Takejima, P.; Kalil, J.; Agondi, R.C. United airway disease: Current perspectives. J. Asthma Allergy 2016, 9, 93–100. [Google Scholar] [CrossRef]
- Brozek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Ryan, D.; van Weel, C.; Bousquet, J.; Toskala, E.; Ahlstedt, S.; Palkonen, S.; van den Nieuwenhof, L.; Zuberbier, T.; Wickman, M.; Fokkens, W. Primary care: The cornerstone of diagnosis of allergic rhinitis. Allergy 2008, 63, 981–989. [Google Scholar] [CrossRef]
- Price, D.; Smith, P.; Hellings, P.; Papadopoulos, N.; Fokkens, W.; Muraro, A.; Murray, R.; Chisholm, A.; Demoly, P.; Scadding, G.; et al. Current controversies and challenges in allergic rhinitis management. Expert Rev. Clin. Immunol. 2015, 11, 1205–1217. [Google Scholar] [CrossRef]
- Lombardi, C.; Musicco, E.; Rastrelli, F.; Bettoncelli, G.; Passalacqua, G.; Canonica, G.W. The patient with rhinitis in the pharmacy. A cross-sectional study in real life. Asthma Res. Pract. 2015, 1, 4. [Google Scholar] [CrossRef]
- Arsoy, G.; Varis, A.; Saloumi, L.M.; Abdi, A.; Basgut, B. Insights on Allergic Rhinitis Management from a Northern Cyprus Perspective and Evaluation of the Impact of Pharmacist-Led Educational Intervention on Patients’ Outcomes. Medicina 2018, 54. [Google Scholar] [CrossRef]
- Canonica, G.W.; Triggiani, M.; Senna, G. 360 degree perspective on allergic rhinitis management in Italy: A survey of GPs, pharmacists and patients. Clin. Mol. Allergy 2015, 13, 25. [Google Scholar] [CrossRef]
- Smith, L.; Brown, L.; Saini, B.; Seeto, C. Strategies for the management of intermittent allergic rhinitis: An Australian study. Health Expect 2014, 17, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Cvetkovski, B.; Kritikos, V.; Price, D.; Yan, K.; Smith, P.; Bosnic-Anticevich, S. Identifying the hidden burden of allergic rhinitis (AR) in community pharmacy: A global phenomenon. Asthma Res. Pract. 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Members of the Workshops. ARIA in the pharmacy: Management of allergic rhinitis symptoms in the pharmacy. Allergic rhinitis and its impact on asthma. Allergy 2004, 59, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Fromer, L.M.; Ortiz, G.; Ryan, S.F.; Stoloff, S.W. Insights on allergic rhinitis from the patient perspective. J. Fam. Pract. 2012, 61, S16–S22. [Google Scholar] [PubMed]
- Bosnic-Anticevich, S.; Costa, E.; Menditto, E.; Lourenco, O.; Novellino, E.; Bialek, S.; Briedis, V.; Buonaiuto, R.; Chrystyn, H.; Cvetkovski, B.; et al. ARIA pharmacy 2018 “Allergic rhinitis care pathways for community pharmacy”: AIRWAYS ICPs initiative (European Innovation Partnership on Active and Healthy Ageing, DG CONNECT and DG Sante) POLLAR (Impact of Air POLLution on Asthma and Rhinitis) GARD Demonstration project. Allergy 2019, 74, 1219–1236. [Google Scholar] [CrossRef]
- Scadding, G.K.; Kariyawasam, H.H.; Scadding, G.; Mirakian, R.; Buckley, R.J.; Dixon, T.; Durham, S.R.; Farooque, S.; Jones, N.; Leech, S.; et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin. Exp. Allergy 2017, 47, 856–889. [Google Scholar] [CrossRef]
- Kuehl, B.L.; Abdulnour, S.; O’Dell, M.; Kyle, T.K. Understanding the role of the healthcare professional in patient self-management of allergic rhinitis. SAGE Open Med. 2015, 3, 2050312115595822. [Google Scholar] [CrossRef]
- Price, D.B.; Smith, P.K.; Harvey, R.J.; Carney, A.S.; Kritikos, V.; Bosnic-Anticevich, S.Z.; Christian, L.; Skinner, D.; Carter, V.; Durieux, A.M. Real-life treatment of rhinitis in Australia: A historical cohort study of prescription and over-the-counter therapies for patients with and without additional respiratory disease. Pragmatic Obs. Res. 2018, 9, 43–54. [Google Scholar] [CrossRef]
- Smith, P.; Price, D.; Harvey, R.; Carney, A.S.; Kritikos, V.; Bosnic-Anticevich, S.Z.; Christian, L.; Skinner, D.; Carter, V.; Durieux, A.M.S. Medication-related costs of rhinitis in Australia: A NostraData cross-sectional study of pharmacy purchases. J. Asthma Allergy 2017, 10, 153–161. [Google Scholar] [CrossRef]
- Mehuys, E.; Gevaert, P.; Brusselle, G.; Van Hees, T.; Adriaens, E.; Christiaens, T.; Van Bortel, L.; Van Tongelen, I.; Remon, J.P.; Boussery, K. Self-medication in persistent rhinitis: Overuse of decongestants in half of the patients. J. Allergy Clin. Immunol. Pract. 2014, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, O.; Calado, S.; Sa-Sousa, A.; Fonseca, J. Evaluation of allergic rhinitis and asthma control in a Portuguese community pharmacy setting. J. Manag. Care Spec. Pharm. 2014, 20, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Hellings, P.W.; Agache, I.; Amat, F.; Annesi-Maesano, I.; Ansotegui, I.J.; Anto, J.M.; Bachert, C.; Bateman, E.D.; Bedbrook, A.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Phase 4 (2018): Change management in allergic rhinitis and asthma multimorbidity using mobile technology. J. Allergy Clin. Immunol. 2019, 143, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Hennessy, S. Pharmacist care and clinical outcomes for patients with reactive airways disease. JAMA 2002, 288, 1642–1643. [Google Scholar] [CrossRef]
- Milosavljevic, A.; Aspden, T.; Harrison, J. Community pharmacist-led interventions and their impact on patients’ medication adherence and other health outcomes: A systematic review. Int. J. Pharm. Pract. 2018, 26, 387–397. [Google Scholar] [CrossRef]
- Westerlund, T.; Andersson, I.L.; Marklund, B. The quality of self-care counselling by pharmacy practitioners, supported by IT-based clinical guidelines. Pharm. World Sci. PWS 2007, 29, 67–72. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- O’Connor, J.; Seeto, C.; Saini, B.; Bosnic-Anticevich, S.; Krass, I.; Armour, C.; Smith, L. Healthcare professional versus patient goal setting in intermittent allergic rhinitis. Patient Educ. Couns. 2008, 70, 111–117. [Google Scholar] [CrossRef]
- Todorova, A.; Tsvetkova, A.; Mihaylova, S.; Andreevska, K.; Kondova, A.; Arnaoudova, M. The impact of pharmaceutical care on improving the quality of life in patients with allergic rhinitis. CBU Int. Conf. Proc. 2017, 5, 6. [Google Scholar] [CrossRef]
- Vandenplas, O.; Vinnikov, D.; Blanc, P.D.; Agache, I.; Bachert, C.; Bewick, M.; Cardell, L.O.; Cullinan, P.; Demoly, P.; Descatha, A.; et al. Impact of Rhinitis on Work Productivity: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1274–1286. [Google Scholar] [CrossRef]
- Scadding, G.K.; Scadding, G.W. Diagnosing Allergic Rhinitis. Immunol. Allergy Clin. North. Am. 2016, 36, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Nolte, H.; Nepper-Christensen, S.; Backer, V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir. Med. 2006, 100, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Juel-Berg, N.; Darling, P.; Bolvig, J.; Foss-Skiftesvik, M.H.; Halken, S.; Winther, L.; Hansen, K.S.; Askjaer, N.; Heegaard, S.; Madsen, A.R.; et al. Intranasal corticosteroids compared with oral antihistamines in allergic rhinitis: A systematic review and meta-analysis. Am. J. Rhinol. Allergy 2017, 31, 19–28. [Google Scholar] [CrossRef]
- Urrutia-Pereira, M.; Bittencourt, R.; Fernandez, C.; Cruz, A.A.; Simon, L.; Rianelli, P.; Solé, D. Knowledge of allergic rhinitis and its impact on asthma among pharmacists (ARIA guidelines for pharmacists): A comparative pilot study in Brazil and Paraguay. Arq Asma Alerg Imunol. 2018, 2, 8. [Google Scholar] [CrossRef]
| PubMed | Web of Science | Cochrane Central Register of Controlled Trials | ||||
|---|---|---|---|---|---|---|
| Search | Result | Search | Result | Search | Result | |
| 1 | Pharmacy | 369,988 | TOPIC:(Pharmacy) | 39,661 | Pharmacy | 12,793 |
| 2 | Community pharmacy | 25,790 | TOPIC:(Community Pharmacy) | 7911 | Community pharmacy | 1619 |
| 3 | Pharmaceutical services | 75,375 | TOPIC:(Pharmaceutical services) | 5499 | Pharmaceutical services | 1332 |
| 4 | Pharmaceutical care | 94,491 | TOPIC:(Pharmaceutical care) | 16,051 | Pharmaceutical care | 4205 |
| 5 | Pharmacist * | 33,612 | TOPIC:(Pharmacist) | 30,192 | Pharmacist * | 3914 |
| 6 | Clinical pharmacy | 87,664 | TOPIC:(Clinical Pharmacy) | 9309 | Clinical pharmacy | 10,802 |
| 7 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 | 439,230 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 | 74,702 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 | 17,638 |
| 8 | Allergic rhinitis | 29,036 | TOPIC:(Allergic Rhinitis) | 22,524 | Allergic rhinitis | 6743 |
| 9 | Hay fever | 15,255 | TOPIC:(Hay fever) | 4196 | Hay fever | 714 |
| 10 | 8 OR 9 | 30,080 | 8 OR 9 | 25,647 | 8 OR 9 | 6977 |
| 11 | 7 AND 10 | 260 | 7 AND 10 | 117 | 7 AND 10 | 33 |
| Author & Year of Publication | Country | Objective | Type of Study | Outcomes | Tools used to Evaluate the Outcomes | Number of Participants | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Arsoy, G. et al. (2018) [10] | Cyprus (Northern Cyprus) | Evaluate the effectiveness of educational intervention by pharmacists to improve the care of patients with AR | Randomized controlled trial (RCT) | Quality of life (QoL) Symptom severity | Mini Rhinoconjunctivitis Quality of Life Questionnaire (Mini RQLQ©) Analog visual scales (VAS) | Pharmacists (n = 70) Patients (n = 63) * | The pharmaceutical intervention significantly improved the quality of life of patients. Both groups experienced an improvement in symptom severity. | Small number of pharmacists and patients |
| O’ Connor, J. et al. (2007) [28] | Australia (Sydney) | Examine the impact of community pharmacist goal setting on AR management versus patient setting | Mixed method parallel-group study, with randomization of participating pharmacies | Quality of life (QoL) Symptom severity | Mini Rhinoconjunctivitis Quality of Life Questionnaire (Mini RQLQ©) Analog visual scales (VAS) | Pharmacists (n = 8) Patients (n = 47) # | The intervention group showed greater improvements in symptom severity than the control group. | Small number of pharmacists and patients, only metropolitan Sydney community pharmacies |
| Todorova, A. et al. (2017) [29] | Bulgaria (Varna) | Assess the impact of pharmaceutical care on AR management | Case study | Quality of life (QoL) | SF-12v2 Health Survey | Patients (n = 63) | The pharmaceutical intervention significantly improved the quality of life of patients with AR | Small number of patients, only city of Varna |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
José, J.; Cvetkovski, B.; Kritikos, V.; Tan, R.; Bosnic-Anticevich, S.; Lourenço, O. Interventions Delivered in the Community Pharmacy to Manage Allergic Rhinitis- A Systematic Review of the Literature. Pharmacy 2020, 8, 80. https://doi.org/10.3390/pharmacy8020080
José J, Cvetkovski B, Kritikos V, Tan R, Bosnic-Anticevich S, Lourenço O. Interventions Delivered in the Community Pharmacy to Manage Allergic Rhinitis- A Systematic Review of the Literature. Pharmacy. 2020; 8(2):80. https://doi.org/10.3390/pharmacy8020080
Chicago/Turabian StyleJosé, Jéssica, Biljana Cvetkovski, Vicky Kritikos, Rachel Tan, Sinthia Bosnic-Anticevich, and Olga Lourenço. 2020. "Interventions Delivered in the Community Pharmacy to Manage Allergic Rhinitis- A Systematic Review of the Literature" Pharmacy 8, no. 2: 80. https://doi.org/10.3390/pharmacy8020080
APA StyleJosé, J., Cvetkovski, B., Kritikos, V., Tan, R., Bosnic-Anticevich, S., & Lourenço, O. (2020). Interventions Delivered in the Community Pharmacy to Manage Allergic Rhinitis- A Systematic Review of the Literature. Pharmacy, 8(2), 80. https://doi.org/10.3390/pharmacy8020080







